Abstract
Context:
Use of BRAF V600E inhibitors to restore thyroid iodide-handling gene expression and radioactive iodine (RAI) avidity is an attractive therapeutic strategy for RAI-refractory thyroid cancer, but recent initial clinical responses were modest. Given histone deacetylation at the sodium/iodide symporter promoter by histone deacetylase (HDAC) as a mechanism, simultaneously targeting BRAF V600E and HDAC could be a more effective strategy.
Objectives:
The objective of the study was to test whether suppressing both BRAF V600E and HDAC could more effectively induce thyroid gene expression and RAI uptake in thyroid cancer cells.
Research Design:
We tested the BRAF V600E inhibitor PLX4032 (vemurafenib) and the HDAC inhibitor SAHA (vorinostat), two major anticancer drugs currently approved for clinical use, in inducing thyroid gene expression and RAI uptake in thyroid cancer cells.
Results:
PLX4032 alone induced a modest expression of thyroid genes and RAI uptake preferentially in thyroid cancer cells harboring BRAF V600E. SAHA showed an effect in a genetic-independent manner in all the cells. A robust synergistic effect on thyroid gene expression and RAI uptake was observed in BRAF V600E-positive thyroid cancer cells when the two inhibitors were simultaneously used. This was dramatically enhanced further by TSH; triple combination of PLX4032, SAHA, and TSH showed the most robust effect on thyroid gene expression and RAI uptake in cells harboring BRAF V600E. Abundant sodium/iodide symporter protein expression in thyroid cancer cells under these conditions was confirmed by immunofluorescent microscopy.
Conclusions:
Simultaneously suppressing BRAF V600E and HDAC, particularly when cotreated with TSH, induced a far more robust expression of thyroid genes and RAI uptake in thyroid cancer cells than suppressing BRAF V600E alone. Triple combination of PLX4032, SAHA, and TSH is a specific robust regimen to restore RAI avidity in RAI-refractory BRAF V600E-positive thyroid cancer, which warrants clinical trials to confirm.
Radioactive iodine (RAI) therapy is a standard treatment for differentiated thyroid cancer (1, 2), which is based on the ability of follicular thyroid cells to take up iodide. This treatment is ineffective in RAI-refractory thyroid cancer. Such thyroid cancer is incurable if it is also surgically inoperable. This is the main cause of thyroid cancer-related morbidity and mortality. A major mechanism underlying RAI refractoriness of thyroid cancer is the aberrant silencing of iodide-handling genes in thyroid cancer cells, such as sodium-iodide symporter (NIS) and TSH receptor (TSHR); the former is normally responsible for iodide transport across the cell membrane into the cell, and the latter up-regulates this and related molecular processes (3). This iodide-handling machinery is negatively regulated by the MAPK pathway in thyroid cancer, in which the BRAF V600E mutation plays a prominent role (4, 5).
BRAF V600E is the most common oncogenic mutation in thyroid cancer (6). In 2005, we for the first time demonstrated an association between this mutation and RAI refractoriness of thyroid cancer (7). We subsequently demonstrated functionally a robust role of BRAF V600E in the silencing of thyroid genes and induced their reexpression by eliminating BRAF V600E or suppressing the MAPK pathway using a MAPK kinase (MEK) inhibitor in a thyroid cell model (8). We also demonstrated that suppression of the MAPK pathway using MEK inhibitors could induce thyroid gene expression and radioiodine uptake in thyroid cancer cells (9) and other cancer cells (8, 10). These and other studies helped the conceptual development that suppressing the BRAF/MAPK pathway by targeting BRAF V600E or MEK could be clinically effective in restoring RAI avidity in RAI-refractory thyroid cancer. Indeed, a recent study demonstrated that the MEK inhibitor selumetinib could partially restore RAI avidity in RAI-refractory thyroid cancer in patients (11). A more recent study demonstrated that the BRAF V600E inhibitor dabrafenib could also partially induce radioiodine uptake in RAI-refractory thyroid cancer in patients (12). Although these clinical studies are encouraging, RAI avidity induced using single agents in these studies occurred only in some patients and the therapeutic effectiveness of RAI treatment was limited.
We previously demonstrated that histone deacetylation at the promoter of the NIS gene by histone deacetylase (HDAC) is an important mechanism in the silencing of thyroid genes by the BRAF V600E/MAPK pathway (13). Histone acetylation at the promoter area is a well-established mechanism in the up-regulation of genes, which, through chromatin remodeling, opens up the access of gene promoters to transcription factors (14). We and others have demonstrated that HDAC inhibitors could induce expression of thyroid genes and RAI uptake in thyroid cancer cells (9, 15, 16) and even in certain nonthyroid epithelial cancer cells (8, 10, 17). Therefore, we hypothesize that simultaneously suppressing BRAF V600E and HDAC may likely have a synergistic effect on thyroid gene expression and RAI uptake in thyroid cancer cells.
In the present study, we tested specifically whether combination of the BRAF V600E inhibitor PLX4032 (vemurafenib) and the HDAC inhibitor SAHA (vorinostat), two major anticancer drugs approved for clinical use, would be a particularly effective and practically applicable regimen in restoring thyroid gene expression and RAI uptake in RAI-refractory thyroid cancer cells.
Materials and Methods
Cell culture
The thyroid cancer cell lines SW1736 and C643 were originally from Dr N. E. Heldin (University of Uppsala, Uppsala, Sweden); OCUT1 from Dr Naoyoshi Onoda (Osaka City University Graduate School of Medicine, Osaka, Japan); K1 from Dr David Wynford-Thomas (University of Wales College of Medicine, Cardiff, United Kingdom); BCPAP from Dr Massimo Santoro (University of Federico II, Naples, Italy); TPC1 from Dr Alan P. Dackiw (The Johns Hopkins University, Baltimore, Maryland) (18); and FTC133 from Dr George Brabant (University of Manchester, Manchester, United Kingdom) (19). The BCPAP, K1, OCUT1, and SW1736 cell lines harbored the BRAF V600E mutation, whereas C643, TPC1, FB1, and FTC133 harbored the wild-type BRAF. They were all grown at 37°C with 5% CO2 and 10% fetal bovine serum in RPMI 1640 medium except for FB1cells, which were cultured in DMEM medium with 10% fetal bovine serum. Under these culture conditions, cells were treated with the BRAFV600E inhibitor PLX4032 (selleckchem) at 0.5 μM and the HDAC inhibitor SAHA (Sigma) at 2 μM individually or in combination for the indicated times. Dimetyl sulfoxide (DMSO) was used in parallel as the vehicle control. In some experiments, after the first 24-hour treatment with the indicated inhibitors, bovine TSH (TSHβ; Sigma) at 20 mU/mL was added for an additional 48 hours to stimulate the expression of thyroid-specific genes or RAI uptake.
Western blotting assay
Cells were lysed in a radioimmunoprecipitation assay buffer with standard protease inhibitors (Santa Cruz Biotechnology). Cellular proteins were collected by centrifugation of lysates at 4°C for 20 minutes, subjected to 10% or 15% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes. The membranes were probed with primary antibodies, including antiphospho-ERK1/2 (4370S; Cell Signaling Technology), anti-ERK1 (sc-94; Santa Cruz Biotechnology), anti-Ac-Histone H3 (sc-8655R; Santa Cruz Biotechnology), and antihistone H3 (4499; Cell Signaling Technology). Antigen-antibody complexes were visualized using horseradish peroxidase-conjugated antirabbit secondary antibody (Cell Signaling Technology) and the enhanced chemiluminescence Western blotting analysis system.
RNA extraction and real-time quantitative RT-PCR analysis
Total RNA was isolated from cells using TriPure reagent according to the instructions of the manufacturer (Roche). RNA samples were quantified using Maltiskan Spectrum, and A260/A280 ratios between 1.8 and 2.0 were used to ensure the quality of the samples. Total RNA (1 μg) was converted to cDNA on an iCycler thermal cycler (Bio-Rad Laboratories) using oligodeoxythymidine and SuperScript II according to the instructions of the manufacturer (SuperScript first-strand synthesis kit; Invitrogen). Real-time quantitative RT-PCR analysis was performed to evaluate the expression of thyroid genes on an ABI Prism 7900HT sequence detector (Applied Biosystems) using FastStart universal SYBR green master mix according to the instructions of the manufacturer (Roche). Glyceraldehyde-3-phosphate dehydrogenase was run in parallel to standardize the input cDNA. The primers designed for thyroid-specific genes, NIS, thyroperoxidase (TPO), TSHR, thyroglobulin (Tg), and the methods used to calculate relative expression levels of these genes were as described previously (9, 17).
Immunofluorescent detection of NIS protein
After the first 24 hours of treatment with PLX4032 and SAHA, TSH was added to cells for 48 hours (PLX4032 and SAHA were maintained for the 48 h). Cells were then fixed with 1% paraformaldehyde for 10 minutes and blocked with 1% BSA for 40 minutes at room temperature. Cells were incubated with VJ1 hNISmAb (a gift from Dr Sabine Costagliola at the Free University of Brussels, Brussels, Belgium) at a dilution of 1:20 in PBS containing 1% BSA at 4°C overnight. Cells were washed thrice with PBS and incubated with goat antirabbit-Alexa 488 (A-11001; Life Technologies) diluted at 1:200 in 1% BSA at room temperature for 1 hour. Cells were washed again with PBS thrice, followed by incubation with 5 μg/mL 4′,6-diamidino-2-phenylindole in PBS for 15 minutes. Fluorescent microscopic examination using a fluorescent microscopy (Nikon Corp) was performed to examine NIS protein expression.
RAI uptake assay
RAI uptake assay was performed as described previously (9, 17). Briefly, after a 72-hour treatment with the indicated agents, cells were incubated with 1 μCi Na125I and 5 μM nonradioactive NaI in 1 mL serum-free medium on 12-well plates at 37°C for 1 hour. To determine nonspecific radioiodine binding, parallel cells similarly treated with the agents were preincubated with the NIS blocker NaClO4 at 300 μM for 30 minutes prior to Na125I treatment. The medium was aspirated and cells were quickly washed three times with Hanks' balanced salt solution (Sigma-Aldrich). Cells were then trypsinized with 100 μL of 0.25% trypsin and lysed in 1 mL 0.33 M NaOH containing 1% sodium dodecyl sulfate. Parallel plates of cells identically treated with the agents but without radioiodine incubation were used to determine the number of cells at the end of the experiment. The radioactivity associated with cells was counted with a γ-counter and presented as counts per minute per 106 cells after correction for nonspecific radioiodine binding of cells in the presence of NaClO4. The average nonspecific binding was approximately 2.5 × 104 cpm/well.
Statistical analysis
All the experiments were similarly done at least two or three times. Data were compared using a one-way ANOVA followed by the Tukey's test. Unless indicated, data shown in the figures are representatives. The distributions of fold changes of NIS expression or RAI uptake in response to the indicated treatments over control were summarized by box plots, and the values between groups were compared using a Mann-Whitney U two-sample test.
Results
Induction of robust expression of thyroid iodide-handling genes by simultaneous suppression of BRAF V600E and HDAC
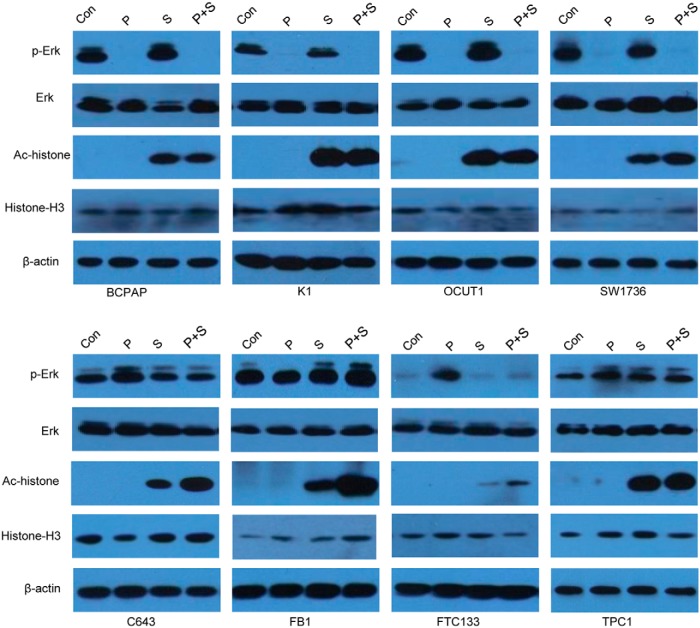
We tested the effect of the BRAF V600E inhibitor PLX4032 and the HDAC inhibitor SAHA on the expression of thyroid genes in eight thyroid cancer cell lines, including four harboring BRAF V600E and four harboring the wild-type BRAF. As shown in Figure 1, treatment of cells with PLX4032 at 0.5 μM for 48 hours preferentially inhibited the phosphorylation of ERK in BRAF V600E-positive cells, whereas it somewhat enhanced ERK phosphorylation in cells harboring the wild-type BRAF. SAHA at 2 μM for 48 hours dramatically enhanced the global acetylation of histone H3 in all the cells, irrespective of the BRAF status. PLX4032 itself did not have effect on global histone H3 acetylation as shown previously (13), but, interestingly, PLX4032 enhanced the effect of SAHA on global histone acetylation in cells harboring the wild-type BRAF (Figure 1).
Figure 1.
Effects of PLX4032 and SAHA on the activities of the MAPK pathway and HDAC in thyroid cancer cells. The four cells (BCPAP, K1, OCUT1, and SW1736) in the upper panel harbored BRAF V600E and the four cells (C643, FB1, FTC133, and TPC1) in the lower panel harbored the wild-type BRAF. Cells were treated with 0.5 μM PLX4032 and/or 2 μM SAHA for 48 hours. DMSO was used as the vehicle control. After treatments, cells were lysed for Western blotting analyses. Phosphorylated ERK (p-ERK), total ERK, acetylated histone, and total histone were detected with specific anti-p-ERK, antitotal ERK, antiacetylated histone H3 (Ac-histone), and antihistone H3 antibodies, respectively. Immunoblotting with antibodies against β-actin was used for quality control. Con, control with DMSO; P, PLX4032; S, SAHA.
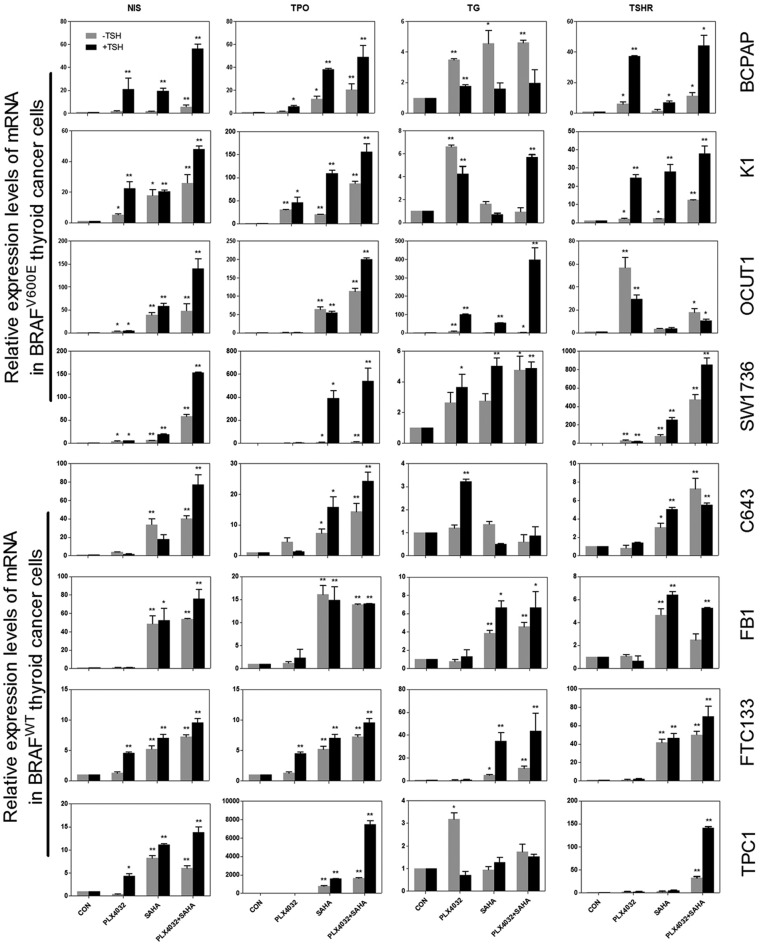
We next tested the expression of thyroid genes induced by PLX4032 and SAHA, alone or in combination. As shown in Figure 2, expression of NIS, TSHR, TPO, and Tg in BRAF V600E-haboring cells was restored to various extents by PLX4032, whereas this effect was generally modest in cells harboring the wild-type BRAF. SAHA showed a profound effect on the expression of thyroid genes in most cells, regardless of their BRAF status. Combination of PLX4032 with SAHA induced a more robust expression of thyroid genes in most of the cells, particularly in cells harboring BRAF V600E. As shown in Figure 3, when the fold change in thyroid gene expression with PLX4032 and SAHA over the control (DMSO treatment) was analyzed, NIS expression induced by PLX4032 alone was more profound in cells harboring BRAF V600E than cells harboring the wild-type BRAF (P = .003). In contrast, SAHA induced NIS expression in a BRAF V600E-independent manner, whether used alone or in combination with PLX4032 or in combination with TSH (P = .166, P = .488, and P =. 065, respectively). A synergistic effect of PLX4032 and SAHA was observed in most BRAF V600E-positive cells; for example, the expression of NIS mRNA in SW1736 cells was increased by 3.86 times after PLX4032 treatment and 6.38 times after SAHA treatment, whereas it was increased by 57.9 times after the combination treatment with the two. This synergism was reflected by the fact that the effect of the combination treatment with the two inhibitors was much higher than the sum of the effects of each individual inhibitor alone. A slight additive effect was observed for the two inhibitors in some wild-type BRAF cells.
Figure 2.
Effects of PLX4032, SAHA, and TSH on thyroid gene expression in thyroid cancer cells. The upper four thyroid cancer cell lines harbored BRAF V600E and the lower four harbored the wild-type BRAF. Cells were treated with 0.5 μM PLX4032 and/or 2 μM SAHA for 72 hours, with/without 20 mU/mL TSH (black bar) added at the 24-hour time point and continued for 48 hours. Data are presented as the mean ± SD of values from three assays. *, P < .05, **, P < .01 for comparison with control.
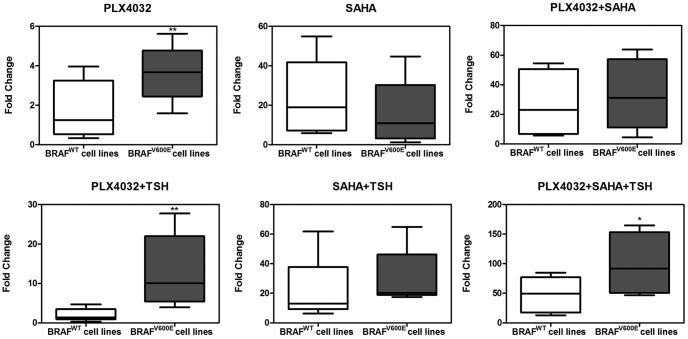
Figure 3.
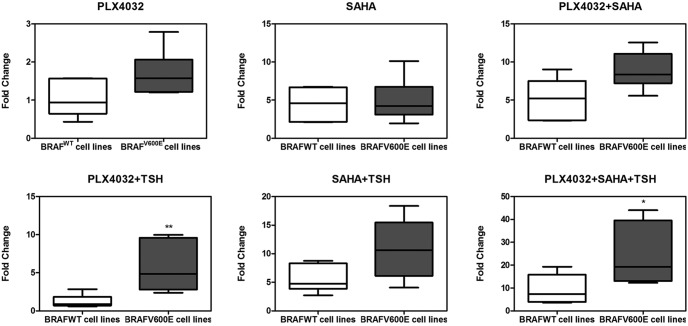
Increased NIS expression over control in response to PLX4032, SAHA and TSH in thyroid cancer cells: BRAF V600E-dependent effect of PLX4032. Box plots are used to show the fold changes of NIS expression in response to the indicated treatment over control (DMSO only). Shown in each panel are results in two groups of cells: four cells harboring the wild-type BRAF (BRAFWT) (C643, FB1, FTC133, and TPC1) and four cells harboring BRAF V600E (BRAFV600E) (SW1736, OCUT1, K1, and BCPAP). The outer bars of the box plots indicate the range of minimum and maximum values, and the bold line indicates the median value. Compared with the wild-type BRAF group, the BRAF V600E group was generally associated with higher responses, particularly robustly with PLX4032 plus TSH or PLX4032 + SAHA + TSH. The cell treatment conditions and concentrations of the agents are as described in the legend to Figure 2. *, P < .05, **, P < .01 for comparison between the wild-type BRAF and BRAF V600E groups.
TSH robustly enhanced thyroid gene expression induced by PLX4032 and SAHA
Because TSHR plays an important role in up-regulating iodide-handling genes in thyroid cells, we investigated whether TSH could further stimulate the expression of thyroid genes in thyroid cancer cells. After the first 24-hour treatment with PLX4032 and SAHA, TSH was added to further treat the cells. As shown in Figures 2 and 3, TSH stimulation dramatically enhanced the effects of PLX4032 and SAHA on the expression of thyroid genes, including its own receptor TSHR, in most cells. Either with PLX4032 or with combined PLX4032 and SAHA, TSH had a robust further effect on thyroid gene expression. This effect of TSH was particularly more profound in cells harboring BRAF V600E than wild-type BRAF cells (Figure 3), again demonstrating a BRAF mutation-dependent effect. The effect of TSH on thyroid gene expression required the presence of PLX 4032 or SAHA as TSH alone virtually had no effect on thyroid gene expression (Figure 2). As seen in the absence of TSH described above, in the presence of TSH, there was also a robust synergistic effect between PLX4032 and SAHA on thyroid gene expression in cells harboring BRAF V600E; for example, with simultaneous treatment of SW1736 cells with TSH, the expression of NIS mRNA was increased by 6.0 times after PLX4032 treatment and 18.7 times after SAHA treatment, whereas it was increased by 153.6 times after the combination treatment with the two inhibitors (Figure 2). The highest magnitude of thyroid gene expression was seen with the simultaneous triple treatment with PLX4032, SAHA, and TSH in BRAF V600E-haboring cells (Figures 2 and 3).
NIS protein expression in thyroid cancer cells and its localization to the cell membrane induced by suppressing BRAF V600E and HDAC
With mRNA expression of thyroid genes demonstrated above, we next sought to confirm the corresponding protein expression using NIS as a representative because it plays the most important role in thyroid cell iodide uptake. We examined NIS protein expression by immunofluorescent microscopy using the SW1736 cell line as a representative cell. As shown in Figure 4, although there was virtually no basal NIS protein expression, robust expression of NIS protein was induced by simultaneous treatment of cells with PLX4032 and SAHA; this NIS expression was prominently seen in the cytoplasm. Consistent with the above finding of the effect of TSH on mRNA expression of NIS, after additional treatment of cells with TSH, NIS protein expression became even more robust, and, importantly, NIS was now seen to be more clearly localized in the peripheral areas of the cell, suggesting increased cell membrane localization (Figure 4).
Figure 4.
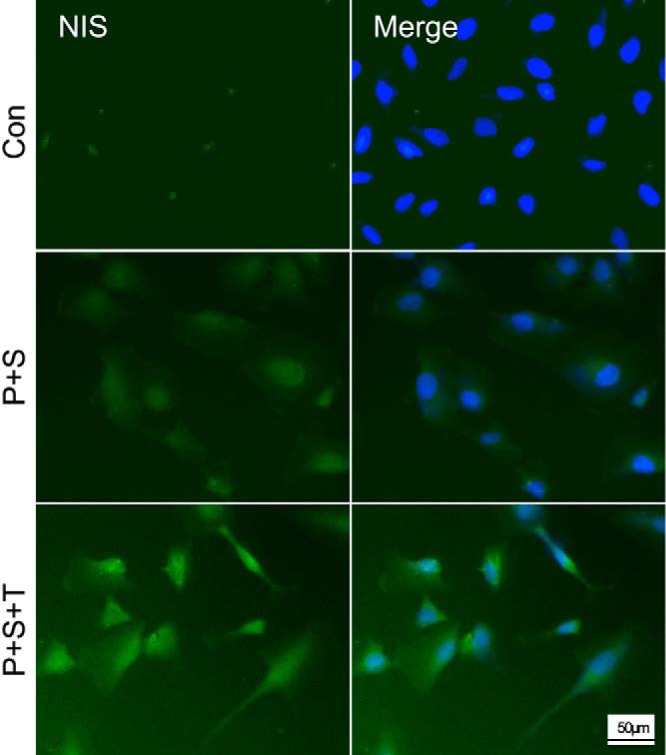
Immunofluorescent microscopic analysis of NIS protein expression in SW1736 cells. Cells were treated with control DMSO (Con), PLX4032 (P), SAHA (S), and TSH (T) as indicated and with the conditions and concentrations described in the legend to Figure 2. Double-immunofluorescent microscopy was displayed, with the blue color representing 4′,6-diamidino-2-phenylindole nuclear staining and the green color representing NIS protein expression. The abundant NIS expression in cells treated with the indicated agents was in striking contrast with the control cells, which showed only minimal expression. The most robust expression of NIS was seen with the triple treatment with PLX4032, SAHA, and TSH, in which cell membrane localization of NIS could be appreciated (represented by the increased peripheral staining of green color of the cell). Scale bar, 50 μm.
Induced RAI in thyroid cancer cells
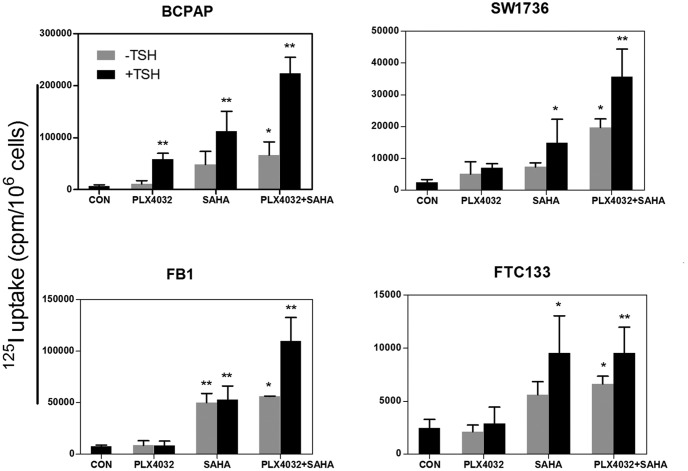
We next tested the functional relevance of induced thyroid gene expression by examining the ability of cells to take up RAI in four selected thyroid cancer cells, including BCPAP and SW1736 cells that harbored BRAF V600E and FTC133 and FB1 cells that harbored the wild-type BRAF. As shown in Figure 5, PLX4032 alone induced a modest RAI uptake, SAHA induced a stronger uptake, and simultaneous treatment with the two inhibitors induced an even stronger uptake. With each of these inhibitors, TSH robustly enhanced RAI uptake, whereas TSH alone had a minimal effect on RAI uptake. When comparing the two groups of cells with different BRAF genotypes (Figure 6), BRAF V600E was generally associated with a higher RAI uptake, particularly robustly when cells were treated with PLX4032 + TSH or PLX4032 + SAHA + TSH. The most robust and highest-magnitude radioiodine uptake was seen with the triple combination of PLX4032, SAHA, and TSH in cells harboring the BRAF V600E mutation (Figures 5 and 6). Overall, the patterns of radioiodine uptake, such as the magnitude, the augmentation by TSH, and the BRAF mutation dependence of the effect of PLX4032, were all consistent with the thyroid gene expression patterns described above.
Figure 5.
Induced radioactive iodine uptake in thyroid cancer cells. Cells used included BCPAP and SW1736, which harbored BRAF V600E mutation, and FB1 and FTC133, which harbored the wild-type BRAF. An 125I uptake assay was performed as described in Materials and Methods. Cells were treated with control DMSO (CON), PLX4032, SAHA, and TSH as indicated and with conditions and concentrations described in the legend to Figure 2. Data on NIS-specific uptake are presented as the mean ± SD of values from three assays. *, P < .05, **, P < .01 for comparison with the control.
Figure 6.
Increased radioactive iodine uptake over control in response to PLX4032, SAHA, and TSH in thyroid cancer cells: BRAF V600E-dependent effect of PLX4032. Box plots are used to show the fold changes of radioiodine uptake in response to the indicated treatment over the control (DMSO). Shown in each panel are the results from two groups of cells: two cells harboring the wild-type BRAF (BRAFWT) (FB1 and FTC133) and two cells harboring BRAF V600E (BRAFV600E) (BCPAP and SW1736), with each group representing six assay measurements from the two cells. The outer bars of the box plots indicate the range of minimum and maximum values, and the bold line indicates the median value. Compared with the wild-type BRAF group, the BRAF V600E group was generally associated with stronger responses, particularly robustly with PLX4032 plus TSH or PLX4032 + SAHA + TSH. The cell treatment conditions and concentrations of the agents are as described in the legend to Figure 2 and in Materials and Methods. *, P < .05, **, P < .01 for comparison between the wild-type BRAF and BRAF V600E groups.
Discussion
RAI-refractory thyroid cancer represents a major therapeutic challenge in thyroid cancer medicine. Recent progresses in understanding the molecular pathogenesis of thyroid iodide-handling machinery in thyroid cancer have provided exciting promises for novel therapeutic strategies targeting the BRAF V600E/MAPK pathway to restore RAI avidity (8–10, 17). The recent clinical demonstration that targeting BRAF V600E using a specific inhibitor could induce RAI uptake in otherwise RAI-refractory PTC in patients is particularly exciting (12). This represents an exciting initial success of clinical translation from the initial preclinical demonstration of the role of BRAF V600E in the aberrant silencing of thyroid genes and loss of RAI avidity in thyroid cancer (7, 8). BRAF V600E inhibitors, such as PLX4032, have shown remarkable therapeutic effects in melanoma and nonmelanoma human cancers harboring the BRAF V600E mutation (20, 21). Recent early-phase clinical studies on PLX4032 in patients with thyroid cancer harboring BRAF V600E showed promising therapeutic effects, good patient tolerability, and acceptable adverse effect profiles (22, 23). Thus, BRAF V600E inhibitors will likely become important anticancer drugs for thyroid cancer in the future. As such, induction of RAI avidity using BRAF V600E inhibitors for adjuvant RAI ablation in conjunction with their conventional anticancer treatments could become a particularly effective therapy for RAI-refractory thyroid cancer. The use of a BRAF V600E inhibitor alone, however, will unlikely be sufficient for such a therapeutic strategy to be effective for thyroid cancer, given the modest effects of BRAF V600E inhibitor alone on thyroid gene expression and RAI uptake demonstrated in the present study. In fact, in the recent clinical studies, the use of single-agent BRAF V600E inhibitor induced only modest RAI avidity and limited therapeutic effects of RAI in RAI-refractory thyroid cancer (12). Thus, new approaches are needed to improve the effectiveness of this promising therapeutic strategy.
By demonstrating that simultaneous suppression of HDAC could robustly synergize the stimulatory effects of BRAF V600E inhibitor on thyroid gene expression and RAI uptake in thyroid cancer cells, the present study provides a new strategy to improve the currently pursued RAI avidity-restoring treatment of thyroid cancer. The results of the present study can be interpreted by our previous demonstration that histone deacetylation by HDAC at the promoter region of NIS is a mechanism in its aberrant silencing; BRAF V600E-activated MAPK pathway could cause histone deacetylation at the NIS promoter, and suppression of BRAF V600E by PLX4032 could increase histone acetylation at the promoter of NIS (13). Like PLX4032, which has been approved for clinical use as an anticancer drug, SAHA is also an approved anticancer drug. Although a clinical study on SAHA in thyroid cancer patients failed to show a direct anticancer effect, the study demonstrated good patient tolerability and adverse effect profiles (24). This makes SAHA potentially clinically applicable when used for the induction of RAI avidity in thyroid cancer patients.
It is remarkable that TSH could robustly enhance the thyroid gene expression and RAI uptake in thyroid cancer cells induced by BRAF V600E and HDAC inhibitors in the present study. TSH, through activating the TSHR, is a master regulator in up-regulating the iodide-handling machinery in thyroid cells (3). In the present study, expression of TSHR was also robustly induced by BRAF V600E and HDAC inhibitors, which could in fact be enhanced by TSH itself. Thus, there was a positive feedback effect of TSH on the expression of TSHR induced by suppressing BRAF V600E and HDAC. The present study also showed that TSH not only enhanced the expression of thyroid genes but also promoted localization of NIS to the cell membrane, which is an important and necessary step for NIS-mediated RAI uptake into the cell. These results provide a strong basis for the synergistic effects of TSH with the BRAF V600E and HDAC inhibitors on the up-regulation of thyroid iodide-handling machinery and RAI uptake in thyroid cancer cells. Elevation of serum TSH, whether achieved through the use of recombinant human TSH or through T4 withdrawal to enhance the pituitary secretion of TSH, is a standard measure to enhance RAI ablation therapy for thyroid cancer (25). The present study suggests that use of elevated TSH will also be an important component of the strategy using inhibitors of the BRAF V600E/MAK kinase pathway and HDAC to induce RAI avidity for RAI ablation treatment of otherwise RAI-refractory thyroid cancer.
Our and others' previous studies demonstrated that BRAF V600E inhibitors inhibit thyroid cancer cells in a BRAF V600E mutation-dependent manner; they preferentially inhibited thyroid cancer cell proliferation when the cells harbored the mutation (26, 27). The present study is consistent with these results in that the effects of the BRAF V600E inhibitor on thyroid gene expression and RAI uptake were more profound in BRAF mutation-positive thyroid cancer cells, particularly when cotreated with TSH. Thus, BRAF mutation status can be useful in guiding the selection of thyroid cancer patients for the induction of RAI avidity and RAI treatment using the approaches described in the present study. It is interesting that the effects of dual treatment with PLX4032 and SAHA on thyroid gene expression and RAI uptake were more pronounced than the treatment with either alone, even in cells harboring the wild-type BRAF. This seems to accompany the finding that PLX4032 somehow enhanced SAHA-induced global histone acetylation in wild-type BRAF cells (Figure 1). One possible explanation is the interaction of BRAF V600E inhibitor with wild-type RAF dimers. resulting in transactivation of the drug-free protomer, causing the activation of the MAPK pathway (28). In fact, PLX4032 indeed increased the phosphorylation of ERK in several cells harboring the wild-type BRAF in the present study (Figure 1). Activation of the MAPK pathway can increase global histone acetylation, whereas it decreases histone acetylation at the NIS promoter (13). Future studies are needed to elucidate the mechanism underlying the synergistic effects of PLX4032 and SAHA on thyroid gene expression and to investigate how the combination of PLX4032 and SAHA may affect thyroid cancer cell growth; inhibition of thyroid cancer cells by such drug treatments, when combined with radioiodine treatment, may represent an ideal therapeutic strategy.
In summary, the present study demonstrates that simultaneous suppression of HDAC to increase histone acetylation can dramatically synergize thyroid gene expression and RAI uptake in thyroid cancer cells induced by inhibiting BRAF V600E, and TSH, which by itself has minimal effect, can robustly augment this effect. This is particularly the case in thyroid cancer cells harboring the BRAF V600E mutation. The specific drugs tested here, the BRAF V600E inhibitor PLX4032 and the HDAC inhibitor SAHA, are two major clinically approved anticancer drugs and are clinically applicable in thyroid cancer, making the present study immediately clinically relevant. It is expected that the triple combination of PLX4032, SAHA, and TSH in conjunction with RAI ablation may become a specific therapy regimen for effective treatment of RAI-refractory thyroid cancer, particularly BRAF V600E mutation-positive cancer. A clinical trial testing this therapy is warranted.
Acknowledgments
We thank Drs N. E. Heldin, Naoyoshi Onoda, David Wynford-Thomas, Massimo Santoro, Alan P. Dackiw, and George Brabant for kindly providing us the thyroid cancer cell lines. We also thank Dr Sabine Costagliola (Free University of Brussels) for generously providing us the VJ1 hNISmAb.
This work was supported by National Institutes of Health Grants R01CA113507 and R01CA189224 (to M.X.).
Disclosure Summary: M.X. received royalties as a coholder of a licensed US patent related to BRAF V600E mutation in thyroid cancer. The other authors have nothing to disclose.
Footnotes
- DMSO
- dimethyl sulfoxide
- HDAC
- histone deacetylase
- MEK
- MAPK kinase
- RAI
- radioactive iodine
- TSHR
- TSH receptor.
References
- 1. Maxon HR, Thomas SR, Hertzberg VS, et al. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med. 1983;309:937–941. [DOI] [PubMed] [Google Scholar]
- 2. Schlumberger MJ, Torlantano M. Papillary and follicular thyroid carcinoma. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:601–613. [DOI] [PubMed] [Google Scholar]
- 3. Spitzweg C, Morris JC. The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin Endocrinol (Oxf). 2002;57:559–574. [DOI] [PubMed] [Google Scholar]
- 4. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. [DOI] [PubMed] [Google Scholar]
- 7. Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. [DOI] [PubMed] [Google Scholar]
- 8. Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13:1341–1349. [DOI] [PubMed] [Google Scholar]
- 9. Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. 2010;95:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou P, Liu D, Ji M, et al. Induction of thyroid gene expression and radioiodine uptake in melanoma cells: novel therapeutic implications. PLoS One. 2009;4:e6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21:1028–1035. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Z, Liu D, Murugan AK, Liu Z, Xing M. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer. 2014;21:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Annunziato AT, Hansen JC. Role of histone acetylation in the assembly and modulation of chromatin structures. Gene Expr. 2000;9:37–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furuya F, Shimura H, Suzuki H, et al. Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroglobulin. Endocrinology. 2004;145:2865–2875. [DOI] [PubMed] [Google Scholar]
- 16. Pugliese M, Fortunati N, Germano A, et al. Histone deacetylase inhibition affects sodium iodide symporter expression and induces 131I cytotoxicity in anaplastic thyroid cancer cells. Thyroid. 2013;23:838–846. [DOI] [PubMed] [Google Scholar]
- 17. Liu Z, Xing M. Induction of sodium/iodide symporter (NIS) expression and radioiodine uptake in non-thyroid cancer cells. PLoS One. 2012;7:e31729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka J, Ogura T, Sato H, Hatano M. Establishment and biological characterization of an in vitro human cytomegalovirus latency model. Virology. 1987;161:62–72. [DOI] [PubMed] [Google Scholar]
- 19. Goretzki PE, Frilling A, Simon D, Roeher HD. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 1990;118:48–63. [DOI] [PubMed] [Google Scholar]
- 20. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dadu R, Shah K, Busaidy NL, et al. Efficacy and tolerability of vemurafenib in patients with BRAF(V600E)-positive papillary thyroid cancer: M. D. Anderson Cancer Center off label experience. J Clin Endocrinol Metab. 2015;100:E77–E81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid. 2013;23:1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woyach JA, Kloos RT, Ringel MD, et al. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. In press. [DOI] [PubMed] [Google Scholar]
- 26. Salerno P, De Falco V, Tamburrino A, et al. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab. 2010;95:450–455. [DOI] [PubMed] [Google Scholar]
- 27. Xing J, Liu R, Xing M, Trink B. The BRAFT1799A mutation confers sensitivity of thyroid cancer cells to the BRAFV600E inhibitor PLX4032 (RG7204). Biochem Biophys Res Commun. 2011;404:958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]