Abstract
Context:
Congenital hyperinsulinism (HI) is the most common cause of hypoglycemia in children. The risk of permanent brain injury in infants with HI continues to be as high as 25–50% due to delays in diagnosis and inadequate treatment. Congenital HI has been described since the birth of the JCEM under various terms, including “idiopathic hypoglycemia of infancy,” “leucine-sensitive hypoglycemia,” or “nesidioblastosis.”
Evidence Acquisition:
In the past 20 years, it has become apparent that HI is caused by genetic defects in the pathways that regulate pancreatic β-cell insulin secretion.
Evidence Synthesis:
There are now 11 genes associated with monogenic forms of HI (ABCC8, KCNJ11, GLUD1, GCK, HADH1, UCP2, MCT1, HNF4A, HNF1A, HK1, PGM1), as well as several syndromic genetic forms of HI (eg, Beckwith-Wiedemann, Kabuki, and Turner syndromes). HI is also the cause of hypoglycemia in transitional neonatal hypoglycemia and in persistent hypoglycemia in various groups of high-risk neonates (such as birth asphyxia, small for gestational age birthweight, infant of diabetic mother). Management of HI is one of the most difficult problems faced by pediatric endocrinologists and frequently requires difficult choices, such as near-total pancreatectomy and/or highly intensive care with continuous tube feedings. For 50 years, diazoxide, a KATP channel agonist, has been the primary drug for infants with HI; however, it is ineffective in most cases with mutations of ABCC8 or KCNJ11, which constitute the majority of infants with monogenic HI.
Conclusions:
Genetic mutation testing has become standard of care for infants with HI and has proven to be useful not only in projecting prognosis and family counseling, but also in diagnosing infants with surgically curable focal HI lesions. 18F-fluoro-L-dihydroxyphenylalanine (18F-DOPA) PET scans have been found to be highly accurate for localizing such focal lesions preoperatively. New drugs under investigation provide hope for improving the outcomes of children with HI.
Introduction: A Bit of History
This Perspective on congenital hyperinsulinism (HI) disorders is especially timely for the Journal of Clinical Endocrinology and Metabolism's 75th Anniversary because the origins of the Journal in 1941 and the earliest recognized children are essentially contemporaneous. Some of the first pediatric patients with hypoglycemia at St Louis Children's Hospital reviewed by Hartmann and Jaudon in 1937 probably had congenital HI (1). Irvine McQuarrie reported the first detailed descriptions of congenital HI in his Presidential Address to the American Pediatric Society in 1953 under the title “Idiopathic Spontaneously Occurring Hypoglycemia in Infants” (2). Dr. McQuarrie stressed the urgency of bringing this topic to the attention of pediatricians because of encountering four children who had “suffered irreparable brain damage” from severe spontaneous hypoglycemia and “were victims of delayed diagnosis and inadequate early therapy.” Among 25 cases with “idiopathic hypoglycemia” seen over the previous 12 years at the University of Minnesota Hospitals, 11 had affected siblings or cousins, which suggested the possibility that their disorder might be genetic or familial. At the time, Dr. McQuarrie considered it unlikely that insulin was the cause of “idiopathic hypoglycemia of infancy” because of the lack of evidence of a pancreatic tumor or of islet hyperplasia, but this has subsequently been proven to be wrong (3, 4). Many of McQuarrie's other descriptions of the disorder still ring true today, including, unfortunately, the continued high frequency of brain damage due to delays in both diagnosis and treatment of infants with congenital HI (5, 6).
Shortly after McQuarrie's report on “idiopathic hypoglycemia,” Cochrane et al (7) in Toronto described children with hypoglycemia that could be precipitated by a high protein-low carbohydrate diet or by feeding specific amino acids, especially leucine; this was later termed “protein-sensitive hypoglycemia” or “leucine-sensitive hypoglycemia.” Shortly thereafter, studies of children with leucine-sensitive hypoglycemia by Yalow and Berson (8) in 1960, using their newly discovered insulin RIA, demonstrated elevated plasma concentrations of insulin, thus not only proving that insulin was indeed the cause of “idiopathic hypoglycemia of infancy,” but also, as shown in Figure 1, demonstrating the importance of amino acids in addition to glucose as fuels controlling insulin secretion. Further evidence that insulin was responsible for “idiopathic hypoglycemia of infancy” was provided by the report of Drash and Wolff (9) in 1964 that diazoxide, an antihypertensive drug with the side effect of suppressing insulin release from β-cells (Figure 1), could be effective in controlling hypoglycemia in some (although not all) children with “leucine-sensitive hypoglycemia” (9). In the absence of other therapies, near-total pancreatectomy was the only choice for children with diazoxide-unresponsive HI to control hypoglycemia.
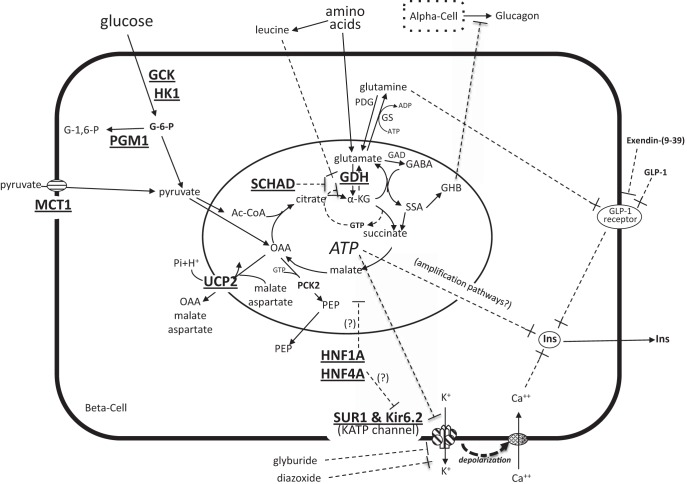
Figure 1.
Monogenic disorders of congenital HI. Diagram of β-cell fuel-mediated insulin secretion showing 11 genes currently associated with congenital HI (bold and underlined). Glucose oxidation stimulates insulin secretion via a “triggering pathway” by inhibiting ATP-sensitive KATP channels, leading to plasma membrane depolarization, activation of voltage-gated calcium channels, elevation of cytosolic calcium, and release of insulin from stored granules. Insulin secretion is also promoted by various “amplification pathways” once cytosolic calcium is elevated, eg, by mitochondria-derived signals or by membrane receptors such as the GLP-1 receptor. Amino acids stimulate insulin secretion through several mechanisms, especially leucine, which allosterically activates GDH enzymatic activity to increase oxidative deamination of glutamate to α-KG; GDH activity is allosterically inhibited by GTP and by the SCHAD enzyme protein. During glucose-stimulated insulin secretion, the rise in α-KG increases flux through a gamma amino butyric acid shunt and generates γ-hydroxybutyrate (GHB), which is released as a paracrine inhibitor of glucagon secretion from α-cells. HI-associated genes include: GCK (glucokinase), HK1 (hexokinase 1), PGM1 (phosphoglucomutase 1), MCT1 (monocarboxylate transporter 1), UCP2 (uncoupling protein 2), SCHAD (short-chain 3-hydroxyacyl-CoA dehydrogenase), GDH (glutamate dehydrogenase), HNF1A (hepatocyte nuclear factor 1A), HNFA (hepatocyte nuclear factor 4A), SUR1 (sulfonylurea receptor 1), Kir6.2 (inwardly rectifying potassium channel 6.2). Other abbreviations: OAA, oxaloacetate; PEP, phosphoenolpyruvate; α-KG, α-ketoglutarate; GAD, glutamic acid decarboxylase; GABA, γ-aminobutyrate, GHB, γ-hydroxybutyrate; SSA, succinic semialdehyde; Ins, insulin.
During the 1970s and 1980s, it became popular to label children with HI as having “nesidioblastosis,” presumed to be an embryological anomaly of β-cells proliferating from ductal epithelium (10); later studies by Rahier et al (11) in 1981 and other workers, however, forced the concept of nesidioblastosis to be abandoned by showing that it was merely a pattern normally found in early infancy (11, 12). In 1995, application of emerging technologies of genetic cloning and DNA sequencing led to the first discovery of a genetic cause for congenital HI: inactivating mutations in the two subunits that form the β-cell plasma membrane ATP-dependent potassium channel (KATP) (13–17). Subsequent rapid advances over the past two decades have led to the discovery of 11 genes associated with congenital HI (Table 1) and to greatly improved methods of diagnosis and treatment. This Perspective will focus on these recent advances in congenital HI and their implications for understanding β-cell biology and the opportunities for better methods of diagnosis and therapy.
Table 1.
Disorders of Congenital HI
| Gene | Locus | Mode | Triggers | Diazoxide Response | ↑NH3 | |
|---|---|---|---|---|---|---|
| Monogenic HI | ||||||
| KATP-Channel (SUR1/Kir6.2 subunits) | ABCC8/KCNJ11 | 11p15.1 | ||||
| Recessive KATP-HI | ABCC8/KCNJ11 | 11p15.1 | Rec | F/P | − | |
| Dominant KATP-HI | ||||||
| Diazoxide-unresponsive | ABCC8 (only?) | 11p15.1 | Dom | F/P | − | |
| Diazoxide-responsive | ABCC8/KCNJ11 | 11p15.1 | Dom | F/P | + | |
| Focal KATP-HI (pat. 11p UPD + pat. rec. KATP mutation) | ABCC8/KCNJ11 | 11p15.1 | Sporadic | F/P | − | |
| GDH | GLUD1 | 10q23.3 | Dom | F/P/Leu | + | + |
| GCK | GCK | 7p13 | Dom | F | − | |
| SCHAD | HADH1 | 4q25 | Rec | F/P/Leu | + | |
| UCP2 | UCP2 | 11q13.4 | Dom | F/Glu | + | |
| HNF4A | HNF4A | 20q13.12 | Dom | F | + | |
| HNF1A | HNF1A | 12q24.31 | Dom | F | + | |
| Pyruvate transporter (MCT1) | SLC16A1 | 1p13.2 | Dom | Exercise | − | |
| HK1 | HK1 | 10q22.1 | Dom | F/Glu | + | |
| PGM1 | PGM1 | 1p31.3 | Rec | Glu | − | |
| Syndromic forms of HIa | ||||||
| BWS (pat. 11p UPD) | IGF2/H19/CDKN1C/KCNQ1 | 11p15.4 | Sporadic | F | +/− | |
| Kabuki (mosaic) | KMT2D/KDM6A | 12q13.12/Xp11.3 | Sporadic | F | +/− | |
| Turner (mosaic X loss) | ? | X | Sporadic | F | +/− | |
| Congenital disorders of glycosylation | Rec | F | ||||
| CDG1a | PMM2 | 16p13.2 | Rec | F | + | |
| CDG1b | MPI | 15q24.1 | Rec | F | ? | |
| CDG1t (see PGM1 above) | ||||||
| Congenital HI-mimickers | ||||||
| Autoimmune hypoglycemia (anti-insulin or anti-insulin receptor antibodies) | Acquired | F/Glu | − | |||
| AKT2 (mosaic) | AKT2 | 19q13.2 | Sporadic | F | − | |
| ACT3 (mosaic) | AKT3 | 1q43-q44 | Sporadic | F | − | |
| Insulin receptor inactivation | INSR | 19p13.2 | Dom | Glu | − | |
| Neonatal HI | ||||||
| Transitional HI in normal newborns | All newborns | F | + | |||
| Perinatal-stress HI (mostly high-risk neonates, but some without apparent stress) | Transient | F | +/− |
Abbreviations: F, fasting; P, protein; Leu, leucine; G, glucose; Dom, dominant; Rec, recessive.
Partial list; see Ref. 72 for other syndromic forms of HI.
Pathways of β-Cell Insulin Secretion
As shown in Figure 1, the release of insulin from storage granules in pancreatic β-cells is chiefly controlled by the metabolism of two essential fuels, glucose and amino acids. Oxidation of these fuels drives insulin secretion via a “triggering” pathway by which elevation of the ATP:ADP ratio causes inhibition of the plasma membrane KATP channel, a heterotetramer composed of two subunits, SUR1 (sulfonylurea receptor) and Kir6.2 (potassium pore). Closure of KATP channels leads to depolarization of the plasma membrane, activation of voltage-gated calcium channels, elevation of cytosolic Ca2+, and release of insulin into the circulation. Other “amplification” pathways can increase insulin release once cytosolic Ca2+ is elevated, including mitochondria-derived molecules, parasympathetic innervation, and receptors for intestinal incretins (especially the glucagon-like peptide-1 [GLP-1] receptor). As shown in Figure 1, sulfonylurea drugs, such as glyburide, stimulate insulin secretion by inhibiting the KATP channel, whereas diazoxide, a KATP channel activator, suppresses insulin release. Somatostatin analogs, such as octreotide, inhibit insulin release downstream of the KATP channel and can be useful in patients who fail to respond to diazoxide.
As shown in Figure 1, all of the 11 known genetic defects that cause monogenic HI (Table 1) affect the triggering pathway of insulin release. The most common defects, here referred to as KATP-HI, involve inactivating mutations of the SUR1 or Kir6.2 subunits of the KATP channel. Children with complete KATP defects are unresponsive to diazoxide and often require pancreatectomy to control hypoglycemia. Defects that interfere with inhibitory control of glutamate dehydrogenase (GDH) by GTP or by protein-protein interaction of GDH with short-chain 3-hydroxyacyl-CoA dehydrogenase (SCHAD) cause HI by increasing oxidation of amino acids. Children with GDH-HI or SCHAD-HI have increased insulin responsiveness to leucine, an allosteric activator of GDH, and therefore manifest protein as well as fasting-induced hypoglycemia (Figure 1). Because GDH lies upstream of the KATP channel in the “triggering” pathway, children with GDH-HI and SCHAD-HI (in contrast to children with KATP-HI) are responsive to treatment with diazoxide. Other monogenic HI defects upstream of the KATP channel (Figure 1) also respond to diazoxide, with the notable exception of children with activating mutations of glucokinase (GCK) HI; these mutations lower the threshold for glucose-stimulated insulin secretion and can override the suppressive effect of diazoxide (18).
Two of the rarer forms of HI involve expression of genes that are common to most tissues, but whose expression in β-cells is normally “disallowed” to ensure appropriate control of insulin release: monocarboxylate transporter 1 (MCT1) HI and hexokinase 1 (HK1) HI. The absence of MCT1 (and also of lactate dehydrogenase [LDH]) is essential to prevent release of insulin when pyruvate and lactate are increased during anaerobic exercise. The absence of HK1, which has a higher affinity for glucose than GCK, prevents normal β-cells from releasing insulin at low glucose concentrations.
Several of the genes associated with monogenic congenital HI are also involved in monogenic forms of diabetes (maturity-onset diabetes of the young [MODY]). These include inactivating mutations of GCK that cause MODY2; inactivating mutations of hepatocyte nuclear factor (HNF) 1A and HNF4A, which can present as congenital HI in neonates but later evolve in early adulthood into insulinopenic diabetes (MODY1 and MODY3); and activating mutations of the KATP channel subunits, which can cause either severe permanent neonatal diabetes or a mild increased susceptibility to adult-onset diabetes.
Clinical Features of Congenital HI
Estimates of the incidence of congenital HI range from 1 in 50 000 births in Holland to as high as 1 in 2500 in Saudi Arabia (related to high rates of consanguinity) (19, 20). Among Ashkenazi Jews, the incidence, based on the carrier frequency for two recessive KATP-HI founder mutations, has been estimated to be 1 in 10 816 (probably approximately 30% higher when cases of focal HI are included) (21). These incidence rates underestimate the frequency of congenital HI because they do not include other forms of the disorder listed in Table 1. Some infants with HI have recognizable clinical features of prenatal hyperinsulinemia, such as fetal overgrowth or hypertrophic cardiomyopathy (similar to infants of diabetic mothers) (3, 22). Hypoglycemia is present at birth, but recognition is often delayed because symptoms of hypoglycemia in neonates can be subtle, and low concentrations of plasma glucose are often confused with the commonly observed transitional HI of normal neonates (23). As a consequence, seizures and irreversible brain injury continue to be major concerns in children afflicted with HI (5, 6). Some of those affected may escape detection in infancy and not be recognized to have symptomatic hypoglycemia until adulthood (especially common in dominant forms of HI) (24).
Clinical Diagnosis of HI
The diagnosis of HI in children, as in adults, relies heavily on markers of inappropriate insulin effects (eg, suppressed ketogenesis) because plasma insulin concentrations are frequently not clearly elevated (25, 26). Diagnosis is most reliably based on a closely monitored provocative fasting test that includes: 1) frequent sampling of plasma concentrations of glucose, insulin, β-hydroxybutyrate, and free fatty acids; and 2) terminating the test when glucose concentrations decline toward 50 mg/dL (2.8 mmol/L) with a determination of the glycemic response to glucagon (25–27). This can be accomplished quickly, and preliminary results can be obtained immediately at the bedside using point-of-care meters for measuring plasma glucose and β-hydroxybutyrate (tests of urine ketones are of little value).
Treatment of HI
Diazoxide is the first-line drug for controlling hypoglycemia in HI, but it is ineffective in some forms (KATP-HI, GCK-HI) (28, 29). Apart from hypertrichosis, side effects of diazoxide are generally limited to fluid retention, which, in infants needing high rates of iv dextrose, often requires prophylaxis with furosemide or other potent diuretics to avoid heart failure (3, 30). Octreotide may be useful in diazoxide-unresponsive patients but is often ineffective due to down-regulation of the somatostatin receptor (31), and it carries a risk of causing necrotizing enterocolitis and death (32). Calcium channel blockers, such as nifedipine, are not useful clinically. Severe cases of congenital HI can have markedly increased rates of glucose utilization (5–10 times normal or greater) and may be unresponsive to either diazoxide or octreotide; such infants may require intensive management with tube feedings, near-total pancreatectomy (for those with diffuse disease), or localized excision (for those with focal disease) (33–35).
Congenital Forms of HI (Table 1)
Monogenic forms of congenital HI
KATP-HI form
The most common and most severe of the monogenic forms of HI is caused by inactivating mutations of the two subunits of the β-cell plasma membrane KATP channel (13–17). KATP-HI causes both fasting and protein-sensitive hypoglycemia, but unlike HI due to activating mutations of GDH, the target for leucine-stimulated insulin release (Figure 1), patients with KATP-HI are not leucine-sensitive. In KATP-HI, as shown in Figure 1, glutamine may mediate protein sensitivity via the “amplification” pathway of the GLP-1 receptor (36, 37). In the absence of functional KATP channels, hypoglycemia in these children is usually unresponsive to treatment with diazoxide (Figure 1). KATP defects account for approximately 97% of the identified mutations in diazoxide-unresponsive HI (the remainder being mutations in GCK) (29). There are four distinct forms of KATP-HI. 1) Recessive KATP mutations (missense, nonsense, or splicing defects) result in complete absence of KATP channels by blocking steps before trafficking of channels to the plasma membrane (38, 39). 2) Dominant KATP mutations are missense defects that permit normal trafficking of subunits into plasma membrane KATP channels, but the mutant subunits (either SUR1 or Kir6.2) act as dominant negatives within the hetero-octomeric KATP complex to impair channel activity; mutations that most severely impair channel activity are unresponsive to diazoxide (39, 40). 3) In contrast, dominant KATP mutations that permit greater residual KATP channel activity can be diazoxide-responsive. Together, dominant mutations of KATP and of GDH account for approximately half of all cases of diazoxide-responsive HI (24, 29). 4) Although the first three forms of KATP-HI produce diffuse HI affecting all pancreatic islets, a focal form of HI can occur as a consequence of two genetic “hits”: a paternally transmitted recessive KATP mutation, together with a postzygotic second event of loss of maternal heterozygosity for the 11p15.1 region. This results in paternal isodisomy for the KATP mutation as well as for the adjacent Beckwith-Wiedemann syndrome (BWS) locus, which contains imprinted genes that lead to islet overgrowth (IGF2, H19, CDKN1C) (41). Half of the children with diazoxide-unresponsive HI have focal KATP-HI and are potentially curable by surgery if the lesion can be found and excised (34, 42, 43). Preoperative 18F-fluoro-L-dihydroxyphenylalanine (18F-DOPA) PET scans have proven useful for locating the small (0.5–1 cm) focal lesions of islet adenomatosis (44–46).
GDH-HI (HI/hyperammonemia [HA] syndrome)
Dominant missense-activating mutations of GDH are one of the most common identifiable causes of diazoxide-responsive congenital HI (29). Up to 80% of cases are due to de novo mutations. Disease-causing mutations impair inhibitory control of GDH activity by GTP (Figure 1), leading to increased glutamate oxidation and hypoglycemia during fasting, and especially after high-protein meals (47–50). Leucine, an allosteric activator of GDH, can rapidly provoke hyperinsulinemic hypoglycemia in these children. In addition to HI, GDH-HI patients have chronic mild elevations of plasma ammonia (HI-HA syndrome); the HA reflects increased renal ammonia production due to overactivity of GDH in the kidney (51). Affected children also have central nervous system (CNS) manifestations of absence epilepsy and behavioral disturbances that may reflect the increased GDH enzymatic activity in the brain (52).
Glucokinase-HI
The third most common gene associated with congenital HI is GCK. Dominant missense-activating mutations of GCK lower the glucose threshold for insulin secretion, resulting in fasting hyperinsulinemic hypoglycemia (53). Affected children often have large birthweights and can present with severe hypoglycemia at birth. Although in vitro studies of GCK mutants suggest a wide range of severity, most patients tend to maintain fairly stable glucose levels in the range of 50–60 mg/dL; they usually respond poorly to diazoxide and may require either surgery or treatment with continuous intragastric dextrose (18).
SCHAD-HI form
SCHAD is a mitochondrial fatty acid β-oxidation enzyme that has been associated with recessive diazoxide-responsive congenital HI in a small number of children. Initial cases were identified by virtue of distinctive abnormal fatty acid metabolites: 3-hydroxybutyryl-carnitine in serum and 3-hydroxyglutarate in urine (54, 55). As noted above, the mechanism of SCHAD-HI involves loss of an inhibitory protein-protein interaction between SCHAD and GDH (Figure 1) (56). The HI phenotype is similar to that of GDH-HI (fasting and leucine/protein-sensitive hypoglycemia), but without the associated HA or CNS disturbances of GDH-HI.
Uncoupling protein 2 (UCP2)-HI
Two children have been reported to have diazoxide-responsive dominant HI associated with inactivating mutations of UCP2 in 2008 (57). Recent work by Palmieri and colleagues (58) shows that UCP2 acts not by uncoupling mitochondrial oxidative phosphorylation, but rather as a carrier that removes four-carbon intermediates from the mitochondrial matrix to suppress glucose oxidation and stimulate glutamine oxidation (Figure 1). In UCP2-HI, the reduced UCP2 activity presumably enhances glucose oxidation by facilitating pyruvate entry into the tricarboxylic acid cycle leading to excessive release of insulin and hypoglycemia. In the original report, HI appeared to resolve by early childhood; however, three cases of UCP2-HI seen at the Children's Hospital of Philadelphia have demonstrated persistence of HI beyond the first decade.
HNF4A-HI and HNF1A-HI
Genetic defects in these two closely related genes are considered together because dominant inactivating mutations in these transcription factors have long been associated with maturity-onset diabetes of the young (MODY1 and MODY3), but were recently demonstrated to also cause HI in early infancy. The link between these genes and HI was first identified by Hattersley and colleagues (59), who noted that increased birthweight and neonatal hypoglycemia were common in HNF4A MODY carriers. Although it first appeared that HI was limited to HNF4A, subsequent reports indicate that inactivating mutations of HNF1A can also cause congenital HI, perhaps because these two transcription factors are part of a regulatory loop controlling the expression of each other (60). Since mutations of these transcription factors also affect development and function of other organs, extrapancreatic manifestations may occur, such as the report of a child with a mutation in HNF4A-HI and features of renal Fanconi syndrome and hepatic glycogen storage (Fanconi-Bickel syndrome due to reduced expression of the GLUT2 glucose transporter) (60). The HI associated with HNF4A and HNF1a mutations may be transient in early infancy or may persist into later childhood and is usually responsive to diazoxide. Affected children are at risk of developing diabetes in their second or third decades; the diabetes is usually highly responsive to treatment with oral sulfonylureas, such as glyburide. The mechanisms responsible for early manifestations of excess insulin and later development of islet failure are not known.
MCT1-HI (exercise-induced HI)
Thirteen patients in three families have been described by Meissner and Otonkoski and their colleagues (61–64) with mutations in the upstream promoter regions of the pyruvate transporter, MCT1. (N.B., inactivating mutations in this gene were recently reported in children with recurrent ketoacidosis due to defective ketone transport [65].) The affected individuals presented with recurrent episodes of hyperinsulinemic hypoglycemia after vigorous exercise. As noted above, expression of MCT1 and LDH are normally “disallowed” in β-cells to prevent stimulation of insulin release by pyruvate and lactate (66). In patients with MCT1-HI, normal suppression of MCT1 expression is lost, leading to a rapid rise in insulin secretion during anaerobic exercise when plasma concentrations of pyruvate increase. Because diazoxide treatment may not prevent acute episodes, treatment depends largely on prevention with carbohydrates during or following exercise. Although no further cases have been reported since 2007, the disorder is probably underdiagnosed.
HK1-HI form
A dominant form of diazoxide-responsive HI has recently been mapped to the HK1 locus in one of the families with “idiopathic hypoglycemia of infancy” originally described by McQuarrie in 1953 (2, 67). In this large, four-generation pedigree, symptoms of hypoglycemia presented early in infancy, which were severe enough to cause seizures and permanent brain damage in some cases. As noted above, HK1 is normally “forbidden” from expression in β-cells to prevent stimulation of insulin release under conditions of low plasma glucose (Figure 1). In affected members of this family, three novel variants were identified in noncoding regions of HK1 that might potentially interfere with the normal suppression of HK1 expression in β-cells.
PGM1-HI form
Recessive inactivating mutations of phosphoglucomutase 1 (PGM1) were recently reported to cause a form of glycogenosis accompanied by hypoglycemia, short stature, cleft palate, weakness, and abnormal protein glycosylation (68). PGM1 catalyzes the reversible interconversion of glucose-6-phosphate and glucose-1-phosphate and is essential for the formation and degradation of glycogen (Figure 1). Although affected children have fasting hyperketotic hypoglycemia, similar to glycogenoses such as glycogen phosphorylase deficiency, they can also manifest symptomatic postprandial hyperinsulinemic hypoglycemia due to exaggerated insulin responses to glucose (69). The latter indicates that PGM1 plays an important role in regulating insulin responses to glucose that has not previously been recognized.
Syndromic forms of congenital HI
Beckwith-Wiedemann syndrome-HI
BWS is a fetal overgrowth syndrome caused by genetic defects affecting an imprinted region on 11p15.4 that is nearby, but telomeric of the two KATP channel genes. This BWS locus contains maternally expressed growth suppressor genes (H19 and CDKN1C), a maternally expressed voltage-gated potassium channel (KCNQ1), and a paternally expressed growth promoting gene (IGF2). Genetic defects that lead to imbalance of the maternally expressed and paternally expressed genes are usually postzygotic mosaic changes and can include point mutations of the two BWS locus imprinting-control regions, ICR1 and ICR2, or chromosomal rearrangements producing 11p loss of heterozygosity (ie, paternal uniparental isodisomy [UPD] for the 11p BWS region). HI occurs in about half of BWS neonates; it is transient in many cases but may be persistent and diazoxide unresponsive in a small proportion. The latter more severe forms of HI in BWS appear to be particularly associated with paternal 11p UPD and can be severe enough to require pancreatectomy (70). Although focal KATP HI also involves paternal 11p UPD, it is usually limited to a small area of islet overgrowth in which insulin secretion is constitutively turned on due to the absence of KATP channels (isodisomy for a paternally transmitted recessive KATP mutation). The typical focal KATP HI lesion can be considered an example of 11pUPD BWS “in situ.” The more typical 11pUPD BWS form of HI is associated with a larger area of islet adenomatosis involving half or more of the pancreas and is usually accompanied by manifestations in other organs (eg, hemihypertrophy, macroglossia). In rare cases of 11pUPD BWS-HI that also have a paternally inherited recessive KATP channel mutation, hypoglycemia may be extremely severe and difficult to manage.
Kabuki make-up syndrome
This syndrome is characterized by dysmorphic facial features reminiscent of Kabuki actors and has been associated with HI in a significant proportion of cases. Most, but not all, have responded to treatment with diazoxide. Two genes have been linked to Kabuki syndrome: monoallelic de novo mutations of lysine-specific methyltransferase 2D (MLL2, encoded by KMT2D), and lysine-specific demethylase 6A (KDM6A on Xp11.3)—two closely related genes that affect histone methylation in promoter and transcription initiation sites of target genes. Severe and persistent congenital HI has been observed in some infants with Kabuki syndrome, although the mechanism(s) remain unknown (71).
HI associated with Turner syndrome
Congenital HI has been reported to occur in infants with Turner syndrome at a higher incidence than in normal children (72, 73). Although some cases have responded to diazoxide treatment, some have required pancreatectomy. The mechanism has not been elucidated, but one can speculate that it might involve a locus on the X-chromosome.
Congenital disorders of glycosylation (CDG)
Three of the genetic defects that disrupt protein glycosylation have been associated with congenital HI (68, 74): CDG1a (phosphomannomutase 2 deficiency), CDG1b (mannosephosphate isomerase deficiency), and CDG1t (phosphoglucomutase 1 deficiency). PGM1 deficiency is discussed above among the monogenic forms of congenital HI, although it has manifestations beyond β-cell dysfunction. The CDG disorders can be identified by screening plasma proteins, such as transferrin, for abnormal glycosylation by isoelectric focusing or mass spectrometry.
Additional syndromic forms of HI have been summarized elsewhere, including Sotos, Simpson-Golabi-Behmel, Costellos, Trisomy 13, Timothy, and Usher (74).
Disorders That Mimic Congenital HI
Table 1 lists several forms of hypoglycemia that, although rare, may be confused with HI: autoimmune hypoglycemia caused by endogenous antibodies either to insulin or to the insulin receptor (75–78); postzygotic activating mutations of AKT2 and AKT3 in the downstream pathways of insulin action that are also associated with hemihypertrophy (79–81); and insulin receptor-inactivating mutations that can cause postprandial hypoglycemia, possibly due to delayed insulin clearance (82).
Transitional and Perinatal Stress-Induced Neonatal HI
Since the 1930s, it has been recognized that hypoglycemia is common in normal newborn infants immediately after delivery. In addition, high-risk neonates (including small for gestational age, birth asphyxia, maternal toxemia, erythroblastosis fetalis, maternal diabetes) may have hyperinsulinemic hypoglycemia for extended periods after birth, although evidence of a triggering stress factor may not be apparent in some of these infants (1, 83). In normal infants, neonatal hypoglycemia quickly resolves by 1–2 days after birth. The mechanism appears to be HI because ketogenesis and lipolysis remain suppressed and there are inappropriately large glycemic responses to glucagon or epinephrine. This transitional neonatal HI may reflect persistence of a lower glucose threshold for insulin release that is necessary to maintain fetal growth (23). In high-risk neonates, prolonged hypoglycemia also appears to be due to HI, although it is unclear whether this involves a separate mechanism or is merely an exaggeration of the HI that is common in all newborns (83–85). In normal newborns, the mild HI during the first day or two of life usually does not require treatment. However, the more severe and prolonged HI in high-risk neonates often requires interventions, ranging from early feeding to prolonged treatment with diazoxide or continuous feedings. These two common forms of HI in neonates have to be considered in developing strategies for earlier recognition and treatment of infants with congenital HI and other persistent hypoglycemia disorders during the first 2 days of life (86).
Special emphasis needs to be placed on the prolonged hyperinsulinemic hypoglycemia seen in infants born to mothers with either permanent or gestational diabetes. Maternal diabetes is a very common, well-recognized risk factor for neonatal hypoglycemia. The mechanism has usually been attributed to a form of “rebound” hypoglycemia, reflecting sudden withdrawal from exposure to high concentrations of plasma glucose in the maternal circulation. The fact that HI often persists for several days or longer after delivery, however, indicates a more chronic disturbance of insulin regulation. It is possible that the underlying mechanism is the same as that involved in the prolonged HI associated with other forms of perinatal stress.
Perspective on Future Developments in Congenital HI
Newborn screening for HI
Reliable newborn screening protocols for early detection of congenital HI are urgently needed because the rate of permanent brain injury in these children has not been reduced over the past 50 years. To encourage earlier detection and treatment of persistent congenital hypoglycemia disorders, new guidelines for hypoglycemia in neonates, infants, and children were recently published by the Pediatric Endocrine Society (86).
Genetic mutation testing is now well established as the standard of care to define best approaches to treatment of congenital HI. Special testing may be necessary in some cases, using next-generation sequencing or other procedures, to exclude large in-dels or deep intronic (cryptic mutations). Parent-of-origin testing should routinely be included at the time of patient testing. This is particularly essential if focal KATP HI is a possibility because the demonstration of a paternally transmitted, monoallelic, recessive mutation in ABCC8 or KCNJ11 is highly accurate for predicting a potentially curable focal lesion (97% sensitivity, 92% specificity) (29). Rapid turnaround time for genetic tests is uniquely important in congenital HI because these babies are desperately ill, and treatment decisions, such as whether to do a 18F-fluoro-L-dihydroxyphenylalanine (18F-DOPA) PET scan or pancreatectomy, need to be made within a few days. New methods make it feasible to obtain results in less than a week, preferably within 2 or 3 days.
Although over 150–200 HI mutations in the two KATP genes are known, saturation of disease-causing mutations in these and other HI genes is far from complete. Interpretation of genetic results remains difficult because over 30% of disease-causing KATP mutations continue to be novel, and computerized in-silico analysis programs remain undependable (29). Novel variants are especially problematic for the KATP channel genes where variants may be activating (causing diabetes) or inactivating (causing HI), may be expressed dominantly or recessively, and may be diazoxide-responsive or unresponsive. Genetic testing is also essential for recognition of syndromic forms of HI, such as BWS or CDG disorders. Because over half of patients with diazoxide-responsive HI do not have an identifiable mutation, additional genetic loci for HI are likely to be discovered. Some cases without an identified mutation may harbor a postzygotic mutation in a known dominant gene; these might be amenable to detection in isolated islets or by advanced next-generation sequencing (87).
Investigational treatments for congenital HI currently being explored include the following. 1) Long-acting somatostatin preparations that can be injected once monthly have been trialed in older infants who are on stable regimens of sc octreotide (88). Whether they are appropriate for initial therapy or in very young infants is uncertain (2). Exendin-(9–39), a reverse agonist of the GLP-1 receptor (Figure 1), has been shown to correct hypoglycemia in the mouse model of KATP-HI when given by chronic sc infusion (37). Short-term studies suggest that it can raise plasma glucose in patients with KATP-HI and are currently being extended to affected neonates (89). 3) The immunosuppressive mammalian target of rapamycin inhibitor, sirolimus, was reported to control hypoglycemia in four infants with diazoxide-unresponsive HI (90). Limitations of the study include the fact that it was uncontrolled and that the only case with clearly documented genetic KATP-HI continued to require treatment with octreotide plus iv glucagon. Long-term safety of sirolimus, especially for long-term therapy in young infants with HI, also remains a concern.
Insights from disorders of congenital HI
The genetic forms of congenital HI in children provide the following unique and often unexpected insights into both normal and pathological mechanisms of islet regulation in humans that deserve future investigation.
Mutations of the KATP channel SUR1 and Kir6.2 subunits illustrate the greater importance of the “triggering pathway” for insulin secretion in humans compared to mice and its link to both HI and diabetes mellitus (37, 91). Inactivating KATP mutations are the most common cause of congenital HI, whereas the more recently discovered KATP-activating mutations produce a spectrum of diabetes phenotypes that are readily treated with sulfonylureas, ranging from permanent neonatal diabetes with developmental delay and epilepsy (DEND syndrome) to a common “polymorphism” associated with increased risk for adult-onset diabetes.
Exercise-induced HI and HK1-associated HI dramatically illustrate the necessity of “disallowing” β-cell expression of specific common genes to permit normal insulin regulation (66). These two examples suggest that immaturities in fetal β-cell control of “disallowed” genes, such as HK1 or MCT1 and LDH, deserve study to explain transitional neonatal hypoglycemia (23, 66).
Discovery of the HI-HA syndrome demonstrates the surprisingly key role for GDH and its allosteric inhibition by GTP in controlling amino acid-stimulated insulin secretion and CNS function. Kibbey et al (92) have pointed out the significance of GTP-specific succinyl-CoA synthetase and of the enigmatic mitochondrial form of phospoenolpyruvate carboxykinase (PEP-CK) to provide a mechanism of removing GTP from the mitochondrial matrix (Figure 1). Studies of impaired glucagon secretion in KATP-HI and GDH-HI have helped to uncover a novel paracrine mechanism for regulating α-cell glucagon secretion, not involving insulin or zinc, via an inhibitory neurotransmitter, γ-hydroxybutyrate, generated from β-cells during glucose oxidation (Figure 1) (93, 94).
The hypoglycemia phenotype of GCK-HI activation of GCK and the MODY2 diabetes associated with GCK inactivating mutations confirm the central importance of this enzyme in determining the glucose setpoint for insulin release. Observations of enlarged islets in pancreas from patients with GCK-HI raise the possibility that glucose flux through GCK serves as a signal for β-cell expansion that might find application for treating type 2 diabetes (95, 96). In addition, the association between paternal isodisomy for 11p in focal KATP-HI, BWS-HI, and some insulinomas suggests that the 11p growth regulatory genes (IGF2, H19, and CDKN1C) may play essential roles in β-cell replication (70, 97, 98).
Acknowledgments
The author gratefully acknowledges the invaluable support of the members of The Children's Hospital of Philadelphia Hyperinsulinism Center and, especially, Drs. Diva De Leon, ChangHong Li, Kara Boodhansingh, and Arupa Ganguly.
This work was supported in part by National Institutes of Health Grant 4R37DK056268.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- BWS
- Beckwith-Wiedemann syndrome
- CDG
- congenital disorders of glycosylation
- CNS
- central nervous system
- GCK
- glucokinase
- GDH
- glutamate dehydrogenase
- GLP-1
- glucagon-like peptide-1
- HA
- hyperammonemia
- HI
- hyperinsulinism
- HK1
- hexokinase 1
- HNF
- hepatocyte nuclear factor
- KATP
- ATP-dependent potassium (channel)
- LDH
- lactate dehydrogenase
- MCT1
- monocarboxylate transporter 1
- MODY
- maturity-onset diabetes of the young
- PGM1
- phosphoglucomutase 1
- SCHAD
- short-chain 3-hydroxyacyl-CoA dehydrogenase
- UCP2
- uncoupling protein 2
- UPD
- uniparental isodisomy.
References
- 1. Hartmann AF, Jaudon JC. Hypoglycemia. J Pediatr. 1937;11:1–36. [Google Scholar]
- 2. McQuarrie I. Idiopathic spontaneously occurring hypoglycemia in infants; clinical significance of problem and treatment. AMA Am J Dis Child. 1954;87:399–428. [PubMed] [Google Scholar]
- 3. Stanley CA, Baker L. Hyperinsulinism in infants and children: diagnosis and therapy. Adv Pediatr. 1976;23:315–355. [PubMed] [Google Scholar]
- 4. Pagliara AS, Karl IE, Haymond M, Kipnis DM. Hypoglycemia in infancy and childhood. II. J Pediatr. 1973;82:558–577. [DOI] [PubMed] [Google Scholar]
- 5. Steinkrauss L, Lipman TH, Hendell CD, Gerdes M, Thornton PS, Stanley CA. Effects of hypoglycemia on developmental outcome in children with congenital hyperinsulinism. J Pediatr Nurs. 2005;20:109–118. [DOI] [PubMed] [Google Scholar]
- 6. Lord K, Radcliffe J, Gallagher PR, Adzick NS, Stanley CA, De León DD. High risk of diabetes and neurobehavioral deficits in individuals with surgically treated hyperinsulinism. J Clin Endocrinol Metab. 2015;100:4133–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cochrane WA, Payne WW, Simpkiss MJ, Woolf LI. Familial hypoglycemia precipitated by amino acids. J Clin Invest. 1956;35:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39:1157–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drash A, Wolff F. Drug therapy in leucine-sensitive hypoglycemia. Metabolism. 1964;13:487–492. [DOI] [PubMed] [Google Scholar]
- 10. Yakovac WC, Baker L, Hummeler K. β Cell nesidioblastosis in idiopathic hypoglycemia of infancy. J Pediatr. 1971;79:226–231. [DOI] [PubMed] [Google Scholar]
- 11. Rahier J, Wallon J, Henquin JC. Cell populations in the endocrine pancreas of human neonates and infants. Diabetologia. 1981;20:540–546. [DOI] [PubMed] [Google Scholar]
- 12. Palladino AA, Stanley CA. Nesidioblastosis no longer! It's all about genetics. J Clin Endocrinol Metab. 2011;96:617–619. [DOI] [PubMed] [Google Scholar]
- 13. Aguilar-Bryan L, Nichols CG, Wechsler SW, et al. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. [DOI] [PubMed] [Google Scholar]
- 14. Thomas PM, Cote GJ, Wohllk N, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. [DOI] [PubMed] [Google Scholar]
- 15. Nestorowicz A, Wilson BA, Schoor KP, et al. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet. 1996;5:1813–1822. [DOI] [PubMed] [Google Scholar]
- 16. Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1809–1812. [DOI] [PubMed] [Google Scholar]
- 17. Nestorowicz A, Inagaki N, Gonoi T, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997;46:1743–1748. [DOI] [PubMed] [Google Scholar]
- 18. Sayed S, Langdon DR, Odili S, et al. Extremes of clinical and enzymatic phenotypes in children with hyperinsulinism caused by glucokinase activating mutations. Diabetes. 2009;58:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruining GJ. Recent advances in hyperinsulinism and the pathogenesis of diabetes mellitus. Curr Opin Pediatr. 1990;2:758–765. [Google Scholar]
- 20. Mathew PM, Young JM, Abu-Osba YK, et al. Persistent neonatal hyperinsulinism. Clin Pediatr (Phila). 1988;27:148–151. [DOI] [PubMed] [Google Scholar]
- 21. Glaser B, Blech I, Krakinovsky Y, et al. ABCC8 mutation allele frequency in the Ashkenazi Jewish population and risk of focal hyperinsulinemic hypoglycemia. Genet Med. 2011;13:891–894. [DOI] [PubMed] [Google Scholar]
- 22. Huang T, Kelly A, Becker SA, Cohen MS, Stanley CA. Hypertrophic cardiomyopathy in neonates with congenital hyperinsulinism. Arch Dis Child Fetal Neonatal Ed. 2013;98:F351–F354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanley CA, Rozance PJ, Thornton PS, et al. Re-evaluating “transitional neonatal hypoglycemia”: mechanism and implications for management. J Pediatr. 2015;166:1520–1525.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinney SE, MacMullen C, Becker S, et al. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118:2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanley CA, Baker L. Hyperinsulinism in infancy: diagnosis by demonstration of abnormal response to fasting hypoglycemia. Pediatrics. 1976;57:702–711. [PubMed] [Google Scholar]
- 26. Finegold DN, Stanley CA, Baker L. Glycemic response to glucagon during fasting hypoglycemia: an aid in the diagnosis of hyperinsulinism. J Pediatr. 1980;96:257–259. [DOI] [PubMed] [Google Scholar]
- 27. Ferrara C, Patel P, Becker S, Stanley CA, Kelly A. Biomarkers of insulin for the diagnosis of hyperinsulinemic hypoglycemia in infants and children. J Pediatr. 2016;168:212–219. [DOI] [PubMed] [Google Scholar]
- 28. Sayed S, Matschinsky FM, Stanley CA. Hyperinsulinism due to activating mutations of glucokinase. In: De Léon DD, Stanley CA, eds. Monogenic Hyperinsulinemic Hypoglycemia Disorders. Geneva, Switzerland: Karger; 2012:146–157. [Google Scholar]
- 29. Snider KE, Becker S, Boyajian L, et al. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98:E355–E363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshida K, Kawai M, Marumo C, et al. High prevalence of severe circulatory complications with diazoxide in premature infants. Neonatology. 2014;105:166–171. [DOI] [PubMed] [Google Scholar]
- 31. Thornton PS, Alter CA, Katz LE, Baker L, Stanley CA. Short- and long-term use of octreotide in the treatment of congenital hyperinsulinism. J Pediatr. 1993;123:637–643. [DOI] [PubMed] [Google Scholar]
- 32. Laje P, Halaby L, Adzick NS, Stanley CA. Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatr Diabetes. 2010;11:142–147. [DOI] [PubMed] [Google Scholar]
- 33. Lovvorn HN, 3rd, Nance ML, Ferry RJ, Jr, et al. Congenital hyperinsulinism and the surgeon: lessons learned over 35 years. J Pediatr Surg. 1999;34:786–792; discussion 792–793. [DOI] [PubMed] [Google Scholar]
- 34. Adzick NS, Thornton PS, Stanley CA, Kaye RD, Ruchelli E. A multidisciplinary approach to the focal form of congenital hyperinsulinism leads to successful treatment by partial pancreatectomy. J Pediatr Surg. 2004;39:270–275. [DOI] [PubMed] [Google Scholar]
- 35. Hardy OT, Hernandez-Pampaloni M, Saffer JR, et al. Diagnosis and localization of focal congenital hyperinsulinism by 18F-fluorodopa PET scan. J Pediatr. 2007;150:140–145. [DOI] [PubMed] [Google Scholar]
- 36. Kelly A, Ng D, Ferry RJ, Jr, et al. Acute insulin responses to leucine in children with the hyperinsulinism/hyperammonemia syndrome. J Clin Endocrinol Metab. 2001;86:3724–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De León DD, Li C, Delson MI, Matschinsky FM, Stanley CA, Stoffers DA. Exendin-(9–39) corrects fasting hypoglycemia in SUR-1−/− mice by lowering cAMP in pancreatic β-cells and inhibiting insulin secretion. J Biol Chem. 2008;283:25786–25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shyng SL, Ferrigni T, Shepard JB, et al. Functional analyses of novel mutations in the sulfonylurea receptor 1 associated with persistent hyperinsulinemic hypoglycemia of infancy. Diabetes. 1998;47:1145–1151. [DOI] [PubMed] [Google Scholar]
- 39. Yan FF, Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL. Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes. 2007;56:2339–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macmullen CM, Zhou Q, Snider KE, et al. Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1. Diabetes. 2011;60:1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glaser B, Ryan F, Donath M, et al. Hyperinsulinism caused by paternal-specific inheritance of a recessive mutation in the sulfonylurea-receptor gene. Diabetes. 1999;48:1652–1657. [DOI] [PubMed] [Google Scholar]
- 42. Laje P, Stanley CA, Palladino AA, Becker SA, Adzick NS. Pancreatic head resection and Roux-en-Y pancreaticojejunostomy for the treatment of the focal form of congenital hyperinsulinism. J Pediatr Surg. 2012;47:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suchi M, Thornton PS, Adzick NS, et al. Congenital hyperinsulinism: intraoperative biopsy interpretation can direct the extent of pancreatectomy. Am J Surg Pathol. 2004;28:1326–1335. [DOI] [PubMed] [Google Scholar]
- 44. Hardy OT, Hernandez-Pampaloni M, Saffer JR, et al. Accuracy of [18F]Fluorodopa positron emission tomography for diagnosing and localizing focal congenital hyperinsulinism. J Clin Endocrinol Metab. 2007;92:4706–4711. [DOI] [PubMed] [Google Scholar]
- 45. Laje P, States LJ, Zhuang H, et al. Accuracy of PET/CT scan in the diagnosis of the focal form of congenital hyperinsulinism. J Pediatr Surg. 2013;48:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Otonkoski T, Näntö-Salonen K, Seppänen M, et al. Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes. 2006;55:13–18. [PubMed] [Google Scholar]
- 47. Stanley CA, Lieu YK, Hsu BY, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338:1352–1357. [DOI] [PubMed] [Google Scholar]
- 48. MacMullen C, Fang J, Hsu BY, et al. Hyperinsulinism/hyperammonemia syndrome in children with regulatory mutations in the inhibitory guanosine triphosphate-binding domain of glutamate dehydrogenase. J Clin Endocrinol Metab. 2001;86:1782–1787. [DOI] [PubMed] [Google Scholar]
- 49. Fang J, Hsu BY, MacMullen CM, Poncz M, Smith TJ, Stanley CA. Expression, purification and characterization of human glutamate dehydrogenase (GDH) allosteric regulatory mutations. Biochem J. 2002;363:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li C, Matter A, Kelly A, et al. Effects of a GTP-insensitive mutation of glutamate dehydrogenase on insulin secretion in transgenic mice. J Biol Chem. 2006;281:15064–15072. [DOI] [PubMed] [Google Scholar]
- 51. Treberg JR, Clow KA, Greene KA, Brosnan ME, Brosnan JT. Systemic activation of glutamate dehydrogenase increases renal ammoniagenesis: implications for the hyperinsulinism/hyperammonemia syndrome. Am J Physiol Endocrinol Metab. 2010;298:E1219–E1225. [DOI] [PubMed] [Google Scholar]
- 52. Raizen DM, Brooks-Kayal A, Steinkrauss L, Tennekoon GI, Stanley CA, Kelly A. Central nervous system hyperexcitability associated with glutamate dehydrogenase gain of function mutations. J Pediatr. 2005;146:388–394. [DOI] [PubMed] [Google Scholar]
- 53. Glaser B, Kesavan P, Heyman M, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–230. [DOI] [PubMed] [Google Scholar]
- 54. Clayton PT, Eaton S, Aynsley-Green A, et al. Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of β-oxidation in insulin secretion. J Clin Invest. 2001;108:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Molven A, Matre GE, Duran M, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. 2004;53:221–227. [DOI] [PubMed] [Google Scholar]
- 56. Li C, Chen P, Palladino A, et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem. 2010;285:31806–31818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. González-Barroso MM, Giurgea I, Bouillaud F, et al. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS One. 2008;3:e3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vozza A, Parisi G, De Leonardis F, et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA. 2014;111:960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pearson ER, Boj SF, Steele AM, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007;4:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stanescu DE, Hughes N, Kaplan B, Stanley CA, De León DD. Novel presentations of congenital hyperinsulinism due to mutations in the MODY genes: HNF1A and HNF4A. J Clin Endocrinol Metab. 2012;97:E2026–E2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meissner T, Otonkoski T, Feneberg R, et al. Exercise induced hypoglycaemic hyperinsulinism. Arch Dis Child. 2001;84:254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Otonkoski T, Kaminen N, Ustinov J, et al. Physical exercise-induced hyperinsulinemic hypoglycemia is an autosomal-dominant trait characterized by abnormal pyruvate-induced insulin release. Diabetes. 2003;52:199–204. [DOI] [PubMed] [Google Scholar]
- 63. Otonkoski T, Jiao H, Kaminen-Ahola N, et al. Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic β cells. Am J Hum Genet. 2007;81:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Otonkoski T, Meissner T. Exercise-induced hyperinsulinism: a failure of monocarboxylate transporter 1 expression silencing. In: Stanley CA, De León DD, eds. Monogenic Hyperinsulinemic Hypoglycemia Disorders. Geneva: Karger; 2012:172–181. [Google Scholar]
- 65. van Hasselt PM, Ferdinandusse S, Monroe GR, et al. Monocarboxylate transporter 1 deficiency and ketone utilization. N Engl J Med. 2014;371:1900–1907. [DOI] [PubMed] [Google Scholar]
- 66. Quintens R, Hendrickx N, Lemaire K, Schuit F. Why expression of some genes is disallowed in β-cells. Biochem Soc Trans. 2008;36:300–305. [DOI] [PubMed] [Google Scholar]
- 67. Pinney SE, Ganapathy K, Bradfield J, et al. Dominant form of congenital hyperinsulinism maps to HK1 region on 10q. Horm Res Paediatr. 2013;80:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tegtmeyer LC, Rust S, van Scherpenzeel M, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med. 2014;370:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ackermann AM, Li C, Freeze HH, Ficicioglu C, Kaestner KH, Stanley CA. Hypoglycemia due to lower threshold of glucose-stimulated insulin secretion in phosphoglucomutase 1 deficiency. Platform Presentation at: Annual Meeting of the Pediatric Academic Societies, April 25–28, 2015; San Diego, CA. [Google Scholar]
- 70. Kalish JM, Boodhansingh KE, Bhatti TR, et al. Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J Med Genet. 2016;53:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Geneviève D, Amiel J, Viot G, et al. Atypical findings in Kabuki syndrome: report of 8 patients in a series of 20 and review of the literature. Am J Med Genet A. 2004;129A:64–68. [DOI] [PubMed] [Google Scholar]
- 72. Alkhayyat H, Christesen HB, Steer J, Stewart H, Brusgaard K, Hussain K. Mosaic Turner syndrome and hyperinsulinaemic hypoglycaemia. J Pediatr Endocrinol Metab. 2006;19:1451–1457. [DOI] [PubMed] [Google Scholar]
- 73. Gibson CE, Boodhansingh KE, Ganguly AE, Stanley CA. Congenital hyperinsulinism in Turner's syndrome. Poster LBS 024-030 presented at: 97th Annual Meeting of the Endocrine Society, March 5–8, 2015; San Diego, CA. [Google Scholar]
- 74. Kapoor RR, James C, Hussain K. Hyperinsulinism in developmental syndromes. Endocr Dev. 2009;14:95–113. [DOI] [PubMed] [Google Scholar]
- 75. Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore). 2009;88:141–153. [DOI] [PubMed] [Google Scholar]
- 76. Redmon JB, Nuttall FQ. Autoimmune hypoglycemia. Endocrinol Metab Clin North Am. 1999;28:603–618, vii. [DOI] [PubMed] [Google Scholar]
- 77. Alves C, Constança J, De León DD, Snider K, Stanley C. A novel atypical presentation of insulin autoimmune syndrome (Hirata's disease) in a child. J Pediatr Endocrinol Metab. 2013;26:1163–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takayama-Hasumi S, Eguchi Y, Sato A, Morita C, Hirata Y. Insulin autoimmune syndrome is the third leading cause of spontaneous hypoglycemic attacks in Japan. Diabetes Res Clin Pract. 1990;10:211–214. [DOI] [PubMed] [Google Scholar]
- 79. Arya VB, Flanagan SE, Schober E, Rami-Merhar B, Ellard S, Hussain K. Activating AKT2 mutation: hypoinsulinemic hypoketotic hypoglycemia. J Clin Endocrinol Metab. 2014;99:391–394. [DOI] [PubMed] [Google Scholar]
- 80. Hussain K, Challis B, Rocha N, et al. An activating mutation of AKT2 and human hypoglycemia. Science. 2011;334:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nellist M, Schot R, Hoogeveen-Westerveld M, et al. Germline activating AKT3 mutation associated with megalencephaly, polymicrogyria, epilepsy and hypoglycemia. Mol Genet Metab. 2015;114:467–473. [DOI] [PubMed] [Google Scholar]
- 82. Højlund K, Hansen T, Lajer M, et al. A novel syndrome of autosomal-dominant hyperinsulinemic hypoglycemia linked to a mutation in the human insulin receptor gene. Diabetes. 2004;53:1592–1598. [DOI] [PubMed] [Google Scholar]
- 83. Hoe FM, Thornton PS, Wanner LA, Steinkrauss L, Simmons RA, Stanley CA. Clinical features and insulin regulation in infants with a syndrome of prolonged neonatal hyperinsulinism. J Pediatr. 2006;148:207–212. [DOI] [PubMed] [Google Scholar]
- 84. Collins JE, Leonard JV. Hyperinsulinism in asphyxiated and small-for-dates infants with hypoglycaemia. Lancet. 1984;2:311–313. [DOI] [PubMed] [Google Scholar]
- 85. Collins JE, Leonard JV, Teale D, et al. Hyperinsulinaemic hypoglycaemia in small for dates babies. Arch Dis Child. 1990;65:1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Thornton PS, Stanley CA, De Leon DD, et al. Recommendations from the Pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr. 2015;167:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Henquin JC, Sempoux C, Marchandise J, et al. Congenital hyperinsulinism caused by hexokinase I expression or glucokinase-activating mutation in a subset of β-cells. Diabetes. 2013;62:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Le Quan Sang KH, Arnoux JB, Mamoune A, et al. Successful treatment of congenital hyperinsulinism with long-acting release octreotide. Eur J Endocrinol. 2012;166:333–339. [DOI] [PubMed] [Google Scholar]
- 89. Calabria AC, Li C, Gallagher PR, Stanley CA, De León DD. GLP-1 receptor antagonist exendin-(9-39) elevates fasting blood glucose levels in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel. Diabetes. 2012; 61:2585–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Senniappan S, Alexandrescu S, Tatevian N, et al. Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N Engl J Med. 2014;370:1131–1137. [DOI] [PubMed] [Google Scholar]
- 91. Li C, Nissim I, Chen P, et al. Elimination of KATP channels in mouse islets results in elevated [U-13C]glucose metabolism, glutaminolysis, and pyruvate cycling but a decreased γ-aminobutyric acid shunt. J Biol Chem. 2008;283:17238–17249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li C, Liu C, Nissim I, et al. Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine. J Biol Chem. 2013;288:3938–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kibbey RG, Choi CS, Lee HY, et al. Mitochondrial GTP insensitivity contributes to hypoglycemia in hyperinsulinemia hyperammonemia by inhibiting glucagon release. Diabetes. 2014;63:4218–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kassem S, Bhandari S, Rodríguez-Bada P, et al. Large islets, β-cell proliferation, and a glucokinase mutation. N Engl J Med. 2010;362:1348–1350. [DOI] [PubMed] [Google Scholar]
- 96. Tornovsky-Babeay S, Dadon D, Ziv O, et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in β cells. Cell Metab. 2014;19:109–121. [DOI] [PubMed] [Google Scholar]
- 97. Avrahami D, Li C, Yu M, et al. Targeting the cell cycle inhibitor p57Kip2 promotes adult human β cell replication. J Clin Invest. 2014;124:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bhatti TR, Ganapathy K, Huppmann AR, et al. Histologic and molecular profile of pediatric insulinomas: evidence of a paternal parent-of-origin effect [published online January 12, 2016]. J Clin Endocrinol Metab. doi:10.1210/jc.2015–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]