Abstract
Context:
Serum free cortisol (SFF) responses to cosyntropin simulation test (CST) may more accurately assess adrenal function than total cortisol (TF).
Objective:
The objective of the study was to evaluate the diagnostic utility of SFF responses during a 250-μg CST.
Design:
We recruited healthy volunteers (HV; n = 27), patients with primary and secondary adrenal insufficiency (n = 19 and n = 24, respectively), and subjects with Child-Pugh class A cirrhosis (CH; n = 15). Each received 250 μg cosyntropin with measurement of ACTH and corticosteroid binding globulin (CBG) at time 0 and TF and SFF at 0, 30, and 60 minutes. Salivary cortisol was measured at all time points in CH subjects.
Results:
Peak SFF and TF were significantly higher in HVs vs both AI groups (P < .05). Peak SFF and TF (6.8 μg/dL vs 2.2 μg/dL; [188 nmol/L vs 62 nmol/L]; P < .01) were significantly higher in the secondary adrenal insufficiency vs primary adrenal insufficiency patients. The optimal peak SFF criterion to identify adrenal insufficiency patients vs HV was 0.9 μg/dL (25 nmol/L) (sensitivity of 95%, specificity of 100%). Mean CBG and albumin levels were similar among all four groups. CH patients had a higher peak SFF than HV (2.4 vs 2.0 μg/dL; P = .02. In the CH patients, peak salivary cortisol levels correlated well with peak SFF (rs = 0.84, P = .005). CBG levels were similar among the groups.
Conclusion:
We provide normative data for SFF values in HV and AI during the CST. Normal CBG levels in mild cirrhosis did not affect the interpretation of the CST.
Measurement of serum cortisol is an important tool in the diagnosis of adrenal insufficiency. Corticosteroid binding globulin (CBG) and albumin bind cortisol so that normally only approximately 5%–10% circulates as the biologically available unbound (free) cortisol (1). As a result, the total cortisol measurement, which includes both bound and free components, may not accurately reflect adrenal function in critically ill patients with low albumin (2). Klose et al (3) showed that the total cortisol response to cosyntropin (also known as tetrocosactide) also may be falsely abnormal in patients with nephrotic syndrome as a result of decreased CBG levels. These data raise concerns about the ability of total cortisol to assess adrenal function in populations with decreased (or increased) binding protein levels. Serum free cortisol or salivary cortisol (which reflects the free fraction in serum) may be a better measure of adrenal function in such patients, but no normative data are available using directly measured serum free cortisol rather than a calculated value.
In patients with cirrhosis, concentrations of serum proteins, including albumin and CBG, may be decreased. Recent studies in these patients found that the total cortisol response to cosyntropin was subnormal, due to low CBG and albumin levels, in the absence of clinical features of adrenal insufficiency (4–6).
The primary objective of this study was to create a normative database of serum free cortisol responses to cosyntropin, 250 μg, in healthy volunteers (HVs) and to compare this with the responses of patients with primary adrenal insufficiency (PAI), secondary adrenal insufficiency (SAI), and cirrhosis (CH). A secondary objective of the study was to evaluate the diagnostic utility of the serum free and salivary cortisol levels in assessing adrenal sufficiency in cirrhotic patients.
Subjects and Methods
The Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development approved the study protocol for evaluation of adrenal function (NCT00156767). All subjects provided written informed consent. HVs received monetary compensation.
Healthy volunteers
Healthy adults were recruited from three age groups (<40 y, 40–55 y, and > 55 y, evenly split by gender) using community flyers from February 2008 to July 2009. Exclusion criteria included presence of uncontrolled acute or chronic illness, abnormal cell blood count or electrolytes, pregnancy, lactation, recent use of imidazole or glucocorticoid medications, mineralocorticoid antagonists, or potassium supplements, chronic use of nonsteroidal antiinflammatory drugs or the presence of signs or symptoms of adrenal insufficiency (eg, unintentional weight loss, nausea, excessive fatigue, low blood pressure, etc). Well-controlled illnesses (eg, hypertension) were allowed.
Known adrenal insufficiency (AI)
Subjects were enrolled from February 2006 through July 2012. Recruitment advertising included patient contact, community flyers, letters to local endocrinologists and primary care physicians, and an announcement on the web site of the National Adrenal Disorders Foundation. Individuals evaluated in the general endocrine clinic (Clinical Center, Bethesda, Maryland) who were highly suspicious for or with known PAI or SAI were invited to participate. The exclusion criteria for the HV applied to the AI subjects as well, with the exception of signs or symptoms of adrenal insufficiency. Well-controlled chronic illnesses (eg, hypertension) were allowed.
High ACTH values, positive 21-hydroxylase antibodies, and/or clinical or genetic evidence of an autoimmune polyglandular syndrome were used to confirm PAI and an autoimmune etiology. None had a history of bilateral adrenalectomy, infectious disease, hemorrhage, or infiltrative disorders.
SAI was determined by low ACTH values and/or history of pituitary disease or exogenous steroid use and lack of medical history to support PAI. The etiologies of SAI were pituitary surgery or radiation exposure (n = 4), Sheehan's syndrome (n = 1), isolated ACTH deficiency, multiple pituitary hormonal deficiencies of uncertain etiology (n = 4), or exogenous steroid exposure (n = 15). Of the patients with exogenous steroid exposure, 7 of 15 had received an injectable steroid for joint pain, 1 of 15 received iv high-dose steroids during hospitalization, and 6 of 15 had been taking supraphysiological doses of oral replacement steroids (hydrocortisone or prednisone). The remaining patient had a presumed history of lymphocytic hypophysitis, was using a fentanyl patch, and reported taking physiological replacement hydrocortisone doses, although we suspect that she might have been taking extra hydrocortisone doses. All adrenally insufficient patients were taking or were started on physiological replacement doses of glucocorticoid (10–12 mg/m2 of hydrocortisone or prednisone equivalent) before testing. All PAI patients were taking or were started on fludrocortisone with the exception of one patient who managed with a high-salt diet and salt tablets.
Patients with cirrhosis
Subjects were recruited from January 2012 through April 2013. Individuals who presented to the National Institutes of Health (NIH) Hepatology Clinic with previously documented cirrhosis based on liver biopsy, Child-Pugh class A or B, secondary to viral hepatitis, and normal renal function (>60 mL/min by the Modified Diet in Renal Disease equation) were invited to participate. The exclusion criteria stated for the HVs applied to the CH subjects as well. Well-controlled chronic illnesses, including liver cirrhosis, were allowed. All of the patients from the CH group had Child-Pugh A cirrhosis and were otherwise healthy.
Procedures
Staff performed a complete history and physical examination and recorded all medications.
ACTH stimulation testing
AI patients did not take their hydrocortisone or prednisone (or, if applicable, florinef) dose on the morning of testing. A dosage of 250 μg ACTH 1–24 (Cortrosyn; Amphastar Pharmaceuticals) was administered iv between 8:00 and 10:00 am, followed by 10 mL saline. Serum total (competitive chemiluminescent enzyme immunoassay [EIA], Siemens Immulite 2000 Xpi; Department of Laboratory Medicine [DLM]; NIH, Bethesda, Maryland; lower limit of quantitation 1.0 μg/dL) and free cortisol levels, isolated by equilibrium dialysis followed by liquid chromatography-tandem mass spectrometry (Quest Diagnostics; lower limit of quantitation 0.03 μg/dL), were obtained just prior to ACTH injection and 30 and 60 minutes later. A chemistry panel (serum sodium, potassium, glucose, albumin, blood urea nitrogen, and creatinine; DLM, NIH), and CBG (in-house RIA; Esoterix Laboratories) were obtained at baseline. Plasma ACTH (competitive chemiluminescence EIA, Siemens Immulite 2000 Xpi; DLM, NIH) was measured initially at baseline in patients with an unclear diagnosis; subsequently ACTH was measured in all patients with presumed AI.
CH subjects collected salivary samples for cortisol at 0, 30, and 60 minutes. Subjects were told to place a cotton salivette(s) (Sarstedt, Inc) inside their mouth and gently chew and/or suck on it for 1–3 minutes until they became soaked in saliva. Immediately upon test completion, each subject's samples were processed for salivary cortisol levels (Immulite 1000, competitive chemiluminescence EIA; DLM, NIH).
Statistics
All summary values are reported as the mean ± SD. Sensitivity and specificity, based on a specific cutoff value of cortisol, were computed using standard formulas. In general, comparisons of cortisol levels in two groups used the standard two-sample Student's t test (using Welch's version if the SDs in the two groups differed substantially); if the data substantially violated the assumptions for the Student's t test, the exact nonparametric Wilcoxon rank sum test was used. To show the relationship of peak serum free cortisol to peak salivary cortisol in cirrhosis patients, a nonparametric robust linear regression (based on the estimate of Theil and Sen) is shown; also, the correlation is estimated using Spearman's nonparametric correlation, which is robust to deviations from normality for the data points.
To evaluate the effect of using different cutoff values for cortisol on sensitivity and specificity, nonparametric receiver operating characteristic curves were computed, along with their estimated area under the curve, computed by the trapezoidal rule. The area under the curve is an overall, single-number summary (with maximal value of 1 for a perfect measure) for how well a given measure separates observations from two groups, as opposed to the sensitivity/specificity, which evaluate separation at one particular cutoff value. All P values are two sided. All computations used Stata, version 12 (StataCorp, 2011) except for the exact Wilcoxon rank sum computation, which used StatXact, version 6.0 (Cytel Software, 2003).
Results
Subject characteristics
Characteristics of the subjects are presented in Table 1. There was a statistically significant difference in age between the CH and non-CH groups (P = .002).
Table 1.
Demographics and Baseline and Stimulated Cortisol Responses (Mean ± SD) to 250 μg Cosyntropin
| HV (n = 27) | PAI (n = 19) | SAI (n = 24) | CH (n = 15) | |
|---|---|---|---|---|
| Gender (female/male) | 12/15 | 12/7 | 17/7 | 6/9 |
| Age, y | 46 ± 13 | 47 ± 18 | 44 ± 14 | 58 ± 11a |
| BMI, kg/m2 | 25.9 ± 4.5 | 25.0 ± 4.6 | 27.9 ± 5.3 | 29.6 ± 8.3 |
| Baseline total F, μg/dL | 10.1 ± 5.9 | 1.8 ± 1.8b | 3.3 ± 3.9b | 9.5 ± 2.9 |
| Peak total F, μg/dL | 26.7 ± 4.6 | 2.2 ± 3.2c | 6.8 ± 6.3c | 26.2 ± 3.0 |
| Baseline serum free F, μg/dL | 0.4 ± 0.4 | 0.1 ± 0.03d | 0.1 ± 0.1d | 0.4 ± 0.2 |
| Peak serum free F, μg/dL | 2.0 ± 0.6e | 0.1 ± 0.1c,f,g | 0.3 ± 0.3c,f,g | 2.4 ± 0.5e |
| Range peak serum free cortisol, μg/dL | 0.9–2.9 | 0.03–0.3 | 0.03–1.3 | 1.3–3.0 |
| Baseline CBG, mg/dL | 2.7 ± 0.4 | 2.8 ± 0.5 | 2.9 ± 0.5 | 2.8 ± 0.6 |
| Baseline albumin, mg/dL | 3.6 ± 0.3 | 3.7 ± 0.3 (n = 16) | 3.5 ± 0.3 (n = 22) | 3.6 ± 0.4 |
| Baseline ACTH, pg/mL | 17.5 ± 9.9i (n = 24) | 798.1 ± 825.7c,h (n = 14) | 25.0 ± 46.9c.i (n = 23) | 14.9 ± 7.1i (n = 15) |
Abbreviation: F, cortisol. To convert cortisol micrograms per deciliter to nanomoles per liter, multiply by 27.6. To convert ACTH picograms per milliliter to picomoles per liter, multiply by 0.22.
P < .05 CH vs others.
P < .0001 PAI vs HV and CH; P < .0001 SAI vs HV and CH (CH and HV combined because of no difference between the groups, P = 0.68).
P < .05 PAI vs. SAI.
P < .0001 PAI vs HV and CH; P < .0001 SAI vs HV and CH (CH and HV combined because of no difference between the groups, P = .95).
P < .05 HVs vs cirrhotics.
P < .05 HVs vs AI groups.
PAI, 0.1 ± 0.1 = .07 ± 0.063; SAI, 0.3 ± 0.3 = 0.273 ± 0.326 (μg/dL).
P < .05 HV vs PAI.
P > .05 HV vs SAI vs. CH.
Of 26 PAI subjects initially enrolled, seven were excluded from the main analyses. Five women using oral contraceptive pills had high CBG levels and were analyzed separately. One PAI subject had missing free cortisol data and another had been exposed to high-dose steroids. Of the 26 SAI subjects initially enrolled, two had missing free cortisol data. Of the 30 HV subjects, two on oral contraceptive pills had elevated CBG levels and one had missing free cortisol data.
Plasma ACTH, and serum total and free cortisol levels
As expected, mean plasma ACTH levels were significantly higher in PAI (range 25.1–2500.0 pg/mL [5.5–550 pmol/L]; reference range 0.0–46.0 pg/mL [0–10.1 pmol/L]) compared with SAI subjects (range 5.0–215.0 pg/mL [1.1–47.3 pmol/L]) and HVs (range 5.9–46.1 pg/mL [1.3–10.1 pmol/L]). Levels were similar between HV and SAI subjects and HV and cirrhotic subjects (range 5.0–27.8 pg/mL [1.1–6.1 pmol/L]) (Table 1).
Two PAI patients had ACTH levels in the normal range, for which we do not have a good explanation (33.2 and 25.1 pg/mL [7.3 and 5.5 pmol/L]). Both patients reported taking physiological replacement doses of hydrocortisone or prednisone and no other exposure to oral, topical, inhaled, or injectable glucocorticoids. Similarly, these patients denied the use of nonglucocorticoid medications that can inhibit ACTH production (7). Plasma ACTH levels can be affected by the time of the blood draw and handling of the sample; however, in this study the levels were drawn in the morning before the physiological glucocorticoid dose. Mishandling of the sample cannot be excluded as a cause of unexpectedly low values in the current study (7).
In contrast, three patients with SAI had elevated ACTH levels. We believe that one patient with a slightly elevated value (53.3 pg/mL [11.7 pmol/L]) had partial ACTH insufficiency due to a history of transsphenoidal surgery. The remaining two patients both had been treated with supraphysiological doses of steroids several weeks before enrolling into this study and had ACTH levels greater than 100 pg/mL (22 pmol/L). We hypothesize that the hypothalamic-pituitary-adrenal axis was recovering from corticotrope suppression causing increased ACTH production in an effort to stimulated atrophied adrenal gland(s) (7). Despite these ACTH values, we believe these patients were correctly diagnosed based on prior medical history and laboratory findings consistent with the disease.
All HV and CH patients had peak total cortisol values of 18 μg/dL or greater (497 nmol/L) except one subject whose peak value was 17.6 μg/dL (486 nmol/L). All PAI and most SAI patients had values less than 18 μg/dL (497 nmol/L); one SAI patient had total cortisol of 20.3 μg/dL (560 nmol/L) but failed an insulin tolerance test done within 2 days, with a peak total cortisol level of 16.4 μg/dL (453 nmol/L). Mean baseline and peak total and serum free cortisol levels for all groups are shown in Table 1 and Figure 1.
Figure 1.
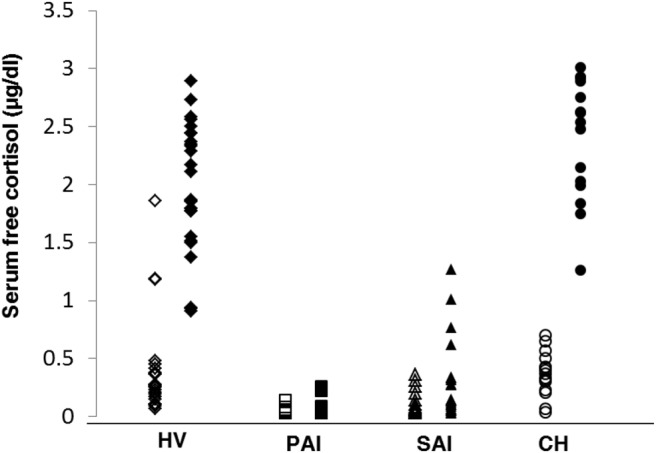
Baseline (open symbols) vs peak (closed symbols) serum free cortisol levels by group. Groups include the following: HVs, PAI, SAI, and CH.
When compared with PAI, the SAI subjects had significantly higher peak serum free cortisol and total cortisol (Table 1; P < .05 for both). When comparing cortisol levels between the AI, HV, and CH groups, the HV and CH groups were combined as there was no significant difference in baseline total or free cortisol levels between the two groups. Baseline total and free cortisol levels in the PAI and SAI groups were significantly lower than those in HV and cirrhotic subjects (P < .0001 for both groups; Table 1).
The optimal peak serum free cortisol criterion to correctly diagnose AI patients vs HVs was 0.9 μg/dL (25 nmol/L) (sensitivity 95%, specificity 100%), as shown in Figure 2.
Figure 2.
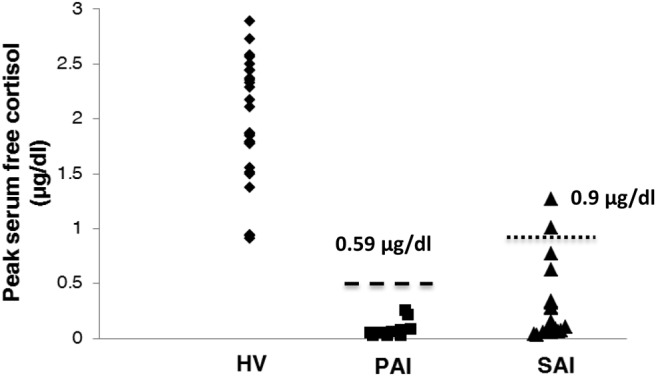
Cutoff values to discriminate between HVs and patients with PAI or SAI. The lower (0.6 μg/dL) and higher (0.9 μg/dL) dashed lines represent cutoff values to determine the healthy volunteers from patients with PAI and SAI, respectively.
Three patients had high baseline serum free cortisol (SFF) results (Figure 1). All subjects were male. The first patient had a baseline SFF of 1.86 μg/dL (51.3 nmol/L), a 30-minute value of 0.64 μg/dL (17.7 nmol/L) and a 60-minute value of 1.61 μg/dL (44.4 nmol/L). It appears as if this patient's results were affected by a labeling or collection error. However, the results could not be further confirmed because of a lack of additional samples. The remaining two patients had baseline SFF values of 1.19 and 1.18 μg/dL (32.8 and 32.5 nmol/L) followed by peak 60-minute values of 2.89 and 1.85 μg/dL (79.8 and 51.1 nmol/L), respectively. Their serum total cortisol levels were 33.3 and 18.2 μg/dL at baseline and 41.1 and 22.6 at 60 minutes, respectively. Both CBG (3.1 and 2.3 mg/dL [1134.3 and 623.7 nmol/L]) and albumin levels (3.7 and 3.5 mg/dL) were normal for these two subjects. Review of these subjects' medical histories and concomitant medications does not suggest an etiology for these unexpectedly high baseline results. We are also unaware of any of these subjects experiencing high levels of stress on the morning of the procedure.
Five PAI patients had elevated CBG (range 4.0–6.4 mg/dL, reference range 2.3–3.9 mg/dL) and were analyzed separately. Their peak total cortisol levels were similar to those with normal CBG (elevated CBG group total cortisol values: 1.0 ± 0.0 (27.6 nmol/L) vs normal CBG group: 1.8 ± 1.8 μg/dL (49.7 nmol/L) (exact Wilcoxon P = .54). The peak total cortisol value was 1.0 μg/dL in the five women with elevated CBG and in 15 of 19 of subjects with normal CBG levels. The other subjects had peak values up to 7.9 μg/dL (218.0 nmol/L).
Comparison of CH with HV subjects
Although CH patients had higher peak serum free cortisol than HVs (2.4 vs 2.0 μg/dL [66 vs 55 nmol/L]; P = .022), there was considerable overlap between the groups (Figure 1). Mean CBG levels in CH (2.8 ± 0.6 mg/dL) did not differ from those of the other groups (Table 1); the mean albumin level in CH was 3.6 mg/dL, with four subjects having albumin values less than 3.5 mg/dL. Mean albumin levels were similar across the groups (Table 1). Peak salivary cortisol levels correlated well with peak serum free cortisol levels in CH patients (rs = 0.84, P = .0001; Figure 3).
Figure 3.
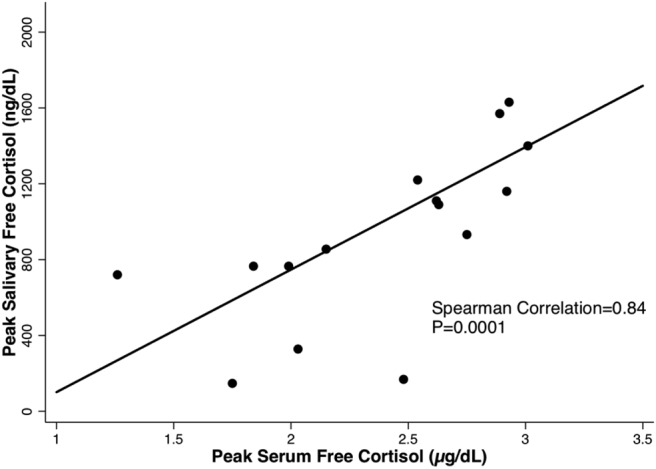
Peak serum vs salivary free cortisol levels in cirrhotic patients.
Discussion
After ACTH administration, because total serum cortisol values exceed CBG capacity, there is a shift toward unbound cortisol. Thus, serum free cortisol likely represents a more accurate assessment of adrenal function, particularly in patient populations with abnormal levels of binding proteins (2, 6). This study provides a normative database for serum free cortisol levels after ACTH stimulation. As might be expected, peak free cortisol was significantly higher in the SAI than the PAI patients. The optimal peak free cortisol criterion to differentiate healthy from adrenally insufficient subjects was 0.9 μg/dL (25 nmol/L).
Whereas patients with Child-Pugh class A cirrhosis had a higher peak free cortisol as compared with healthy volunteers, there was significant overlap and no difference in mean CBG or albumin levels. In contrast, in patients with CBG deficiencies, as in congenital CBG deficiency, there is a shift toward a greater proportion of free cortisol as well as an increase in binding of cortisol to albumin (8). Because there was no significant difference in CBG levels in the cirrhotic patients compared with the healthy volunteers in this study, the role of albumin levels in these patients is diminished. Salivary cortisol levels were measured only in the cirrhotic patients. In this group with largely normal CBG, peak salivary cortisol levels strongly correlated with peak serum free cortisol.
One of the earliest studies to evaluate the utility of serum free cortisol was performed in critically ill patients (2). Patients were divided into two groups according to serum albumin level. Serum free cortisol increased during critical illness compared with healthy individuals and was similar in patients with and without protein abnormalities. However, in ill patients with low serum protein, total cortisol levels were low when compared with the normoproteinemic patient group, both at baseline and after cosyntropin administration. Subsequent studies confirmed these findings and suggested that serum free cortisol more accurately reflects the physiological state in critically ill individuals and in patients with advanced cirrhosis (6, 9, 10).
However, more recently, total cortisol was found to be elevated in critically ill subjects, possibly due to reduced cortisol clearance (11). The differences in the studies might be explained by differences in the patient populations or types of critical illness. For example, in one study, hypoalbuminemia did not affect total cortisol of septic patients but did reduce that of nonseptic patients (12). In a study comparing patients with severe acute hepatitis, nonsevere acute hepatitis, and healthy controls, the median values of baseline (time 0) and 60-minute serum total cortisol values collected during a 250-μg cosyntropin stimulation test did not significantly differ between the groups (13). In the same study, the percentage serum free cortisol at baseline and at 60 minutes increased significantly with the degree of illness (severe acute hepatitis greater than nonsevere acute hepatitis greater than healthy controls). Interestingly, there was no significant difference in serum total cortisol at baseline or 60 minutes between the patients with low and high CBG levels, although serum free cortisol was significantly higher in those with low CBG levels (13).
Serum free cortisol and salivary cortisol also have been evaluated in patients with cirrhosis. In a study by Thevenot et al (4), CBG and albumin levels declined significantly as liver function deteriorated. They studied 95 nonseptic subjects (Child-Pugh class A, n = 34; Child-Pugh class B, n = 29; Child-Pugh class C, n = 32) with measurements of salivary and serum free and total cortisol levels during 250 μg ACTH stimulation testing (4). The patients with lower CBG and albumin levels had lower total cortisol levels compared with those with normal CBG and albumin levels, whereas the stimulated serum free and salivary cortisol levels were similar in both groups. Similar results were found in a study of 88 patients hospitalized for complications related to cirrhosis but not septic shock (Child-Pugh class A, n = 4; Child-Pugh class B, n = 24, Child-Pugh class C, n = 60) using salivary cortisol as a surrogate for serum free cortisol (5).
The study by Thevenot et al (4) showed a trend of increased basal and stimulated serum free and salivary cortisol levels and decreased total cortisol values from Child-Pugh class A to Child-Pugh class C stages. Supportive of these results are other studies in mixed populations of cirrhotics (Child-Pugh classes A, B, and C) that show inverse correlation of CBG with severity of disease, positive correlation between basal but not peak (250 μg and 1 μg cosyntropin stimulation) serum free cortisol levels and severity of disease, lower peak total cortisol levels in patients with cirrhosis, and discordance between serum free and total cortisol in the diagnosis of AI (6, 14). Although cirrhotic patients in the current study had normal CBG levels, our findings show no significant difference in basal serum free cortisol levels and a significant difference in peak serum free cortisol levels compared with healthy volunteers. Higher levels of free cortisol in cirrhotic patients are proposed to be a result of the underlying inflammatory process (4, 13).
The difference in CBG and free cortisol results in the aforementioned studies compared with the current study are likely because of patient population differences. The patients in the previous studies had more advanced liver disease as evidenced by their Child-Pugh class and included patients with alcoholic cirrhosis and those being referred for liver transplant as well as patients with decompensated cirrhosis. In the current study, all of the cirrhotic subjects had Child-Pugh class A cirrhosis as a result of viral hepatitis and were otherwise healthy. It is noted, however, that the serum total and free cortisol levels seen in the Child-Pugh class A subjects in the study by Thevenot et al (4) were higher than that those seen in the current study. Measurement by RIA (Thevenot et al) compared with equilibrium dialysis/liquid chromatography-tandem mass spectrometry (current study) may also contribute to the differences.
The current study shows that Child-Pugh class A subjects with cirrhosis due to viral hepatitis do not appear to suffer from protein abnormalities that might affect interpretation of total serum cortisol levels. Thus, concern for misdiagnosis of AI is mitigated in this patient population, and the measurement of serum free cortisol does not add any clinical value. However, in patients with more severe forms of cirrhosis, serum free cortisol values may be of considerable value in evaluating adrenal function, despite the increased expense. Further evaluation of AI with serum total and serum free cortisol levels classified by the severity of illness, as in this study, could prove to be very useful from both medical care and cost perspectives.
Salivary cortisol has been proposed as a surrogate for serum free cortisol, given the ease of collection and availability of assay. Arafah et al (10) found that although salivary cortisol correlated well with serum free cortisol, the relationship differed between patient groups. The relationship was similar in the healthy subjects and in critically ill patients with normal protein levels (r = 0.91 and r = 0.80, respectively), whereas in critically ill patients who had low serum protein levels, the correlation was much weaker (r = 0.53). In our study, salivary cortisol obtained in the CH group correlated well with serum free cortisol, with an rs = 0.84. Unknown serum factors or assay differences may explain the reduced correlation between serum and salivary free cortisol in patients with decreased serum proteins or cirrhosis. Further investigation is needed to determine what those factors are and how they might dictate the clinician's choice in whether to measure saliva or serum during testing.
The limitations of the current study include a smaller group of CH patients as compared with the other groups. In addition, salivary cortisol was only assessed in the CH group, and we are therefore unable to assess the diagnostic utility of salivary cortisol among the noncirrhotic groups.
In conclusion, our limited data suggest that patients with Child-Pugh class A cirrhosis due to viral hepatitis, with no other comorbidities, can be accurately assessed for AI using total cortisol levels. This study demonstrates that the serum free cortisol response to cosyntropin is an alternative to serum total cortisol for the diagnosis of AI, and its use should be considered in patients with protein abnormalities, specifically those with abnormal CBG levels, whereas total cortisol can be used in patients with normal protein levels. The normative database for serum free cortisol responses to ACTH stimulation may be clinically useful in the assessment of adrenal function. We suggest a cutoff of 0.9 μg/dL (25 nmol/L) for peak serum free cortisol to differentiate patients with AI from healthy subjects.
Acknowledgments
We thank the Eunice Kennedy Shriver National Institute of Child Health and Human Development Clinical Trials Database Team, Unit on Computer Support, for their invaluable support with the data management.
This work was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AI
- adrenal insufficiency
- CBG
- corticosteroid binding globulin
- CH
- cirrhosis
- DLM
- Department of Laboratory Medicine
- EIA
- enzyme immunoassay
- HV
- healthy volunteer
- PAI
- primary adrenal insufficiency
- SAI
- secondary adrenal insufficiency
- SFF
- serum free cortisol.
References
- 1. Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and cortisol-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53:58–68. [DOI] [PubMed] [Google Scholar]
- 2. Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–1638. [DOI] [PubMed] [Google Scholar]
- 3. Klose M, Lange M, Rasmussen AK, et al. Factors influencing the adrenocorticotropin test: role of contemporary cortisol assays, body composition, and oral contraceptive agents. J Clin Endocrinol Metab. 2007;92:1326–1333. [DOI] [PubMed] [Google Scholar]
- 4. Thevenot T, Borot S, Remy-Martin A, et al. Assessment of adrenal function in cirrhotic patients using concentration of serum-free and salivary cortisol. Liver Int. 2011;31:425–433. [DOI] [PubMed] [Google Scholar]
- 5. Galbois A, Rudler M, Massard J, et al. Assessment of adrenal function in cirrhotic patients: salivary cortisol should be preferred. J Hepatol. 2010;52:839–845. [DOI] [PubMed] [Google Scholar]
- 6. Tan T, Chang L, Woodward A, et al. Characterizing adrenal function using directly measured plasma free cortisol in stable severe liver disease. J Hepatol. 2010;53:841–848. [DOI] [PubMed] [Google Scholar]
- 7. Abraham SB, Abel BS, Sinaii N, Saverino E, Wade M, Nieman LK. Primary versus secondary adrenal insufficiency: ACTH-stimulated aldosterone diagnostic cut-off values by tandem mass spectrometry. Clin Endocrinol (Oxf). 2015;83:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta. 2005;359(1–2):189–194. [DOI] [PubMed] [Google Scholar]
- 9. Ho JT, Al-Musalhi H, Chapman MJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91:105–114. [DOI] [PubMed] [Google Scholar]
- 10. Arafah B, Nishiyama F, Tlaygeh H, Hejal R. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92:2965–2971. [DOI] [PubMed] [Google Scholar]
- 11. Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molenaar N, Johan Groeneveld AB, Dijstelbloem HM, et al. Assessing adrenal insufficiency of corticosteroid secretion using free versus total cortisol levels in critical illness. Intensive Care Med. 2011;37:1986–1993. [DOI] [PubMed] [Google Scholar]
- 13. Degand T, Monnet E, Durand F, et al. Assessment of adrenal function in patients with acute hepatitis using serum free and total cortisol. Dig Liver Dis. 2015;47(9):783–789. [DOI] [PubMed] [Google Scholar]
- 14. Fede G, Spadaro L, Tomaselli T, et al. Comparison of total cortisol, free cortisol, and surrogate markers of free cortisol in diagnosis of adrenal insufficiency in patients with stable cirrhosis. Clin Gastroenterol Hepatol. 2014;12(3):504–512. [DOI] [PubMed] [Google Scholar]


