Achieving deep levels of remission is one of the prerequisites to reach long-term survival in solid tumors and hematologic malignancies, and this has also been proved in newly diagnosed multiple myeloma (MM) patients, particularly in the era of novel agents.1–3 Accordingly, both the Spanish and UK groups have shown the prognostic value and clinical relevance of minimal residual disease (MRD) monitoring by multiparameter flow cytometry (MFC) in both newly diagnosed transplant candidates and elderly MM patients treated with novel agents.4–6 However, the value of the depth of response in the relapse setting has been subject to far less investigation than in the up-front setting.7–11 In fact, there are few data exploring different outcomes between patients achieving very good partial response (VGPR) or complete response (CR) with salvage therapy,7–11 and there is no information regarding the prognostic value of achieving immunophenotypic or molecular responses at relapse. If MRD-negativity translated into superior outcomes, like those observed in newly diagnosed patients, then it could become a desirable end point for clinical trials exploring new drugs for relapse/refractory patients.
In the present study, we focused on a total of 52 patients who after clinical relapse achieved CR with salvage therapy. Patients were divided into two categories: 21 rescued with novel agents followed by allogeneic stem cell transplantation (alloSCT; n=21), and 31 patients rescued and achieving CR with novel agents (in all except 6) followed or not by autologous stem cell transplantation (autoSCT) (11 and 14 cases, respectively).
All samples were collected after informed patient consent according to the local ethical committees and the Declaration of Helsinki protocol. Median follow up was 2.7 years (32 months). MFC studies were performed on bone marrow (BM) samples using 4-color monoclonal antibody combinations (FITC/PE/PerCPCy5.5/APC), as described elsewhere.4,5,12 Plasma cells (PCs) were initially identified on the basis of strong CD38 expression and intermediate side scatter signals; discrimination between clonal and normal PCs was performed by the recognition of aberrant phenotypic expression profiles such as simultaneous downregulation of CD19 and CD45, with or without overexpression of CD56. For patients in whom CD45 or CD19 was positively expressed, lack of CD19 or CD45, respectively, dim CD38 intensity and/or bright CD56 staining (equal or higher than that of natural killer cells) allowed identification of clonal PCs in the vast majority of cases; in selected patients (n=7), CD56 was replaced by CD28, CD81 or CD117 since these markers were known to be more informative according to the base-line phenotypic evaluation. Data acquisition was performed in FACSCalibur and FACSCantoII flow cytometers (Becton Dickinson Biosciences, BDB, San Jose, CA, USA) using the FACSDiva 6.1 software (BDB), and a 2-step acquisition procedure allowing for a minimum of 2×105 leukocytes/tube to be selectively stored. Data analysis was performed using the Paint-a-Gate (BDB) and the Infinicyt software (Cytognos SL, Salamanca, Spain). Patients were defined as being MRD-negative when less than 20 clonal PCs were detectable by MFC, at a sensitivity level of 10−4. Time-to-progression (TTP) was measured from the moment of MRD assessment to the date of progression or last visit. Curves were plotted by the Kaplan-Meier method, and the log rank test was used to estimate the statistical significance of differences observed between curves. The Cox proportional hazards model was used to estimate hazard ratios (HR) and 95% confidence intervals (95%CI), as well as to perform a multivariate analysis including patients’ MRD status (dichotomized into negative/positive), type of treatment (dichotomized into yes/no): autoSCT, novel agents; proteasome inhibition, immune modulators, as well as the event of extramedullary relapses (dichotomized into yes/no). The X2 test was used to estimate the statistical significance of differences observed between groups. For all statistical analyses, the SPSS software (v.15.0; SPSS Inc., Chicago, IL, USA) was used.
First, we focused on the more standard treatment population of the 31 patients achieving CR after salvage therapy in the non-alloSCT setting (Table 1). Among them, 13 achieved MRD-negative status (42%) whereas in the remaining 18 (58%) cases persistent MRD was detected by MFC (median 0.2% clonal PC among total BM leukocytes; range 0.01–1.67%). MRD-negative cases showed a median TTP of 75 months, whereas 17 of the 18 MRD-positive CR patients progressed with a median TTP of only 14 months (HR: 2.8; 95%CI: 1.0–7.6; P=0.039) (Figure 1A). Similar results were observed while specifically analyzing patients’ TTP during the first three years after MRD assessment (Figure 1B), with median TTP for MRD-negative cases not yet reached versus 14 months among MRD-positive patients (P=0.037). On the multivariate analysis for TTP that included, in addition to patients’ MRD status, the type of treatment being used (i.e. autoSCT, novel agents; proteasome inhibition, immune modulators) and the event of extramedullary relapses, MRD status showed an HR of 2.96 (95%CI: 0.8–10.7) and a P value equal to 0.098; all other variables were non-significant and showed inferior P values (data not shown). Additional studies in larger series of patients are, therefore, warranted to assess the independent prognostic value of MRD monitoring over the type of salvage therapy and type of relapse in MM (i.e. medullary vs. extramedullary). Afterwards, we focused on the 21 patients in CR after alloSCT, and observed that 10 (48%) failed to eradicate MRD (median of 0.12% clonal PC among total BM leukocytes; range 0.01–0.7%). Surprisingly, there were no significant differences according to the presence versus absence of MRD in TTP (P=0.77) among patients in CR after alloSCT. These observations led us to investigate whether this phenomenon could be associated with a higher incidence of extramedullary relapses (and therefore potentially missed on BM monitoring) after alloSCT. Accordingly, a total of 11 extramedullary relapses were observed among patients in CR after alloSCT, 7 of which among MRD-negative patients. By contrast, only 2 of 31 patients in the non-alloSCT setting had extramedullary relapses (both MRD-positive).
Table 1.
Minimal residual disease rates and prognostic value according to the different treatment schemas used on relapsing multiple myeloma patients.
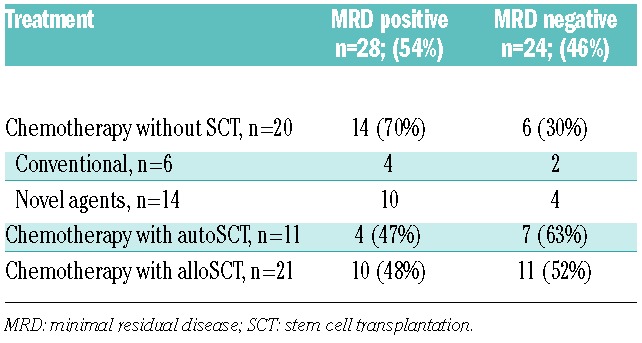
Figure 1.
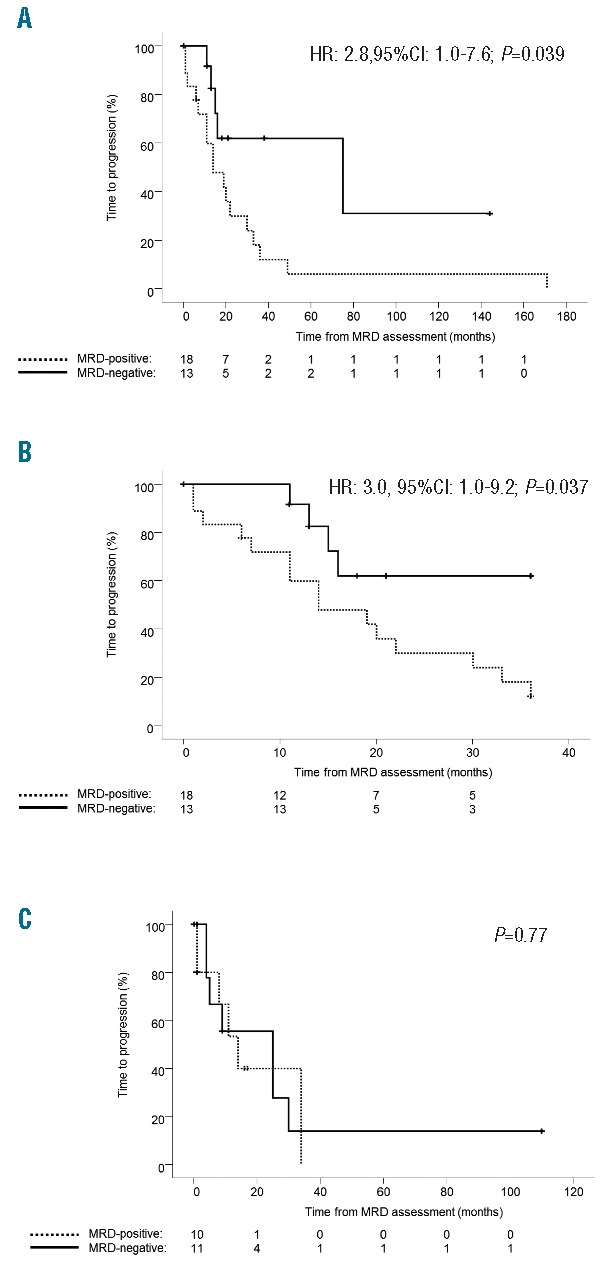
(A) Time-to-progression (TTP) of multiple myeloma (MM) patients in complete response (CR) after salvage chemotherapy with or without autoSCT (n=31), according to the absence [minimal residual disease (MRD)-negative] versus presence of phenotypically aberrant clonal plasma cells (MRD-positive). (B) Specific analysis of TTP during the first three years after MRD assessment among MM patients in CR after salvage chemotherapy with or without autoSCT. (C) TTP of MM patients in CR after salvage chemotherapy with alloSCT (n=21), according to patients’ MRD status.
In summary, we show that CR patients after salvage therapy constitute a heterogeneous subgroup with approximately half of the cases showing persistent MRD and early relapse (approx. one year). Patients with MRD-negativity experience significantly prolonged TTP outside of the alloSCT setting, and further studies with larger series of patients are warranted to confirm if MRD-negativity could become an end point for novel drugs being tested in relapsed/refractory patients. The likelihood of extramedullary relapses even among MRD-negative patients after alloSCT suggests that, at least for this particular therapeutic strategy, response assessment should include combined medullar and extramedullary (PET/CT) measure of MRD.
Footnotes
Funding: this study was supported by the Cooperative Research Thematic Network grants RD12/0036/0058 of the Red de Cancer (Cancer Network of Excellence); Instituto de Salud Carlos III, Spain, Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS: PI060339; 06/1354; 02/0905; 01/0089/01-02; PS09/01897/01370; G03/136; Sara Borrell: CD13/00340); and Asociación Española Contra el Cáncer (GCB120981SAN), Spain. The study was also supported internationally by the International Myeloma Foundation Junior Grant Proposal and the Multiple Myeloma Research Foundation research fellow award.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007; 370(9594):1209–1218. [DOI] [PubMed] [Google Scholar]
- 2.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. [DOI] [PubMed] [Google Scholar]
- 3.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120(1):9–19. [DOI] [PubMed] [Google Scholar]
- 4.Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29(12):1627–1633. [DOI] [PubMed] [Google Scholar]
- 5.Paiva B, Vidriales MB, Cervero J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540–2547. [DOI] [PubMed] [Google Scholar]
- 7.Harousseau JL, Dimopoulos MA, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95(10):1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niesvizky R, Richardson PG, Rajkumar SV, et al. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol. 2008;143(1):46–53. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008;19(6):1160–1165. [DOI] [PubMed] [Google Scholar]
- 10.Pineda-Roman M, Zangari M, van Rhee F, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22(7):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110(10):3557–3560. [DOI] [PubMed] [Google Scholar]
- 12.Paiva B, Gutierrez NC, Rosinol L, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119(3):687–691. [DOI] [PubMed] [Google Scholar]


