Abstract
Despite recent treatment improvements, multiple myeloma remains an incurable disease. Since antibody-dependent cell-mediated cytotoxicity is an important effector mechanism of daratumumab, we explored the possibility of improving daratumumab-mediated cell-mediated cytotoxicity by blocking natural killer cell inhibitory receptors with the human monoclonal anti-KIR antibody IPH2102, next to activation of natural killer cells with the immune modulatory drug lenalidomide. In 4-hour antibody-dependent cell-mediated cytotoxicity assays, IPH2102 did not induce lysis of multiple myeloma cell lines, but it did significantly augment daratumumab-induced myeloma cell lysis. Also in an ex vivo setting, IPH2102 synergistically improved daratumumab-dependent lysis of primary myeloma cells in bone marrow mononuclear cells (n=21), especially in patients carrying the FcγRIIIa-158F allele or the FcγRIIa-131R allele, who bind IgG1 with lower affinity than patients carrying the FcγRIIIa-158V allele or the FcγRIIa-131H allele. Finally, a further synergistically improved myeloma cell lysis with the daratumumab-IPH2102 combination was observed by adding lenalidomide, which suggests that more effective treatment strategies can be designed for multiple myeloma by combining daratumumab with agents that independently modulate natural killer cell function.
Introduction
Multiple myeloma (MM), the progressive malignancy of clonal plasma cells is the second most common hematologic neoplasia1 and accounts for 1.4% of all cancers and for 1.8% of all cancer mortality worldwide.2 Despite encouraging improvements in the survival of MM patients over the last decade, the disease remains incurable, even with combination therapies with effective novel pharmacological agents.2–5 An attractive novel alternative to these treatments is the targeting of MM with therapeutic antibodies, as already standard-of-care in several other hematologic malignancies. Therefore, we generated the CD38-specific human monoclonal antibody, daratumumab (DARA), which induces MM cell death via various mechanisms, including antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).6 Based on these preclinical data, DARA is currently being evaluated in patients with relapsed/refractory MM, with encouraging results.7
In previous studies, we demonstrated that DARA-mediated ADCC can be significantly improved by lenalidomide (LEN), mainly due to the potent capacity of LEN to activate NK cells.8,9 Based on these observations, we hypothesized that the efficacy of DARA-induced, NK cell-mediated ADCC may be further enhanced via modulation of NK-cell regulatory signals transmitted via the inhibitory and activating NK receptors [killer-cell immunoglobulin-like receptors (KIRs)].10,11 Since the signals transmitted by inhibitory KIRs may prevent NK cell-mediated ADCC, even in the presence of an activating receptor-ligand interaction,12 we set out to test the possibility of improving DARA efficacy by blocking inhibitory KIRs.
IPH2102 (formerly 1-7F9 and IPH2101) is a hinge-stabilized, human IgG4 monoclonal antibody that blocks the interaction of the three main inhibitory KIR receptors (KIR2DL-1, -2, -3) with their ligands, the human leukocyte antigen-C (HLA-C) molecules. The predecessor of IPH2102, IPH2101 (a wild-type IgG4 version of the antibody), was shown to increase in vitro NK-cell cytotoxicity against MM cells, but not against normal healthy cells.13,14 Clinical trials conducted with IPH2101 in patients with relapsed/refractory MM and smoldering myeloma revealed that the clinical use of IPH2101 is safe and tolerable at doses that achieve full inhibitory KIR saturation, with disease stabilization as the best observed response to IPH2101.15,16 This suggested that this antibody likely requires inclusion in a combination regimen such as with a potent ADCC-inducing antibody and/or with NK-cell activating agents like LEN. Hence, we explored in a series of ex vivo assays the potential benefits of combining DARA with IPH2102 and LEN. We demonstrate that DARA-induced killing of primary MM cells increases synergistically when combined with these NK-enhancing agents.
Methods
Bone marrow mononuclear cells from MM patients
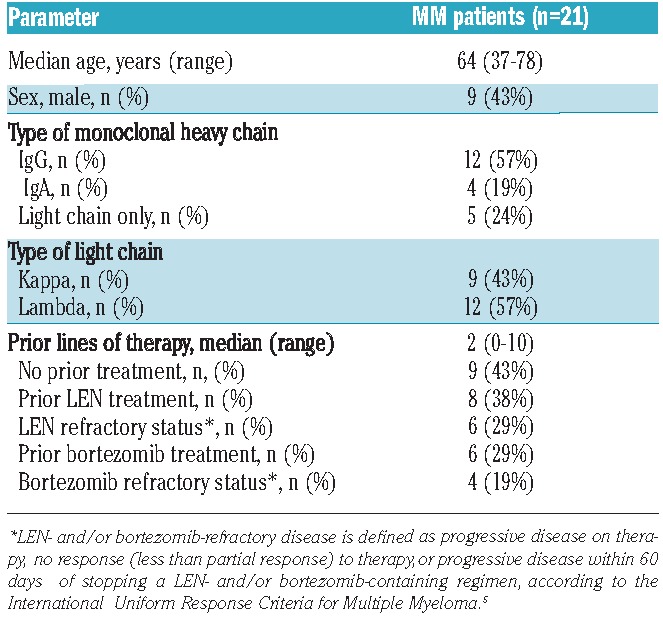
All patients’ samples were collected and stored under protocols approved by the Institutional Review Board. All procedures involving bone marrow material were in accordance with the Declaration of Helsinki and approved by the local medical ethical committee. Mononuclear cells (MNC) from the bone marrow (BM) were isolated by Ficoll-Hypaque density-gradient centrifugation and contained 2%–35% MM cells as detected by flow cytometry. Freshly isolated BM-MNC from patients were immediately used in experiments (see below). Characteristics of the patients’ providing the tested BM-MNC are summarized in Table 1.
Table 1.
Base-line characteristics of the tested MM patients’ BM-MNC aspirates.
MM cell lines
The CD38 positive, luciferase (LUC)-transduced MM cell lines UM9 and RPMI8226 were cultured in RPMI 1640 (Invitrogen Life Technologies, Carlsbad, California, USA), supplemented with 10% fetal bovine serum (FBS; Integro BV, Salisbury, NC, USA) and antibiotics (100 units/mL penicillin, 100 μg/mL streptomycin; both Invitrogen Life Technologies) as previously described.17
Peripheral blood mononuclear cells from healthy donors
All procedures involving material from healthy donors were approved by the local institutional medical ethical committee. Peripheral blood from healthy volunteers was obtained after written informed consent. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density-gradient centrifugation to use as effector cells in ADCC assays.
Immunophenotyping by flow cytometry
Cell surface expression of various receptors was determined by FACS analysis using fluorochrome-conjugated monoclonal antibodies (mAb). PE-conjugated IPH2102 was produced by Innate Pharma. All other mAbs used for phenotyping were purchased from BD Biosciences (Franklin Lakes, New Jersey, USA). Flow cytometry was performed using a FACS-Calibur device (BD Biosciences, Franklin Lakes, New Jersey, USA); data were analyzed using CellQuest software.
Test antibodies
The anti-KIR mAb IPH2102 (formerly 1-7F9/IPH2101) is a hinge-stabilized human IgG4 antibody, anti-KIR2DL1/L2/L3/S1/S2, produced in a CHO cell line and was generated by Innate Pharma (Marseille, France). Polyclonal human IgG4 (Sigma-Aldrich, St. Louis, Missouri, USA) was used as an isotype control for IPH2102. DARA was provided by Genmab (Utrecht, The Netherlands). Human IgG1-b12 against an innocuous antigen (HIV-1 gp120), donated by Genmab, was used as an isotype control for DARA.
Bioluminescence imaging-based ADCC assay using LUC-transduced MM cell lines
LUC-transduced MM cell lines UM9 and RPMI8226 were co-cultured with effector cells (freshly isolated PBMCs from healthy donors) at an effector to target ratio of 25:1 in white opaque 96-well flat bottom plates (Costar) in the presence of solvent control, or IgG1-b12 control antibody (1.0 μg/mL), or DARA (0.0001, 0.001, 0.01, 0.1 and 1.0 μg/mL), and/or IgG4 control antibody (10.0 μg/mL), or IPH2102 (10.0 μg/mL) for 4 h. The survival of LUC+-MM cells was then determined by bioluminescence imaging (BLI), 10 min after addition of the substrate luciferin (125 μg/mL; Promega, Leiden, The Netherlands). Lysis of MM cells was determined using the following formula: % lysis = 1 − (mean BLI counts in treated wells / mean BLI counts in control wells) × 100%.
Flow cytometry-based ADCC assays using BM-MNC aspirates from MM patients
Bone marrow mononuclear cells derived from 21 MM patients, containing 2%–57% CD138+ tumor cells, but also autologous effector cells, were used in flow cytometry-based ADCC assays, as previously described.8,9 BM-MNC were suspended in RPMI+10% FBS and incubated with serial concentrations of DARA, IPH2102, control antibodies and LEN (Celgene, Utrecht, The Netherlands) alone or in combination in 96-well U bottom plates. BM-MNC were incubated in fully humidified 5% CO2-air mixture at 37°C for 48 h. Sample viability at incubation was more than 98%, as assessed by using ToPro-3 (Invitrogen Life Technologies). Surviving MM cells after 48 h were enumerated by single platform flow cytometric analysis of CD138+ cells in the presence of Flow-Count Fluorospheres (Beckman Coulter) and ToPro-3 to determine absolute numbers of viable MM cells. The percentage of daratumumab-mediated ADCC was then calculated using the following formula: % lysis cells = 1 − (absolute number of surviving CD138+ cells in treated wells / absolute number of surviving CD138+ cells in control wells) × 100%. Where indicated, the DARA and IPH2102-specific lysis values were calculated by using the counts of surviving MM cells in IgG1-b12 and IgG4 treated wells, respectively.
Fc gamma receptor polymorphism genotyping
After isolating the patients’ genomic DNA from PBMC, the typing for FcγRIIIa (CD16) polymorphisms (158V/F) on immune effector cells, including NK cells and macrophages, and FcγRIIa (CD32) polymorphisms (131H/R) on immune effector cells, including monocytes, was performed as described by Koene et al.18 and Jiang et al.,19 respectively.
Statistical analysis
Differences between indicated groups were analyzed for significance in two-tailed paired Student t-tests analyses using Prism software (Graphpad Software Inc., v.5). P<0.05 was considered significant. Where indicated, expected lysis values from combinatorial treatments were calculated using the formula: % expected lysis = (% lysis by Agent 1 + % lysis by Agent 2) − % lysis by Agent 1 × % lysis by Agent 2. This formula assumes that there is only an additive effect between the combined agents. Paired t-tests were then used to test the statistical difference between the observed and expected values. The additive interaction was rejected, and synergy was concluded if the observed values were significantly higher than the expected values. In triple combinations, the combination of the first two agents was considered as Agent 1.
Results
IPH2102 increases DARA-mediated ADCC of MM cells
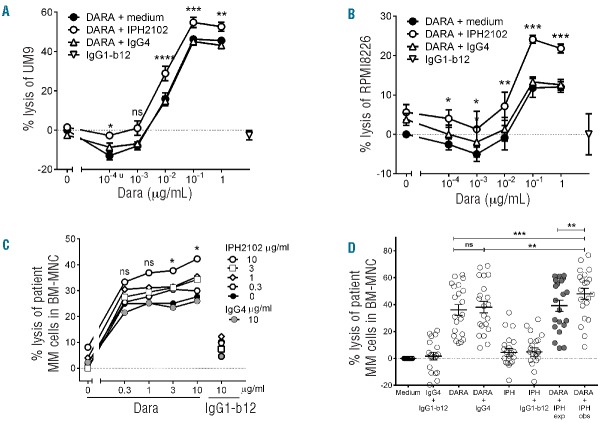
We first investigated the potential of combining DARA with IPH2102 in standard 4-h ADCC assays using MM cell lines UM9 and RPMI8226 as target cells and PBMC from different healthy donors (n=4) as effector cells. An irrelevant polyclonal IgG4 antibody and an irrelevant IgG1 antibody (IgG1-b12) were used as isotype controls. DARA, but not IPH2102, induced ADCC of MM cells in a dose-dependent manner (Figure 1A and B). Interestingly, however, DARA-dependent ADCC was significantly augmented in the presence of IPH2102 (P<0.05), with a somewhat larger effect in the cell line RPMI8226, which is relatively resistant to DARA. These results suggested that blockade of KIR receptors can improve NK cell-mediated lysis by DARA.
Figure 1.
Dose-dependent enhancement of DARA-mediated ADCC by IPH2102. (A and B) 4-hour ADCC assays were performed using PBMC from 4 healthy donors as effector cells. The MM cell lines UM9 (A) and RPMI8226 (B) were used as target cells at an effector to target ratio of 25:1. Data show mean lysis and SEM of 4 experiments. DARA was used at indicated concentrations; IPH2102 was used at 10 μg/mL. IgG4 (10 μg/mL) was used as isotype control for IPH2102. IgG1-b12 was used at 1 μg/mL as isotype control for DARA. P values were calculated by a paired t-test comparing DARA + IPH2102 versus DARA + IgG4. ns: non-significant; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Negative lysis levels indicate MM cell growth. (C) BM-MNC of 10 MM patients were incubated with DARA and/or IPH2102 and/or controls at serial concentrations (0–10 μg/mL) for 48 h. IgG4 was used as isotype control for IPH2102, IgG1-b12 was used as isotype control for DARA. Surviving CD138+ MM cells were enumerated by flow cytometry. Percentage lysis of MM cells was calculated as indicated in the Methods section. The mean lysis values are calculated and depicted. P values were calculated by a paired t-test comparing DARA + IPH2102 versus DARA + IgG4. ns: non-significant; *P<0.05. (D) BM-MNC of 21 MM patients were incubated with DARA and/or IPH2102 and/or control antibodies at 10 μg/mL for 48 h. Data show mean lysis and SEM. The observed (obs) lysis levels of MM cells with DARA-IPH2102 were compared to the expected lysis levels (exp), which were calculated with the assumption that the combinatorial effect is achieved by additive effects as indicated in the Methods section. The statistical differences between the observed and expected levels were tested by a paired t-test. Ns: non-significant; *P<0.05, **P<0.01, ***P<0.001. Every circle represents an individual BM-MNC patient sample. The black bar indicates the group mean±SEM.
To confirm these results in a more physiological setting, we employed our previously described ex vivo flow cytometry-based cytotoxicity assays, in which we measure the survival of primary CD138+ MM cells in patients’ BM-MNC, without separating malignant cells from their microenvironment and autologous effector cells.8 In this setting, incubation of 10 BM-MNC in serial dilution (0–10 μg/mL) of DARA and IPH2102 in a checkerboard fashion, confirmed the dose-dependent induction of MM cell lysis by DARA. Again, IPH2102 induced little or no lysis alone, even at a concentration of 10 μg/mL, which has been shown to saturate all KIR receptors.14,15 However, 10 μg/mL of IPH2102 combined with DARA (>3 μg/mL), significantly enhanced DARA-mediated killing (P<0.05) (Figure 1C). Hence, in an extended analysis, in which we assessed BM-MNC from 21 patients, we tested both antibodies only at a saturating concentration of 10 μg/mL (Figure 1D). Again, IPH2102 alone was not able to induce MM cell lysis, but it significantly improved the DARA-dependent ADCC in these primary patient samples. A mixed model analysis indicated the synergistic action of DARA and IPH2102 to generate an average of 10% extra tumor cell lysis as the observed amount of tumor cell lysis significantly exceeded the expected levels, which were calculated with the assumption that the combinatorial effect was achieved only by additive effects (Figure 1D).
Impact of FcγR polymorphisms on the DARA-IPH2102 combination effect
Although the MM cell lysis was significantly increased by DARA-IPH2102 combination, the observed heterogeneity in the results prompted us to analyze the impact of the FcγRIIIa 158 V-F and FcγRIIa 131 H-R polymorphisms on the outcome. FcγRIIIa (CD16) is expressed on immune effector cells, including NK cells. FcγRIIa (CD32) is expressed on immune effector cells, including monocytes. The homozygous FcγRIIa His 131 and FcγRIIIa Val 158 have a higher affinity for IgG1.20 FcγR polymorphisms influence the efficacy of ADCC21 and might also influence effectiveness of monoclonal antibody-based immunotherapy.22 Genomic DNA material was available from 16 of the tested MM patients and used for typing the FcγRIIa and FcγRIIIa polymorphism. We did not observe a significant association between Fc-receptor polymorphisms and DARA-mediated MM cell lysis in our cohort. However, the synergism between DARA and IPH2102 appeared to occur only in patients carrying an FcγRIIIa-158F allele (V/F and FF) and in patients carrying an FcγRIIa-131R allele (H/R and R/R) (Figure 2A and B). This suggests that the synergistic effects between IPH2102 and DARA are most pronounced in patients carrying an FcγRIIIa or FcγRIIa polymorphism with lower affinity to DARA.
Figure 2.
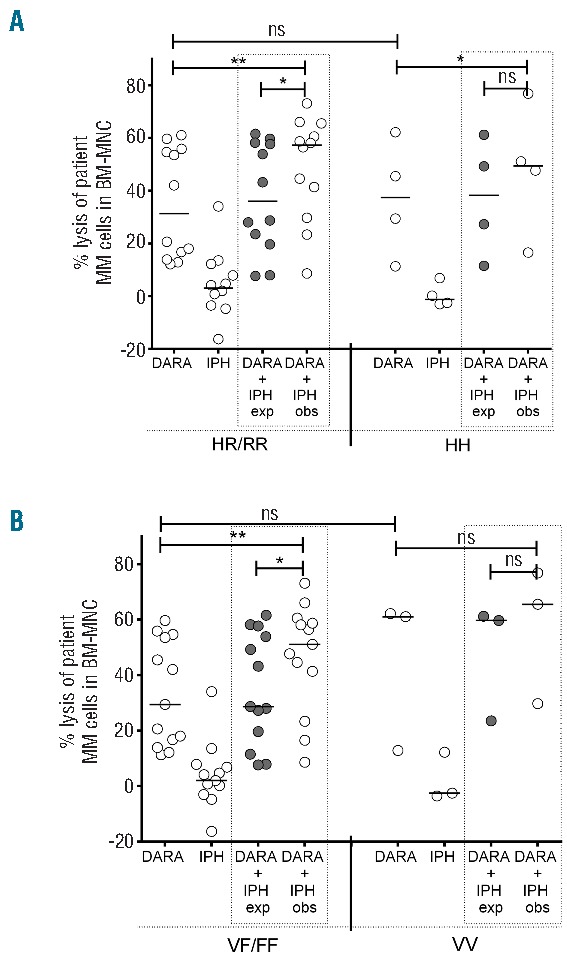
Impact of FcγR polymorphisms on the DARA-IPH2102 combination effect. Genomic DNA available from PBMC from 16 patients tested in Figure 1D were typed for (A) FcγRIIa 131 H/R and (B) FcγRIIIa 158 V/F polymorphisms on effector cells. The data obtained in Figure 1D were then grouped according to the respective polymorphisms. Every circle represents an individual patient sample. The black bars indicate the group median value. The observed (obs) lysis levels of MM cells with DARA-IPH2102 were compared to the expected lysis levels (exp), which were calculated with the assumption that the combinatorial effect is achieved by additive effects as indicated in methods. P values were calculated by a paired t-test. ns: non-significant; *P<0.05, **P<0.01.
IPH2102 further augments DARA-mediated killing of MM in the presence of LEN
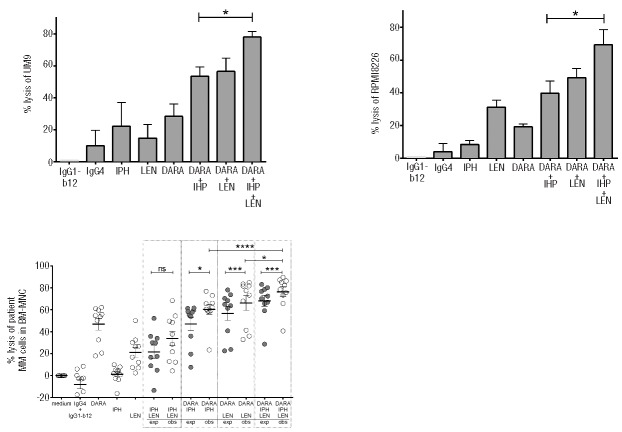
After demonstrating the improvement of DARA-mediated MM cell lysis by IPH2102, we questioned whether adding LEN to this combination could further improve MM cell lysis. In our previous studies, LEN improved DARA-dependent MM cell lysis mainly via the activation of NK cells8,9,23 and this effect was best visible after 48 h incubation of effector cells with LEN. Hence in 4-h ADCC assays, using MM cell lines UM9 and RPMI8226 as target cells and PBMC from different healthy donors (n=4) as effector cells, we not only added LEN in the assay, but also pre-treated the effector PBMC for 48 h with LEN to determine the effect of LEN. As expected, LEN increased the DARA-dependent MM cell lysis in UM9 and RPMI8226 cells, but also significantly up-regulated MM cell lysis in combination with DARA-IPH2102 treatment (Figure 3A and B). To confirm this effect on primary MM cells, we used our 48-h flow cytometry-based MM cell lysis assays and incubated the BM-MNC of 10 MM patients with DARA, IPH2102, control antibodies or LEN, alone or in combinations (Figure 3C). In these assays, DARA, LEN and IPH2102 induced a median MM cell lysis of 54%, 22% and 3%, respectively. Combining DARA with IPH2102 and LEN further increased tumor cell lysis compared to DARA plus IPH2012 alone (P<0.05). With a median lysis level of 81%, the triple combination significantly exceeded the levels observed for DARA+LEN (75%) or DARA+IPH2102 (61%). The MM cell lysis observed for the triple combination were found to be synergistic, since the observed values significantly exceeded the calculated expected values presuming additive effects. Taken together, these data suggest superiority of the DARA, IPH2102 and LEN triple combination compared to respective double combinations of these agents.
Figure 3.
Improvement of DARA-IPH2102 combinatorial effects by LEN. Four-hour ADCC assays were performed using PBMC from healthy donors as effector cells and the MM cell lines UM9 (A) and RPMI8226 (B) as target cells. DARA, IPH2102 and LEN were used at 0.01 μg/mL, 10 μg/mL and 3 μM, respectively. P values are calculated using a paired student t-test. Ns: non-significant; *P<0.05. Data shown represent mean±SEM of 4 experiments (C) BM-MNC of 10 MM patients were incubated with DARA (10 μg/mL), LEN (3 μM), IPH2102 (10 μg/mL), combination treatments or control antibodies for 48 h. The observed (obs) lysis levels of MM cells with DARA-IPH2102-LEN were compared to the expected lysis levels (exp), which were calculated with the assumption that the combinatorial effect is achieved by additive effects as indicated in methods. Black bars indicate the group mean value±SEM. P values are calculated using a paired student t-test. ns: non-significant; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Discussion
In this study, we explored the possibility to further improve DARA-mediated killing of MM cells by combining it with the anti-KIR antibody IPH2102 and the immunomodulatory drug LEN. The rationale behind this exploration is that a further augmentation of the activity of DARA might lead to stronger in vivo responses in order to increase the chances for long-term sustained remissions.
To develop this concept, we previously tested the combination of DARA with LEN and demonstrated that its potent activating effects on NK cells can significantly improve DARA-dependent ADCC of MM cells.8 In this regard, adding IPH2102 to this combination was a logical next step because this novel monoclonal antibody, was shown to increase NK cell lysis of malignant cells by blocking the three main inhibitory KIR receptors (KIR2DL1/2/3) on NK cells.14 It, therefore, could conceivably further potentiate DARA-dependent ADCC of MM cells independent of LEN. In a series of experiments, we discovered that IPH2102 alone, in contrast to earlier studies (see below), did not induce significant lysis of MM cells. However, it did augment DARA-dependent ADCC, and this combinatorial anti-MM effect was further improved by LEN. Our analyses strongly suggest that the enhancement of DARA-dependent ADCC by IPH2012, in the absence or presence of LEN, is established through synergistic action of the agents since the observed levels of combinatorial tumor cell lysis significantly exceeded expected levels, which were calculated with the assumption that the effects were merely additive. A synergistic effect is consistent with the assumed mechanisms of action, because IPH2102 and LEN most probably increase NK cell cytotoxicity via non-overlapping, independent mechanisms. While IPH2102 blocks KIR-inhibitory signaling of NK cells,14 LEN stimulates the proliferation of NK cells and activates them to increase the production of IFN-γ, TNFα and granzyme B.8,23–26
As mentioned, in our experiments, IPH2102 alone induced little or no MM cell lysis (Figures 1–3). This is apparently inconsistent with previous reports, in which IPH2101 was found to induce NK-cell cytotoxicity against lymphoma, acute myeloid leukemia (AML) cells and MM cells.14,27 While this latter study used IPH2101 at a higher (30 μg/mL) concentration than we did (10 μg/mL), this is probably not the reason for the observed apparent discrepancy, since earlier tests have demonstrated that KIRs are fully saturated above 10 μg/mL IPH2102.14 On the other hand, it needs to be noted that the study in the AML setting was performed using purified and IL-2 activated NK cells from HLA-C-matched healthy donors, which were furthermore used at higher NK:tumor ratios (10:1) to induce visible and significant tumor cell lysis above background levels. By contrast, we carried out our experiments mainly in patient-derived BM-MNC, thus without isolating the patient’s own MM cells or NK cells from their natural environment. Consequently, in these experiments we readily achieve a median NK cell: MM cell ratio of 1:1 (data not shown), which generally does not induce significant MM cell lysis without addition of ADCC-inducing antibodies like DARA. Another apparent discrepancy, which might be related to the differences in the experimental approach, is the absence of IPH2102-mediated MM cell lysis when combined with LEN in our setting, while previously IPH-LEN combinations were shown to improve MM cell lysis in both AML and MM settings.13 In addition to the aforementioned experimental differences, our approach applied a lower dose of LEN (3 μM vs. 10 μM in other studies) since in a clinical setting it may be difficult to obtain LEN plasma levels above 3 μM without inducing adverse toxicities.28 Our results, combining DARA with IPH2102, are fully compatible with the recent findings that anti-KIR antibodies augment the efficacy of ADCC-inducing antibodies like rituximab.27 Accordingly, our results advocate that the best strategy to exploit the beneficial effects of IPH2102 may be in combination with ADCC-inducing antibodies. Furthermore, as we also show here, the ADCC-enhancing effects of IPH2102 with DARA can be further potentiated by addition of LEN. In this light, it is interesting that significant synergistic effects between DARA and IPH2102 were especially observed in bone marrow samples from MM patients carrying alleles for the low affinity variants for the activating receptors for ADCC, FcγRIIa or FcγRIIIa (Figure 2).
In summary, this preclinical evaluation suggests that a further increase in the potency of MM treatment strategies can be designed by combining DARA with the immune modulatory agent LEN as well as with the KIR-blocking antibody IPH2102, whereby synergistic elimination of MM tumors by NK cells may be achieved. These preclinical data, together with the preliminary results of the first clinical studies with DARA monotherapy and IPH2101 monotherapy, support the use of a combination of DARA with LEN and IPH2102 in a clinical trial.
Acknowledgments
The authors would like to thank all of the patients and healthy donors who contributed to this study. IPH2102 was donated by Innate Pharma. Daratumumab was donated by Genmab.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Howlader N, Noone AM, Krapcho M. SEER Cancer Statistics Review, 1975–2010. Available http://seer.cancer.gov/csr/1975_2010/. Accessed June 30, 2014.
- 2.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. [DOI] [PubMed] [Google Scholar]
- 3.Barlogie B, van Rhee F, Shaughnessy JD, Jr, Anaissie E, Crowley J. Making progress in treating multiple myeloma with total therapies: issue of complete remission and more. Leukemia. 2008;22:1633–1636. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Weers M, Tai YT, van der Veer M, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848. [DOI] [PubMed] [Google Scholar]
- 7.Lokhorst HM, Laubach JP, Nahi H, et al. Dose-dependent efficacy of daratumumab (DARA) as monotherapy in patients with relapsed or refractory multiple myeloma (RR MM). J Clin Oncol. 32:5s[2014]. 8513. [Google Scholar]
- 8.van der Veer M, de Weers M, van Kessel B, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011; 96:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Veer M, de Weers M, van Kessel B, et al. The therapeutic human CD38 antibody daratumumab improves the antimyeloma effect of newly emerging multidrug therapies. Blood Cancer J. 2011;1:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. [DOI] [PubMed] [Google Scholar]
- 11.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. [DOI] [PubMed] [Google Scholar]
- 12.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson DM, Jr, Bakan CE, Zhang S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118:6387–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romagne F, Andre P, Spee P, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson DM, Jr, Hofmeister CC, Padmanabhan S, et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120:4324–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korde N, Carlsten M, Lee MJ, et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99:e81–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Spek E, Bloem AC, Lokhorst HM, van KB, Bogers-Boer L, van de Donk NW. Inhibition of the mevalonate pathway potentiates the effects of lenalidomide in myeloma. Leuk Res. 2009;33:100–108. [DOI] [PubMed] [Google Scholar]
- 18.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–1114. [PubMed] [Google Scholar]
- 19.Jiang XM, Arepally G, Poncz M, McKenzie SE. Rapid detection of the Fc gamma RIIA-H/R 131 ligand-binding polymorphism using an allele-specific restriction enzyme digestion (ASRED). J Immunol Methods. 1996;199:55–59. [DOI] [PubMed] [Google Scholar]
- 20.Lehrnbecher T, Foster CB, Zhu S, et al. Variant genotypes of the low-affinity Fcgamma receptors in two control populations and a review of low-affinity Fcgamma receptor polymorphisms in control and disease populations. Blood. 1999;94:4220–4232. [PubMed] [Google Scholar]
- 21.van Sorge NM, van der Pol WL, van de Winkel JG. FcgammaR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61:189–202. [DOI] [PubMed] [Google Scholar]
- 22.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. [DOI] [PubMed] [Google Scholar]
- 23.Lioznov M, El-Cheikh J, Jr, Hoffmann F, et al. Lenalidomide as salvage therapy after allo-SCT for multiple myeloma is effective and leads to an increase of activated NK (NKp44(+)) and T (HLA-DR(+)) cells. Bone Marrow Transplant. 2010;45:349–353. [DOI] [PubMed] [Google Scholar]
- 24.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Hideshima T, Akiyama M, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128:192–203. [DOI] [PubMed] [Google Scholar]
- 26.van de Donk NW, Görgün G, Groen RW, et al. Lenalidomide for the treatment of relapsed and refractory multiple myeloma. Cancer Manag Res. 2012;4:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohrt HE, Thielens A, Marabelle A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014; 123(5):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bringhen S, Gay F, Pautasso C, Cerrato C, Boccadoro M, Palumbo A. Evaluation of the pharmacokinetics, preclinical, and clinical efficacy of lenalidomide for the treatment of multiple myeloma. Expert Opin Drug Metab Toxicol. 2012;8:1209–1222. [DOI] [PubMed] [Google Scholar]