Abstract
Context:
Pheochromocytoma is a catecholamine-producing tumor that originates from adrenal chromaffin cells and is capable of secreting various hormones, including ACTH.
Case Description:
A 56-year-old female presented with Cushingoid appearance and diabetic ketoacidosis. Endocrinological examinations demonstrated ectopic ACTH production with hypercortisolemia and excess urinary cortisol accompanied by elevated plasma and urine catecholamines. Computed tomography revealed a large left adrenal tumor with bilateral adrenal enlargement. Metaiodobenzylguanidine scintigraphy revealed abnormal accumulation in the tumor, which was eventually diagnosed as pheochromocytoma with ectopic ACTH secretion with subsequent manifestation of Cushing's syndrome. Ectopic ACTH secretion and catecholamine production were blocked by metyrapone treatment, whereas dexamethasone paradoxically increased ACTH secretion. Left adrenalectomy resulted in complete remission of Cushing's syndrome and pheochromocytoma.
In Vitro Studies:
Immunohistological analysis revealed that the tumor contained two functionally distinct chromaffin-like cell types. The majority of tumor cells stained positive for tyrosine hydroxylase (TH), whereas a minor population of ACTH-positive tumor cells was negative for TH. Furthermore, gene expression and in vitro functional analyses using primary tumor tissue cultures demonstrated that dexamethasone facilitated ACTH as well as catecholamine secretion with parallel induction of proopiomelanocortin (POMC), TH, and phenylethanolamine N-methyltransferase mRNA, supporting a glucocorticoid-dependent positive-feedback loop of ACTH secretion in vivo. DNA methylation analysis revealed that the POMC promoter of this tumor, particularly the E2F binding site, was hypomethylated.
Conclusion:
We present a case of ectopic ACTH syndrome associated with pheochromocytoma. ACTH up-regulation with paradoxical response to glucocorticoid, possibly through the hypomethylation of the POMC promoter, exacerbated the patient's condition.
Pheochromocytomas are functional catecholamine-producing tumors originating from chromaffin cells in the adrenal medulla and extra-adrenal paraganglial regions. Pheochromocytomas are sometimes associated with ectopic production of a wide variety of several hormones or cytokines, including ACTH, adrenomedullin, and IL-6 (1). Cushing's syndrome has been described as a complication of pheochromocytoma with ectopic ACTH production, but reported cases remain limited (1). Here, we describe a case of ACTH-producing pheochromocytoma with clinical features of Cushing's syndrome.
Case Description
A 56-year-old woman presented to a local hospital with impaired consciousness, general malaise, hypertension (153/93 mmHg), tachycardia (134 bpm), and Cushingoid appearance (moon face, central obesity, body mass index of 32.8 kg/m2). Initial laboratory tests revealed diabetic ketoacidosis (plasma glucose concentration, 626 mg/dL [34.7 mmol/L]; pH, 7.207; serum β-hydroxybutyrate concentration, 2840 μmol/L [normal, 0–74]). Computed tomography (CT) revealed a large left adrenal mass. The patient's mental status did not improve, despite treatment for diabetic ketoacidosis. She was referred to our hospital for further investigation and treatment.
Laboratory studies displayed ACTH-dependent hypercortisolemia and elevated plasma and urine catecholamines and their metabolites (Table 1). Enhanced CT revealed a 54 × 51-mm left adrenal tumor and bilateral adrenal gland enlargement (Figure 1, A and B). I123-metaiodobenzylguanidine scintigraphy exhibited intense focal uptake in the tumor region (Figure 1C). We diagnosed this patient with pheochromocytoma with ectopic ACTH secretion and consequent Cushing's syndrome and treated her continuously with phentolamine, landiolol, and metyrapone. Plasma ACTH concentration markedly decreased from 995 pg/mL (day 1) to 18.4 pg/mL (day 35) with dose-dependent reduction of hypercortisolemia after metyrapone administration (Table 1). We performed a dexamethasone suppression test to confirm ectopic ACTH syndrome. Intriguingly, ACTH secretion was paradoxically stimulated by dexamethasone in a dose-dependent manner (Table 2).
Table 1.
Hormone Profiles
| On Admission | Postoperative | Reference Range | |
|---|---|---|---|
| Basal hormone levels | |||
| Serum or plasma | |||
| PG, mg/dL | 341 | 89 | 70–109 |
| HbA1c, % | 10.2 | 5.3 | 4.6–6.2 |
| ACTH, pg/mL | 995 | 34 | 7.2–63.3 |
| CS, μg/dL | 85.6 | 14.7 | 6.2–19.4 |
| AD, pg/mL | 589 | 8 | <100 |
| NA, pg/mL | 1342 | 140 | 100–450 |
| DA, pg/mL | 68 | 8 | <20 |
| Urine | |||
| UFC, μg/d | 1250 | 44 | 11.2–80.3 |
| Ald, μg/d | 2.3 | 8.8 | <10 |
| AD, μg/d | 244.4 | 9.1 | 3.4–26.9 |
| NA, μg/d | 1175.8 | 104.3 | 48.6–168.4 |
| DA, μg/d | 669.8 | 658.1 | 365.0–961.5 |
| UMNE, mg/d | 1.12 | 0.04 | 0.04–0.19 |
| UNMN, mg/d | 1.29 | 0.12 | 0.09–0.33 |
| Day 1 | Day 12 | Day 35 | |
|---|---|---|---|
| Changes during metyrapone treatment | |||
| Metyrapone, g/d | 0 | 4 | 6 |
| ACTH, pg/mL | 995 | 272 | 18.4 |
| CS, μg/dL | 85.6 | 34.8 | <1.0 |
| UMNE, μg/d | ND | 1.33 | 0.44 |
| UNMN, μg/d | ND | 1.54 | 0.54 |
Abbreviations: PG, plasma glucose; CS, cortisol; AD, adrenaline; NA, noradrenaline; DA, dopamine; UFC, urine free cortisol; Ald, aldosterone; UMNE, urine metanephrine; UNMN, urine normetanephrine; ND, no data; HbA1c, glycosylated hemoglobin.
Figure 1.
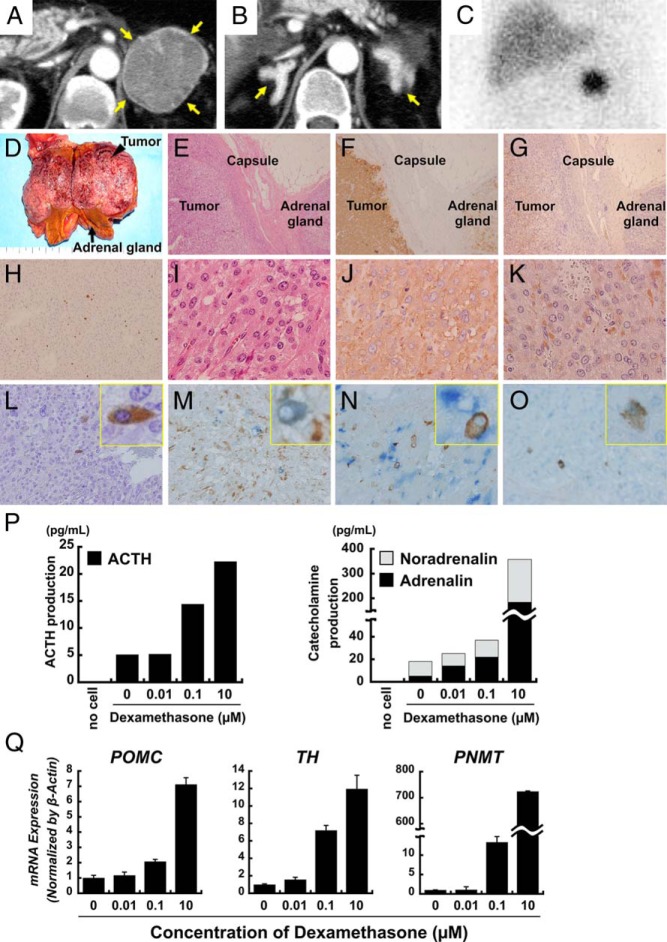
Imaging studies. A and B, Contrast-enhanced CT revealed a 54 × 51-mm left adrenal tumor (A) and bilateral adrenal enlargement (indicated by yellow arrows) (B). C, I123-metaiodobenzylguanidine scintigraphy identified a focal region of intense uptake in the left adrenal tumor. D–O, Pathological findings of pheochromocytoma lesions and attached adrenal tissue in the left adrenal gland. Macroscopic view of the patient's tumor and attached adrenal gland. The adrenal tumor was 53 × 40 × 40 mm, as indicated by the black arrowhead. D, The attached adrenal gland was significantly enlarged (black arrow). E–G, A low-magnification view of the tumor and adjacent adrenal gland. E, Hematoxylin and eosin staining. F, Immunostaining of synaptophysin (in brown). G, Immunostaining of chromogranin A (in brown). H–L, Magnified views of the tumor. H, Ki67 labeling index. I, Hematoxylin and eosin staining. J, Immunostaining of synaptophysin (in brown). K, Immunostaining of chromogranin A (in brown). L, Immunostaining of ACTH (in brown). M–O, Double-immunostaining also was performed, and magnified views are shown with a representative image of a positive cell in the yellow box insets. M, Double-immunostaining for ACTH and chromogranin A revealed expression of chromogranin A (in brown) in ACTH-positive cells (in blue). N, Double-immunostaining for ACTH and TH revealed that ACTH- (in brown) and TH- (in blue) positive cells do not appear to overlap. O, Double-immunostaining for ACTH and GR revealed diffuse expression of GR (in blue) in most tumor cells, including ACTH-positive cells (in brown). P and Q, Effect of dexamethasone on secretion of ACTH and catecholamine from tumor cells in vitro. P, Secreted hormone levels after treatment in tumor cell medium (ACTH concentration on the left, catecholamines on the right). Q, Dose-dependent effect of dexamethasone on the induction of the indicated mRNAs as determined by RT-qPCR.
Table 2.
Dexamethasone Suppression Test
| 1 mg | 2 mg | 8 mg | 8 mg | |
|---|---|---|---|---|
| Before surgical operation | ||||
| ACTH, pg/mL | 18.4 | 22.6 | 93.7 | 241 |
| CS, μg/dL | <1.0 | <1.0 | <1.0 | <1.0 |
| AD, pg/mL | 363 | 400 | 873 | 1395 |
| NA, pg/mL | 1490 | 1308 | 2820 | 3063 |
| After surgical operation | ||||
| ACTH, pg/mL | <5.0 | ND | <5.0 | <5.0 |
| CS, μg/dL | <1.0 | ND | <1.0 | <1.0 |
| AD, pg/mL | <5.0 | ND | <5.0 | 9 |
| NA, pg/mL | 108 | ND | 77 | 78 |
Abbreviations: CS, cortisol; AD, adrenaline; NA, noradrenaline, ND, no data. Dexamethasone suppression test was carefully performed after stabilizing the patient's condition with administration of phentolamine, landiolol, and metyrapone.
Left adrenalectomy, which was performed 41 days after admission due to severe complications (sepsis, gastrointestinal bleeding), resulted in complete remission of pheochromocytoma and Cushing's syndrome (Table 1) including the disappearance of paradoxical ACTH secretion in response to dexamethasone (Table 2). The left adrenal tumor was 53 × 40 × 40 mm, brown in color, and associated with bilateral adrenal gland enlargement (Figure 1D). Histopathology revealed chromaffin-like cells with eosinophilic cytoplasm (Figure 1, E and I). Immunohistochemistry revealed positive staining for synaptophysin (Figure 1, F and J) and chromogranin A (Figure 1, G and K) with approximately 15% of Ki67 labeling index (Figure 1H), confirming pheochromocytoma. ACTH-positive cells were distributed sparsely within the tumor, consistent with ectopic ACTH production (Figure 1L).
We performed double-immunostaining for ACTH and chromogranin A, tyrosine hydroxylase (TH), or glucocorticoid receptor (GR). ACTH-positive cells costained primarily with chromogranin A (Figure 1M), confirming that ACTH-positive cells belonged to the neuroendocrine tumor and possibly arose from the same origin as the pheochromocytoma cells.
Although most tumor cells were TH-positive, ACTH and TH staining appeared to be mutually exclusive, suggesting two types of cells in the tumor (Figure 1N). GR was also detected in most cells, including ACTH-positive cells, consistent with the observed response of ACTH to glucocorticoid (Figure 1O).
Adjacent adrenal cortex tissue was macroscopically hyperplastic with high immune reactivity for 3β-hydroxysteroid dehydrogenase, CYP17, CYP11B1, and CYP21, consistent with ACTH-dependent Cushing's syndrome (Supplemental Figure 1).
Methods
Primary tumor cell culture
Primary tumor cell cultures were prepared as described in the Supplemental Data and treated with increasing concentrations of dexamethasone (0–10 μm).
Genomic DNA and total RNA extraction from frozen tumor tissues
Excised tumor tissues were derived from this patient (case 1), thymic carcinoid with ectopic ACTH syndrome (case 2), and four pheochromocytomas without ACTH syndrome (cases 3–6) (Supplemental Table 1). Genomic DNA and total RNA were extracted using DNAeasy Blood and Tissue Kits and RNAeasy Kits (QIAGEN), respectively.
Gene expression analysis
RT-quantitative PCR (RT-qPCR) experiments were performed as previously described (2). All gene-specific mRNA expression values were normalized by β-actin. Primer information and experimental conditions are described in the Supplemental Data.
Methylation analysis
Genomic DNA samples were subjected to both methylation assay and bisulfite sequencing as described in the Supplemental Data.
In Vitro Analysis
We examined the glucocorticoid effect on ACTH and catecholamine secretion in primary tumor cell cultures (Supplemental Figure 2). Consistent with in vivo observations, cultured cells secreted ACTH and catecholamine, which were markedly increased by dexamethasone in a dose-dependent manner (Figure 1P). Dexamethasone also induced the following mRNAs in a dose-dependent manner: proopiomelanocortin (POMC), a precursor hormone for ACTH; TH, a rate-limiting enzyme in the catecholamine synthesis pathway; and phenylethanolamine N-methyltransferase, a key enzyme in noradrenaline-to-adrenaline conversion (Figure 1Q).
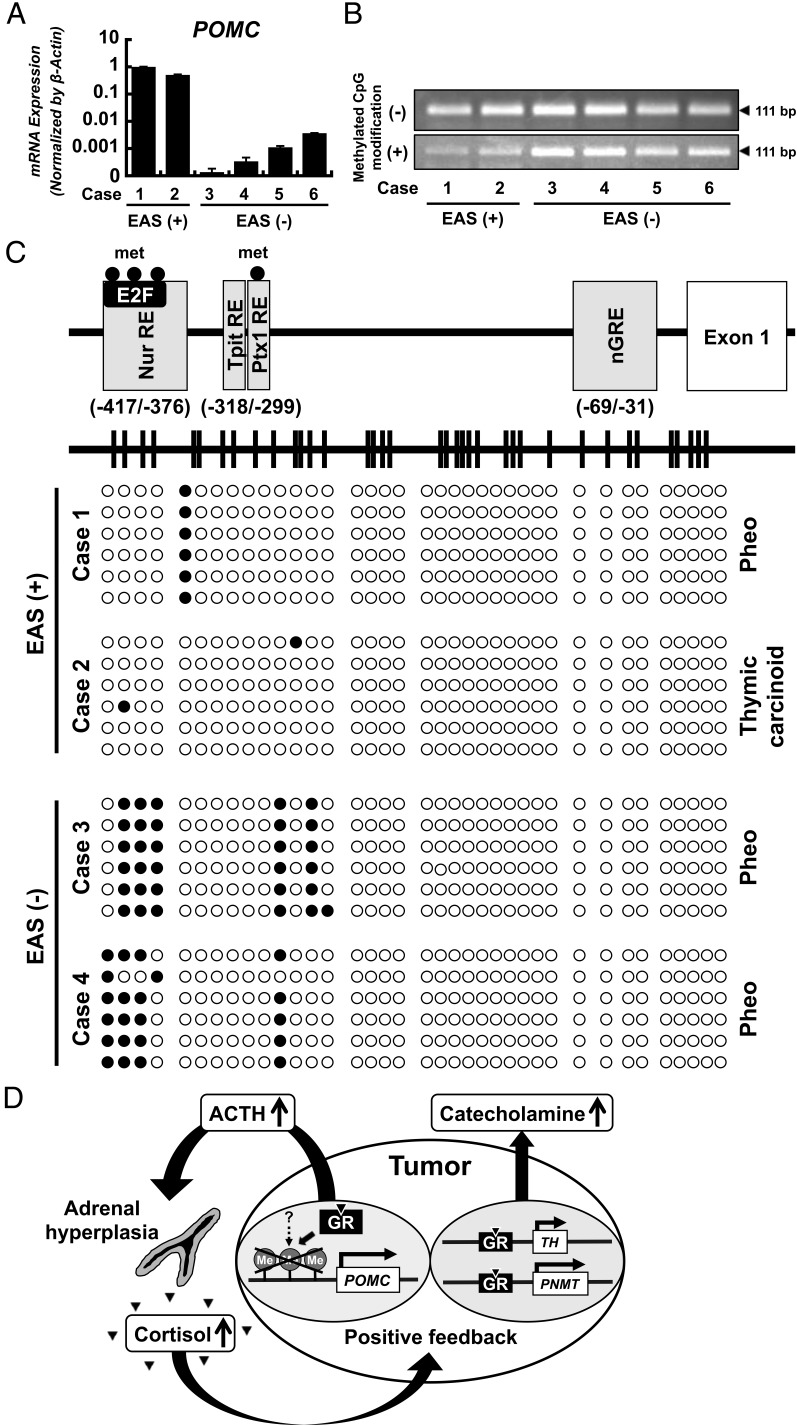
It has been reported that E2F factors bind to the distal region of the POMC promoter (−417/−376), mediating POMC expression in ectopic ACTH syndrome (3). The methylation status of the CpG island of the potential E2F binding region of the POMC promoter has also been shown to correlate with POMC overexpression in thymic carcinoid (4). To address the mechanism of paradoxical regulation of ACTH by glucocorticoid in this tumor, we examined methylation levels on the POMC promoter region in cases 1–6 (Supplemental Table 1) using a methylation-specific PCR assay and bisulfite sequencing. RT-qPCR analysis confirmed very high expression of POMC mRNA in cases 1 and 2 compared to cases 3–6 (Figure 2A), consistent with clinical phenotypes. The methylation-specific assay demonstrated that PCR products were less amplified in cases 1 and 2 compared to cases 3–6 (Figure 2B).
Figure 2.
Expression of POMC mRNA in various neuroendocrine tumor tissues. A, RT-qPCR analysis using tumor tissues revealed that the expression of POMC mRNA was extremely high in the presented case (case 1) and thymic carcinoid with ectopic ACTH syndrome (case 2) compared to pheochromocytomas without ectopic ACTH syndrome (cases 3–6). B, Methylation-specific PCR analysis for the POMC promoter. Electrophoretic bands amplified by methylation-specific PCR for the POMC promoter are shown. The upper panel represents amplification of untreated DNA. The lower panel represents amplification of bisulfite-treated DNA. The PCR products of bisulfite-treated DNA were less amplified in the presented case (case 1) and thymic carcinoid with ectopic ACTH syndrome (case 2) than in the four pheochromocytomas without ectopic ACTH syndrome (cases 3–6), which indicates that the POMC promoter is hypomethylated in ectopic ACTH syndrome, including in the presented case. C, Bisulfite sequencing of CpG islands in the human POMC promoter. The human POMC gene locus is shown with potential responsive elements for transcriptional factors and GR. The horizontal line in the upper panel represents the CpG island to scale, with vertical lines indicating individual CpG dinucleotides. The lower panel displays the CpG methylation density scale, with each row representing a single clone; six clones were selected for sequencing from the presented case (case 1), one thymic carcinoid with ectopic ACTH syndrome (case 2), and two pheochromocytomas without ectopic ACTH syndrome (cases 3 and 4). Each open circle represents an unmethylated CpG site, and each closed circle represents a methylated CpG site. The E2F binding region (−417 to −376 bp) is unmethylated or hypomethylated in the presented case (case 1) and thymic carcinoid with ectopic ACTH syndrome (case 2) compared to the two pheochromocytomas without ectopic ACTH syndrome (cases 3 and 4). D, Model of the glucocorticoid-driven positive-feedback loop that exacerbates this patient's condition. This patient's tumor consisted of two functionally distinct cell types: ACTH-producing cells and catecholamine-producing cells. Ectopic ACTH secretion mediates adrenal hyperplasia with cortisol excess, which leads to glucocorticoid-dependent POMC up-regulation and ACTH secretion, possibly through CpG hypomethylation of the POMC promoter. In addition, hypercortisolemia markedly drives catecholamine secretion. Thus, this patient may have experienced an exacerbation of Cushing's syndrome and pheochromocytoma via a glucocorticoid-dependent positive-feedback loop through GR expression. Abbreviations: EAS, ectopic ACTH syndrome; met, methylation; nGRE, negative glucocorticoid response element; Nur, nuclear receptor subfamily 4; Pheo, pheochromocytoma; Ptx, pituitary homeobox 1; RE, responsive element; Tpit, T-box pituitary-restricted transcription factor.
Subclonings of individual amplified PCR products across the POMC promoter region were performed in cases 1–4. DNA methylation was enriched at distal sites of the POMC gene, especially the E2F binding site, in cases 3 and 4 but not cases 1 and 2 (Figure 2C and Supplemental Figure 3).
Discussion
Cushing's syndrome due to pheochromocytoma with ectopic ACTH production is rare (1). We have described a unique case in which ectopic ACTH secretion and catecholamine production were blocked by metyrapone treatment, whereas both were up-regulated by dexamethasone. Dexamethasone has been shown to increase catecholamine-synthesizing enzyme expression and facilitate catecholamine secretion in primary tumor cells (5). Exogenous glucocorticoid has also been shown to induce pheochromocytoma crisis, possibly due to massive catecholamine secretion accompanied by induction of catecholamine-synthesizing enzymes (6). In contrast, previous studies have suggested that chromaffin cells exert paracrine control on the adrenal cortex and thereby activate steroid synthesis (7). Taken together, these cortical-chromaffin cell interactions may have bidirectionally contributed to this patient's condition.
Diagnosis of ACTH-producing pheochromocytoma was histologically and biochemically confirmed by positive staining with synaptophysin, chromogranin A, and ACTH (Figure 1). Interestingly, TH was expressed in most tumor cells, but sparsely distributed ACTH-positive cells were TH-negative, indicating two independent cell populations diffusely expressing GR in this tumor. In vitro analyses demonstrated that dexamethasone increased ACTH and catecholamine secretion and up-regulated TH, phenylethanolamine N-methyltransferase, and POMC mRNA expression, indicating a glucocorticoid-dependent positive-feedback loop via GR expression, which accelerates ACTH and catecholamine secretion, and exacerbated Cushing's syndrome in this patient (Figure 2D).
Glucocorticoid suppresses POMC transcription via negative-glucocorticoid responsive element (8), which conflicts with ours and other findings that dexamethasone increases both POMC expression and ACTH secretion in pheochromocytoma (9). Several transcription factors implicated in pituitary development induce pituitary POMC transcription via sequence-specific DNA interactions on its promoter (3). In contrast, E2F factors on the distal region of the POMC promoter transactivate POMC in extrapituitary neuroendocrine tumor (3). Furthermore, demethylation of the CpG island of the E2F binding motif on the POMC promoter was correlated with POMC overexpression in thymic carcinoids with ectopic ACTH syndrome (4), consistent with our finding. The molecular mechanisms by which glucocorticoid paradoxically up-regulates POMC expression in cases of demethylated POMC promoter region remain to be elucidated. One possible explanation is that glucocorticoid-dependent repression of DNA-methyltransferase 1, which regulates DNA demethylation (10), may underlie this paradoxical response. Further studies are needed to clarify the pathogenesis of ACTH-secreting pheochromocytoma.
Conclusion
Immunohistological and in vitro studies demonstrated positive feedback of glucocorticoid-inducible ACTH secretion involving hypomethylation of the POMC promoter in a case of pheochromocytoma with ectopic ACTH-dependent Cushing's syndrome.
Acknowledgments
We thank Masha Poyurovsky for thoughtful comments, discussions, and proof-reading, and Erika Sugawara for technical assistance.
This work was supported by the Japan Society for the Promotion of Science (Grants 15K14375 and 26430105), the Takeda Science Foundation, and the Uehara Memorial Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CT
- computed tomography
- GR
- glucocorticoid receptor
- POMC
- proopiomelanocortin
- RT-qPCR
- RT quantitative PCR
- TH
- tyrosine hydroxylase.
References
- 1. Kirkby-Bott J, Brunaud L, Mathonet M, et al. Ectopic hormone-secreting pheochromocytoma: a francophone observational study. World J Surg. 2012;36:1382–1388. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka T, Ohkubo S, Tatsuno I, Prives C. hCAS/CSE1L associates with chromatin and regulates expression of select p53 target genes. Cell. 2007;130:638–650. [DOI] [PubMed] [Google Scholar]
- 3. Pecori Giraldi F, Cassarino F, Pagliardini L, Asnaghi V, Cavagnini F. The human POMC gene promoter: where do we stand? J Endocrinol Invest. 2011;34:454–460. [DOI] [PubMed] [Google Scholar]
- 4. Ye L, Li X, Kong X, et al. Hypomethylation in the promoter region of POMC gene correlates with ectopic overexpression in thymic carcinoids. J Endocrinol. 2005;185:337–343. [DOI] [PubMed] [Google Scholar]
- 5. Wurtman RJ, Axelrod J. Adrenaline synthesis: control by the pituitary gland and adrenal glucocorticoids. Science. 1965;150:1464–1465. [DOI] [PubMed] [Google Scholar]
- 6. Rosas AL, Kasperlik-Zaluska AA, Papierska L, Bass BL, Pacak K, Eisenhofer G. Pheochromocytoma crisis induced by glucocorticoids: a report of four cases and review of the literature. Eur J Endocrinol. 2008;158:423–429. [DOI] [PubMed] [Google Scholar]
- 7. Neri G, Andreis PG, Prayer-Galetti T, Rossi GP, Malendowicz LK, Nussdorfer GG. Pituitary adenylate-cyclase activating peptide enhances aldosterone secretion of human adrenal gland: evidence for an indirect mechanism, probably involving the local release of catecholamines. J Clin Endocrinol Metab. 1996;81:169–173. [DOI] [PubMed] [Google Scholar]
- 8. Drouin J, Sun YL, Chamberland M, et al. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J. 1993;12:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White A, Ray DW, Talbot A, Abraham P, Thody AJ, Bevan JS. Cushing's syndrome due to phaeochromocytoma secreting the precursors of adrenocorticotropin. J Clin Endocrinol Metab. 2000;85:4771–4775. [DOI] [PubMed] [Google Scholar]
- 10. Yang X, Ewald ER, Huo Y, et al. Glucocorticoid-induced loss of DNA methylation in non-neuronal cells and potential involvement of DNMT1 in epigenetic regulation of Fkbp5. Biochem Biophys Res Commun. 2012;420:570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]