Abstract
Context:
Insulin reportedly impairs endothelial function in conduit arteries but improves it in resistance and microvascular arterioles in healthy humans. No studies have assessed endothelial function at three arterial levels in healthy or metabolic syndrome (METSYN) subjects.
Objective:
The objective of the study was to compare endothelial responsiveness of conduit arteries, resistance, and microvascular arterioles to insulin in healthy and METSYN subjects.
Design:
We assessed conduit, resistance, and microvascular arterial function in the postabsorptive and postprandial states and during euglycemic hyperinsulinemia (insulin clamp).
Setting:
The study was conducted at a clinical research unit.
Participants:
Age-matched healthy and METSYN subjects participated in the study.
Interventions:
We used brachial flow-mediated dilation, forearm postischemic flow velocity, and contrast-enhanced ultrasound to assess the conduit artery, resistance arteriole, and microvascular arteriolar endothelial function, respectively. We also assessed the conduit artery stiffness (pulse wave velocity and augmentation index) and measured the plasma concentrations of 92 cardiovascular disease biomarkers at baseline and after the clamp.
Results:
Postabsorptive and postprandial endothelial function was similar in controls and METSYN in all tested vessels. METSYN subjects were metabolically insulin resistant (P < .005). In controls, but not METSYN subjects, during euglycemic hyperinsulinemia, endothelial function improved at each level of arterial vasculature (P < .05 or less for each). Conduit vessel stiffness (pulse wave velocity) was increased in the METSYN group. Twelve of 92 biomarkers differed at baseline (P < .001) and remained different at the end of the insulin clamp.
Conclusions:
We conclude that insulin enhances arterial endothelial function in health but not in METSYN, and this vascular insulin resistance may underlie its increased cardiovascular disease risk.
Insulin acts directly on vascular tissue, including endothelial (1) and smooth muscle cells (2). In resistance arterioles, hyperinsulinemia reportedly enhances nitric oxide (NO)-dependent forearm blood flow in response to brachial arterial acetylcholine (3–6) or methacholine (7) infusions. In addition, obesity (7), metabolic syndrome (6), hypertension (3), and TNFα infusion (4) are reported to diminish insulin's action on resistance arterioles. Similarly, we reported that insulin relaxes muscle microvasculature in humans, and this is impaired in obesity (8, 9). This contrasts with reports of conduit vessel responses to insulin in healthy humans in which two groups have reported that in healthy humans hyperinsulinemia (euglycemic clamp) inhibits endothelial function measured as flow-mediated dilation (FMD) of the brachial artery (10, 11). Those authors hypothesized that hyperinsulinemia by causing endothelial dysfunction might accelerate cardiovascular disease (CVD). In contrast a recent study of obese insulin resistant humans reported no decline in endothelial function (FMD) during 4 hours of euglycemic hyperinsulinemia (12).
Whether conduit vessels respond differently to insulin than resistance or microvascular arterioles or are affected differently by insulin resistance is therefore unclear. In healthy humans no previous studies have measured insulin's vascular actions at each of three levels of the arterial tree. Metabolic syndrome (METSYN) associates with a significant conduit vessel atherosclerotic disease burden (13), principally involving conduit vessel atherosclerosis. Metabolic insulin resistance is strongly linked to METSYN and may be pathogenically associated.
In the current study, we measured insulin's vascular actions at each of three levels of the arterial vasculature (including conduit arteries, resistance, and microvascular arterioles) in healthy adults and in individuals with METSYN as defined by the American Heart Association/National Heart, Lung, and Blood Institute (14). We compared basal, postprandial, and insulin-stimulated vascular function between groups and were particularly interested in ascertaining the congruence of insulin responses across arterial vessels of differing size and whether METSYN selectively affected one or more levels. We measured vascular function in the postabsorptive state and then following a high-fat meal (HFM) and in response to a euglycemic insulin clamp, beginning 2 hours after the meal. This study design recognizes that most humans spend approximately half of their life in the postprandial state and that recent data suggest that even healthy individuals manifest metabolic insulin resistance (15) and/or endothelial dysfunction after a single HFM (16, 17).
We measured three indices of conduit artery health. The first two, augmentation index (AI) and pulse wave velocity (PWV), assess vascular stiffness (18, 19). PWV is not known to be affected by acute dietary changes or insulin infusion. However, AI may also have an insulin-responsive component (20, 21). The third measure, FMD, reflects NO-mediated vascular relaxation and is a direct measure of endothelial function (22, 23). Resistance arteriolar function as measured using brachial artery postischemic flow velocity (PIFV) is in part NO dependent. Microvascular blood volume (MBV), measured using contrast-enhanced ultrasound (CEU), reflects relaxation of terminal arterioles in muscle and also depends on NO production. We recognize that each test used to some extent reflects function at more than one arterial level. Thus, PWV is used as a measure of the elastic property of the aorta but is also influenced by resistance vessels, PIFV is affected predominantly by resistance but also by microvascular arterioles, and FMD reflects principally endothelial in the brachial artery but is influenced by resistance arterioles as well.
Materials and Methods
Human subjects
Studies were performed on 16 control and 18 METSYN subjects 18–60 years of age. Control subjects were healthy, had a body mass index less than 25 kg/m2, were nonsmokers, were on no medications or supplements known to affect vascular function (fish oil, vitamins E and C, aspirin), and had no first-degree relatives with type 2 diabetes mellitus. METSYN subjects (as defined by American Heart Association/National Heart, Lung, and Blood Institute guidelines) (11) were nonsmokers, were not diabetic (glycated hemoglobin [HbA1C] < 6.5), were on no medications (except diuretics for eight hypertensive subjects) or supplements known to affect vascular function (angiotensin converting enzyme inhibitors, angiotensin receptor blockers), and were without chronic disease other than those qualifying them for the study. The study protocol was approved by the University of Virginia Institutional Review Board. All studies were performed in the University of Virginia Clinical Research Unit (CRU).
Experimental protocol
Each subject gave written informed consent at a screening visit. That visit included a history and physical examination; measurement of electrolytes, liver and renal function tests; complete blood count; fasting glucose; HbA1C; a lipid profile; and for females a negative serum pregnancy test. Those meeting the screening criteria were instructed to maintain a low-fat diet for 3 days and to avoid alcohol, exercise, and caffeine for 24 hours prior to admission. Subjects were admitted to the CRU at 7:00 am after fasting from 10:00 pm the evening before admission. We obtained baseline blood samples for glucose, insulin, nitrate, nitrite, free fatty acids (FFAs), and CVD biomarkers. AI and PWV were then measured, followed by FMD and PIFV. Skeletal muscle MBV was determined using CEU (9). After baseline measurements were completed, subjects consumed a high-fat breakfast meal (HFM) consisting of 60% of calories from fat, 14% carbohydrate, and 26% protein (8.5 kcal/kg body weight). Subjects were asked to finish the meal within 20 minutes. Between 100 and 120 minutes after the meal, AI, PWV, FMD, PIFV, and CEU measurements were repeated. A euglycemic insulin clamp was performed between 120 and 240 minutes with vascular measurements obtained between 220 and 240 minutes. Blood for insulin, glucose, and FFAs was obtained every 30 minutes, and nitrate, nitrite, and CVD biomarkers were measured at 0 and 240 minutes.
Measures of conduit and resistance arterial function
AI and PWV were measured by aplanation tonometry using the SphygmoCor system (AtCor Medical). For AI we used the radial artery and the measured AI was corrected to a heart rate of 75 bpm. PWV was measured between the carotid and femoral artery. FMD was determined using a SONOS 7500 ultrasound system (Philips Medical Systems). The brachial artery was imaged approximately 5 cm proximal to the antecubital crease using B-mode ultrasound. Baseline brachial artery diameter and flow were measured and a forearm blood pressure (BP) cuff was inflated to 250 mm Hg for 5 minutes and then deflated. Blood velocity was obtained during the first 20 seconds after cuff deflation and diameter obtained every 10 seconds from 30 to 120 seconds. Analysis for vessel diameter was done offline using Brachial Analyzer (Medical Imaging Applications, LLC).
Measurement of skeletal muscle MBV
CEU was performed using a SONOS 7500 ultrasound system (Philips Medical Systems) with harmonic imaging during the continuous systemic infusion of Definity (Lantheus Medical Imaging) as described previously (9).
Euglycemic hyperinsulinemic clamp
A primed 2-mU/kg·min insulin infusion was started at 120 minutes and decreased to 1 mU/kg·min at 130 minutes and maintained until 240 minutes. During the insulin clamp, to avoid the confounding systemic vasodilatory effect of limb heating (24, 25), we did not use a heated hand to arterialize venous blood. Instead, as previously described (9), the glucose infusion rate was targeted to decrement forearm venous plasma in proportion to the observed rise in glucose infusion rate (GIR).
Biochemical analyses
Comprehensive metabolic panels, HbA1C, complete blood counts, lipid profiles, and pregnancy tests were assayed at the University of Virginia Clinical Chemistry Laboratory. Plasma glucose, insulin, and FFAs were measured by the CRU core laboratory. Plasma nitrate and nitrite were measured in duplicate by heavy metal reduction of nitrite and nitrate to nitric oxide followed by the chemiluminescent reaction of nitric oxide with ozone using the Sievers Model 280i nitric oxide analyzer (GE Analytic Instruments). We measured 92 CVD-associated biomarkers in plasma obtained in the fasting state and at 240 minutes. Samples were analyzed using the Olink Bioscience Proseek CVD cDNA-based proximity extension multiplex system according to the manufacturer's instructions. The values are presented as log 2, and the assay uses a proximity extension method that involves two different antibodies directed at discrete sites against each analyte. These antibodies have oligos attached, which can hybridize to form a template for a polymerase extension that amplifies the signal, and this is quantitated by RT-PCR. Values can be transformed to actual concentrations using transforms on the O-Link Bioscience web site, and there is approximation in this transformation. Inasmuch as all samples are run in a single assay, we present the untransformed data, which reflect the actual assay output.
Statistics
Primary end points were the changes in FMD, PIFV, and MBV. Data are presented as the mean ± SEM. Secondary end points were glucose, insulin, and FFA concentrations as well as GIR. Comparisons were made within and between groups at baseline, at 120 minutes after HFM, and at 240 minutes when the insulin clamp was finished using a two-way, repeated-measures ANOVA or a paired Student's t test where appropriate and Pearson's correlation using Sigmastat 3.2 (Systat Software). Statistical significance was considered when the value was P ≤ .05 for primary and secondary end points. For the multiplex assay, to compensate for the multiple comparisons, P < .001 was treated as significant, except as noted in the Results.
Results
Subject characteristics
Table 1 provides the clinical characteristics of the 18 METSYN and 16 control subjects. The body mass index, systolic and diastolic BP, fasting plasma glucose, and triglyceride concentrations were higher and the high-density lipoprotein (HDL)-cholesterol concentrations lower for METSYN subjects than for controls. Gender, age, and total or low-density lipoprotein-cholesterol concentrations did not differ between groups. Fasting insulin and FFA concentrations were greater in the METSYN group.
Table 1.
Clinical Characteristics of Controls and METSYN Subjects
| Controls (n = 16) | METSYN (n = 18) | P Value | |
|---|---|---|---|
| Gender | F (10), M (6) | F (9), M (9) | NS |
| Age | 43 ± 3 | 46 ± 3 | NS |
| Body mass index, kg/m2 | 23 ± 0.5 | 35 ± 2b | <.001 |
| Waist circumference, cm | <102 (M) | 135 ± 18 (M)a | <.01 |
| <88 (F) | 109 ± 5 (F)a | <.01 | |
| Systolic BP, mm Hg | 112 ± 2 | 131 ± 5b | <.001 |
| BP medications | (8) | ||
| Diastolic BP, mm Hg | 70 ± 2 | 78 ± 3a | <.01 |
| Fasting blood glucose, mg/dL | 88 ± 2 | 98 ± 3b | <.005 |
| Total cholesterol, mg/dL | 191 ± 10 | 196 ± 9 | NS |
| LDL cholesterol, mg/dL | 123 ± 9 | 128 ± 8 | NS |
| Statin treatment | (5) | ||
| HDL cholesterol, mg/dL | 57 ± 3 | 46 ± 2a | <.01 |
| HDL <40, M | (5) | ||
| HDL <50, F | (5) | ||
| Triglycerides, mg/dL | 70 ± 7 | 124 ± 10b | <.001 |
| Baseline insulin, mU/L | 2.7 ± 0.5 | 11.3 ± 2.6b | <.001 |
| Baseline FFAs, mEq/L | 0.68 ± 0.04 | 0.93 ± 0.06b | <.002 |
Abbreviation: LDL, low-density lipoprotein. Values are mean ± SEM.
, P < .01;
, P < .001.
Metabolic responses: plasma glucose, insulin, and FFA concentrations and the GIR
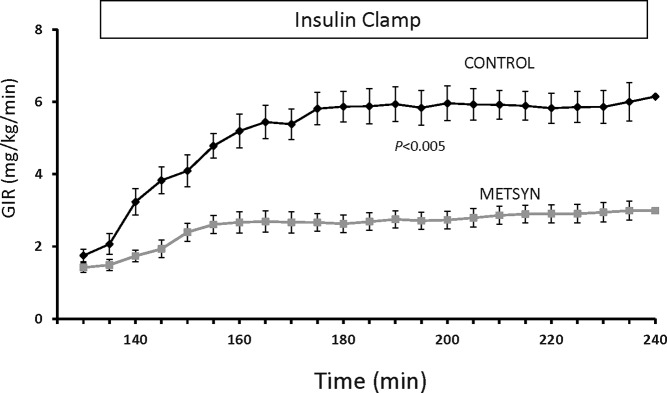
The plasma glucose and insulin concentrations were higher at baseline in the METSYN than in the control group (Table 1) and remained so throughout the postprandial interval and during the insulin clamp (Figure 1, A and B). Serum FFA concentrations (Figure 1C) declined postprandially and with the insulin clamp in both groups but were greater in the METSYN throughout. Despite higher prevailing glucose and insulin concentrations, the GIR required to maintain euglycemia was lower in METSYN than control subjects at steady state (2.9 ± 0.3 mg/kg·min vs 5.8 ± 0.4 mg/kg · min, P < .005, Figure 2), confirming metabolic insulin resistance.
Figure 1.
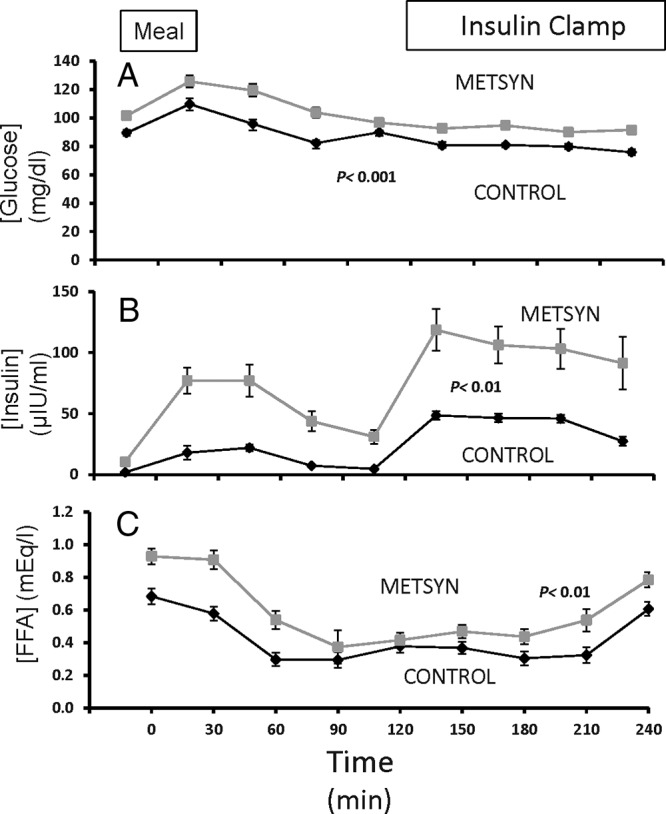
Time course for plasma glucose insulin and FFA changes over the 4 hours of the study. Plasma glucose, insulin, and FFAs were each elevated in the subjects with metabolic syndrome (repeated measures ANOVA).
Figure 2.
The GIR required to maintain basal glucose concentration over the course of the 2-hour euglycemic insulin clamp. The P value refers to the difference between control and metabolic syndrome subjects compared using a repeated-measures ANOVA.
AI and PWV
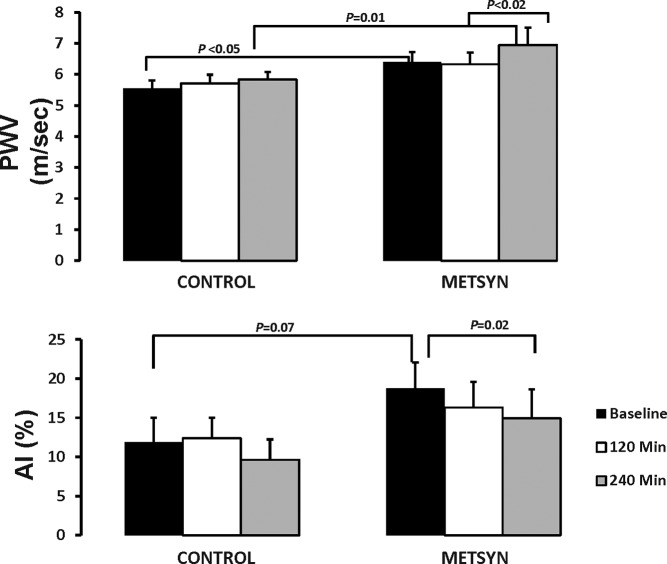
PWV was higher at baseline (6.4 ± 0.3 m/sec vs 5.5 ± 0.3 m/sec, P < .01) and at 240 minutes (7.0 ± 0.6 m/sec vs 5.8 ± 0.2 m/sec, P = .01) in METSYN as compared with controls (Figure 3A). PWV increased between 120 minutes and 240 minutes during the insulin clamp in the METSYN group (6.3 ± 0.4 m/sec vs 7.0 ± 6 m/sec, P = .03) but not in controls. Considering the AI, there were no significant differences between groups at any time point (Figure 3B), However, at baseline the AI was marginally higher in the METSYN group (19% ± 3% vs 12% ± 3%, P = .07). By 240 minutes AI declined only in the METSYN group (19% ± 3% vs 15% ± 4%, P = .02).
Figure 3.
The upper panel illustrates the PWV at baseline, 2 hours after ingesting the HFM and 2 hours later at the end of the insulin clamp. Control subjects are on the left, and metabolic syndrome on the right. The lower panel shows the AI at these same time intervals for the control and METSYN subjects. Comparison across groups was done with a two-way ANOVA and within-group comparisons with a paired t test.
FMD and PIFV
FMD did not differ between groups at baseline or at 120 minutes after the HFM ingestion. By 240 minutes, however, FMD rose in controls (Figure 4A) compared with either the baseline (7.3 ± 1 vs 10.3 ± 15, P = .04) or post-HFM values (5.8% ± 1% vs 10.3% ± 1%, P = .001) and was significantly greater vs METSYN subjects. Within the METSYN group, neither the HFM nor the insulin clamp affected FMD.
Figure 4.
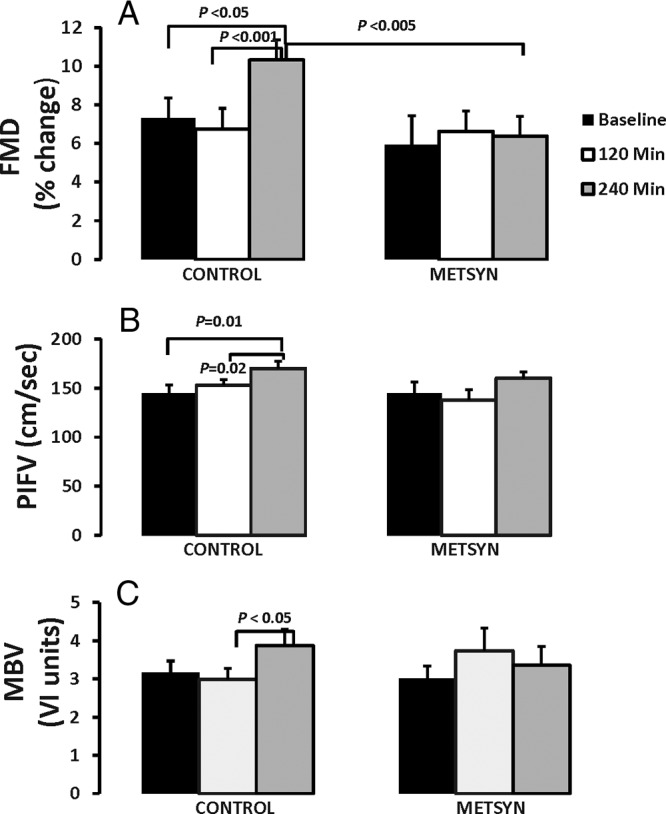
The upper panel illustrates the FMD at baseline, 2 hours after ingesting the HFM, and 2 hours later at the end of the insulin clamp. Control subjects are on the left and METSYN subjects on the right. The middle panel shows the postischemic flow velocity and the lower panel the microvascular blood volume measured by CEU at these same time intervals for the control and METSYN subjects. Comparison across groups was done with a two-way ANOVA and within-group comparisons with a paired t test.
There were no differences in PIFV between groups at any time point (Figure 4B). There were no changes in PIFV in the METSYN group, whereas PIFV increased from baseline to 240 minutes (145 ± 10 cm/sec vs 170 ± 6 cm/sec, P = .01) and between 120 minutes and 240 minutes (153 ± 7 cm/sec vs 170 ± 6 cm/sec, P = .002) in the control group. Whereas brachial artery PIFV increased in controls but not in METSYN, it was not significantly different between the two groups (Supplemental Table 1).
Considering both the control and METSYN groups, we noted a significant positive correlation between FMD and GIR during the insulin clamp (r = 0.487, P = .01). We also noted that FMD and PIFV (both of which are NO mediated events) were significantly positively correlated (r = 0.392, P = .001).
Microvascular blood volume
There was no difference in MBV between the two groups at any time point (Figure 4C). Within the METSYN group, there were no changes in MBV with HFM or the clamp. However, MBV increased in the control group during the insulin clamp (2.99 ± 0.3 vs 3.87 ± 0.4, P = .04).
We measured nitrate and nitrite in plasma at baseline and 240 minutes. Nitrate and nitrite can reflect NO generation, dietary intake, and other factors. There was no difference in serum nitrate plus nitrite at baseline between the two study groups. Nitrate plus nitrite declined significantly and similarly in METSYN (35.7 ± 4.4 μM to 27.6 ± 3.2 μM, P < .001) and controls (39.3 ± 6.7 μM to 27.8 ± 3.9 μM, P < .001) between time 0 and 240 minutes.
CVD-associated biomarkers
Of 92 CVD biomarkers measured in all subjects after an overnight fast, 12 were significantly (P < .001) different between groups (Supplemental Table 2). For nine of these, a highly significant difference was also seen at the end of the study, with three others (receptor for advanced glycation endproducts, IL-6, and tissue plasminogen activator) showing statistically less strongly significant differences at the end of the study. We also noted a significant change for 16 biomarkers between baseline and 240 minutes in the control subjects that were mimicked by changes in 14 of these same biomarkers in the METSYN subjects (Supplemental Table 2). Several biomarkers changed markedly in the controls but not the METSYN subjects (follistatin, receptor for advanced glycation endproducts, TNF receptor superfamily member 6 [fatty acid synthase], IL-6, epidermal growth factor), whereas platelet endothelial cell adhesion molecule-1 changed markedly between 0 and 240 minutes in the METSYN subjects (P = 9 × 10−4) but not in the controls (P = .06) (Supplemental Table 2).
Discussion
The current study provides the first data on endothelial function measured at three distinct levels of the arterial tree in healthy adults and showed a consistent augmentation of endothelial function after a meal and insulin infusion at each level. As noted, previous reports suggest that in healthy subjects insulin provokes conduit artery endothelial dysfunction measured as brachial or femoral artery FMD (10, 11), whereas others report insulin enhances endothelial function in resistance (3, 4, 7) and terminal arteriolar (9) vessels in healthy subjects. Insulin's vasodilatory effect was absent in our METSYN subjects. To our knowledge, the present results are the first demonstrating impaired NO-responsive vascular function at several levels of the arterial vasculature in METSYN. This vascular insulin resistance in METSYN may contribute to its role as a risk factor for accelerated atherosclerosis and arteriosclerosis (13, 26) and small vessel dysfunction (27). The current PWV also indicates that aortic stiffness is increased in individuals with METSYN, as previously shown for obese (28), hypertensive (29), and hyperlipidemic (30) subjects. The higher levels of insulin may in part explain the insulin response of AI seen in the METSYN subjects. AI has previously been reported to improve in response to insulin in insulin-sensitive subjects (31) but not in insulin resistant subjects. The findings with AI reported here diverged from those seen with PWV, which if anything deteriorated specifically in the METSYN subjects. Presently we have no clear explanation for this divergence. However, although both AI and PWV are regarded as indices of vascular stiffness, they clearly differ in many respects, and AI in particular responds to factors other than stiffness (19).
The baseline FMD difference between groups in the current study (1.4%) is similar to the 1% difference previously reported for a larger study of METSYN subjects vs controls (32). The lack of statistical significance was likely due to insufficient power in the current study.
Recent rodent studies have demonstrated that introducing oxidative stress specifically in vascular smooth muscle cells (33) or interfering with insulin signaling specifically in vascular endothelial cells (34) induces whole-body metabolic insulin resistance. These findings raise the possibility that the vasculature may not simply be an adversely affected bystander when metabolic insulin resistance is present but rather is a coconspirator in an evolving process fueled by environmental factors like nutrient overload and inactivity. Other work has shown that specific knockout of the endothelial cell insulin receptor accelerates atherosclerosis in the apolipoprotein E null mouse (35). In aggregate, these murine studies support an important role for vascular insulin action in regulating both vascular health and metabolic insulin action. Insulin's microvascular actions in muscle in particular appear critical to its overall metabolic action (36) in both rodents (37) and humans (9).
It is difficult to reconcile the current FMD results with two prior studies. Arcaro et al reported a progressive decline in brachial and femoral FMD during a 6-hour low- or moderately high-dose euglycemic insulin clamp (10) and concluded that insulin caused endothelial dysfunction. Campia et al (11) also reported that FMD declined during a 4-hour insulin clamp in healthy subjects. Within those healthy subjects, baseline FMD responses were lower in the less insulin-sensitive subjects but declined in both groups with insulin. The subjects in these studies were younger and were fasted overnight, and the hot-hand method was used to conduct the insulin clamp, which extended for 4–6 hours. In one study brachial FMD was measured as the area under the curve of artery diameter over 6 minutes after brief (2 min) wrist ischemia (10). Later studies (including the current one) have followed consensus recommended methods for FMD measurement (38). The short duration of the clamp used here may have missed a later inhibitory effect of insulin on FMD. However, there was no indication in either previous study of an early augmenting action of insulin on FMD that later reversed.
Perkins et al (12) recently reported that FMD was unchanged during 4 hours of euglycemic hyperinsulinemia in obese insulin-resistant subjects, similar to our finding here for postprandial METSYN subjects. They also reported that FMD declined with time when insulin and glucose were held at postabsorptive levels using the pancreatic clamp method. By contrast, Arcaro et al (10) reported FMD declined with insulin vs a saline control.
The insulin-induced brachial artery endothelial dysfunction reported (10, 11) in healthy humans contrasts with the effects of insulin on resistance artery function measured as forearm or leg blood flow during acetyl- or methacholine infusion (4–7). Arcaro et al (10) speculated that the insulin response by large muscular arteries evaluated by FMD might differ from those of resistance vessels. Our results do not support this speculation. Instead, we find that insulin acts similarly at all three levels of the arterial vasculature to enhance NO-dependent vasorelaation. Our study design does not account for changes in any of the vascular end points that might have occurred as a result of the passage of time in the absence of the meal or the insulin infusion or both. We were particularly interested in a model of insulin's action in the postprandial state, either from endogenous insulin or from infused insulin. This design may account for some of the differences between our results and those published previously. However, if anything, we might expect a HFM to diminish NO-dependent vascular actions of insulin.
Both previous studies in which FMD was reported impaired by insulin used the heated-hand method to arterialize blood for the insulin clamp (10, 11). This increases blood flow in both the heated and the contralateral limb (in which FMD was measured) and differentially shifts flow from muscle to skin (24, 25). As a result, FMD under those conditions differs from the usual method in which there is no stimulation of blood flow prior to measurement. We avoided using the heated-hand method and avoided arterial hyperglycemia (which would occur if we simply clamped venous glucose), by allowing a graded decrement in peripheral venous glucose based on the glucose infusion rate and prior direct measures of arterial-venous glucose concentration differences during euglycemic clamp conditions (9). Importantly, studies examining the effect of insulin on resistance arteriolar endothelial function used direct arterial cannulation, and no limb heating was involved.
Whether technical differences in either how FMD is measured or in how the clamp is performed or other factors (age, dietary history, fitness) account for the differing results is uncertain. However, we suggest that the conclusion that insulin causes conduit artery endothelial dysfunction (10, 11) in healthy humans is controversial and open to further investigation.
The plasma nitrate plus nitrite concentrations declined during the course of the insulin clamp in both groups. This has been seen previously; however, stable isotope tracer study results have shown that both the fractional and absolute synthetic rate for nitric oxide increases with hyperinsulinemia in healthy individuals (39). The pathway responsible for the overall decline in NO metabolites is not certain but does not signal a decrease in NO production.
The multiplex biomarker findings highlight both the complexity of the molecular differences between control and metabolic syndrome subjects as well as the dynamic character of the vascular regulation that occurs after the dual stimulation of feeding and hyperinsulinemia. We were impressed by the heterogeneity of the differences between groups with some presumably vascular beneficial as well as some vascular detrimental markers being higher in METSYN subjects. Considering the differences seen at baseline (Supplemental Table 2), it is of interest that circulating levels of adrenomedullin (a vasodilator), IL-1 receptor antagonist (an antiinflammatory protein) and tissue plasminogen activator (an antithrombotic factor) are all significantly higher in metabolic syndrome subjects, perhaps signaling some compensatory process to minimize vascular dysfunction. Conversely, the increases in E-selectin, chemokine ligand-3, and IL-6 may indicate a more proinflammatory state with METSYN. Because some multiplex assays show considerable variance with repeat testing, we were encouraged that 9 of the 12 measures that were statistically different (P < .001) between groups in the postabsorptive samples were also highly significantly different after the meal plus insulin intervention and the remaining three also differed, if a P < .05 was used. We also note the expected significantly higher leptin and lower GH in the obese METSYN subjects.
A somewhat surprising finding was the number of biomarkers that changed significantly in response to the meal ingestion plus hyperinsulinemia. As noted, between the control and metabolic syndrome subjects, there was striking coherence in the direction of these changes (Table 2). This would seem to underscore the importance of controlling for the nutritional status (fasting vs fed) of subjects when comparing patterns of biomarker expression across subjects. Five biomarkers changed in response to meal plus insulin only in the control subjects, whereas only one (platelet endothelial cell adhesion molecule-1) changed only in the metabolic syndrome participants.
In conclusion, we found that in postprandial humans, insulin enhanced endothelial function in large muscular arteries, in resistance arterioles, and in muscle terminal arterioles in controls, whereas METSYN subjects had significantly impaired responses. Insulin also increases vascular stiffness as measured by PWV in METSYN. In aggregate, these findings emphasize that metabolic insulin resistance and vascular insulin resistance are closely linked and vascular insulin resistance likely contributes to the enhanced vascular disease seen in insulin resistant states.
Acknowledgments
We thank Dr R. Meijer and Ms S. Gray for their helpful review of the manuscript.
This work was supported by National Institutes of Health Grant RO1 DK 073759 and a grant from the University of Virginia/AstraZeneca Research Alliance.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AI
- augmentation index
- BP
- blood pressure
- CEU
- contrast-enhanced ultrasound
- CRU
- Clinical Research Unit
- CVD
- cardiovascular disease
- FFA
- free fatty acid
- FMD
- flow-mediated dilation
- GIR
- glucose infusion rate
- HbA1C
- glycated hemoglobin
- HDL
- high-density lipoprotein
- HFM
- high-fat meal
- MBV
- microvascular blood volume
- METSYN
- metabolic syndrome
- NO
- nitric oxide
- PIFV
- postischemic flow velocity
- PWV
- pulse wave velocity.
References
- 1. Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996;98:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johansson GS, Arnqvist HJ. Insulin and IGF-I action on insulin receptors, IGF-I receptors, and hybrid insulin/IGF-I receptors in vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2006;291:E1124–E1130. [DOI] [PubMed] [Google Scholar]
- 3. Taddei S, Virdis A, Mattei P, Natali A, Ferrannini E, Salvetti A. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation. 1995;92:2911–2918. [DOI] [PubMed] [Google Scholar]
- 4. Rask-Madsen C, Dominguez H, Ihlemann N, Hermann T, Kober L, Torp-Pedersen C. Tumor necrosis factor-α inhibits insulin's stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108:1815–1821. [DOI] [PubMed] [Google Scholar]
- 5. Westerbacka J, Bergholm R, Tiikkainen M, Yki-Jarvinen H. Glargine and regular human insulin similarly acutely enhance endothelium-dependent vasodilatation in normal subjects. Arterioscler Thromb Vasc Biol. 2004;24:320–324. [DOI] [PubMed] [Google Scholar]
- 6. Schinzari F, Tesauro M, Rovella V, et al. Generalized impairment of vasodilator reactivity during hyperinsulinemia in patients with obesity-related metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299:E947–E952. [DOI] [PubMed] [Google Scholar]
- 7. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coggins MP, Lindner J, Rattigan S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50:2682–2690. [DOI] [PubMed] [Google Scholar]
- 9. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. [DOI] [PubMed] [Google Scholar]
- 10. Arcaro G, Cretti A, Balzano S, et al. Insulin causes endothelial dysfunction in humans sites and mechanisms. Circulation. 2002;105:576–582. [DOI] [PubMed] [Google Scholar]
- 11. Campia U, Sullivan G, Bryant MB, Waclawiw MA, Quon MJ, Panza JA. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol Heart Circ Physiol. 2004;286:H76–H82. [DOI] [PubMed] [Google Scholar]
- 12. Perkins JM, Joy NG, Tate DB, Davis SN. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am J Physiol Endocrinol Metab. 2015;309(2):E168–E176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. [DOI] [PubMed] [Google Scholar]
- 14. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 15. Nowotny B, Zahiragic L, Krog D, et al. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes. 2013;62:2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ceriello A, Taboga C, Tonutti L, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106:1211–1218. [DOI] [PubMed] [Google Scholar]
- 17. Brock DW, Davis CK, Irving BA, et al. A high-carbohydrate, high-fiber meal improves endothelial function in adults with the metabolic syndrome. Diabetes Care. 2006;29:2313–2315. [DOI] [PubMed] [Google Scholar]
- 18. Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92:595–600. [DOI] [PubMed] [Google Scholar]
- 19. Gurovich AN, Beck DT, Braith RW. Aortic pulse wave analysis is not a surrogate for central arterial pulse wave velocity. Exp Biol Med. 2009;234:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Westerbacka J, Wilkinson I, Cockcroft J, Utriainen T, Vehkavaara S, Yki-Jarvinen H. Diminished wave reflection in the aorta. A novel physiological action of insulin on large blood vessels. Hypertension. 1999;33:1118–1122. [DOI] [PubMed] [Google Scholar]
- 21. Westerbacka J, Vehkavaara S, Bergholm R, Wilkinson I, Cockcroft J, Yki-Jarvinen H. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes. 1999;48:821–827. [DOI] [PubMed] [Google Scholar]
- 22. Gokce N, Keaney JF, Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 23. Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Astrup A, Simonsen L, Bülow J, Christensen NJ. Measurement of forearm oxygen consumption: role of heating the contralateral hand. Am J Physiol. 1988;255:E572–E578. [DOI] [PubMed] [Google Scholar]
- 25. Gallen IW, Macdonald IA. Effect of two methods of hand heating on body temperature, forearm blood flow, and deep venous oxygen saturation. Am J Physiol. 1990;259(5 Pt 1):E639–E643. [DOI] [PubMed] [Google Scholar]
- 26. Chen K, Lindsey JB, Khera A, et al. Independent associations between metabolic syndrome, diabetes mellitus and atherosclerosis: observations from the Dallas Heart Study. Diabetes Vasc Dis Res. 2008;5:96–101. [DOI] [PubMed] [Google Scholar]
- 27. Serne EH, de Jongh RT, Eringa EC, RG IJ, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–211. [DOI] [PubMed] [Google Scholar]
- 28. Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 30. ter Avest E, Holewijn S, Bredie SJ, van Tits LJ, Stalenhoef AF, de Graaf J. Pulse wave velocity in familial combined hyperlipidemia. Am J Hypertens. 2007;20:263–269. [DOI] [PubMed] [Google Scholar]
- 31. Westerbacka J, Uosukainen A, Mäkimattila S, Schlenzka A, Yki-Järvinen H. Insulin-induced decrease in large artery stiffness is impaired in uncomplicated type 1 diabetes mellitus. Hypertension. 2000;35:1043–1048. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki T, Hirata K, Elkind MS, et al. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS). Am Heart J. 2008;156:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Youn J-Y, Siu KL, Lob H, Itani H, Harrison DG, Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes 2014;63(7):2344–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. [DOI] [PubMed] [Google Scholar]
- 35. Rask-Madsen C, Li Q, Freund B, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 11:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301:E252–E263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. [DOI] [PubMed] [Google Scholar]
- 38. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 39. Tessari P, Coracina A, Puricelli L, et al. Acute effect of insulin on nitric oxide synthesis in humans: a precursor-product isotopic study. Am J Physiol Endocrinol Metab. 2007;293:E776–E782. [DOI] [PubMed] [Google Scholar]