Abstract
Context:
HIV patients are at an increased risk for cardiometabolic disease secondary to depot-specific alterations in adipose function, but mechanisms remain poorly understood.
Objective:
The endoribonuclease Dicer has been linked to the modulation of brown and white adipocyte differentiation. We previously demonstrated that Dicer knockout mice undergo transformation of brown adipose tissue to white adipose tissue and develop a lipodystrophic phenotype. We hypothesized reduced Dicer and brown adipose tissue gene expression from nonlipomatous sc fat among HIV patients with a lipodystrophic phenotype.
Design:
Eighteen HIV (nine with and without lipodystrophic changes in fat distribution, characterized by excess dorsocervical adipose tissue [DCAT]) and nine non-HIV subjects underwent punch biopsy of abdominal sc fat to determine expression of Dicer and other adipose-related genes.
Results:
HIV subjects with long-duration antiretroviral use demonstrated excess DCAT vs non-HIV subjects (9.8 ± 1.0 vs 6.6 ± 0.8 cm2, P = .02) with similar body mass index. Dicer expression was decreased in abdominal sc fat of HIV vs non-HIV (4.88 [1.91, 11.93] vs 17.69 [10.72, 47.91], P = .01), as were PPARα, ZIC1, PRDM16, DIO2, and HSP60 (all P ≤ .03). Moreover, the expression of Dicer (2.49 [0.02, 4.88] vs 11.20 [4.83, 21.45], P = .006), brown fat (PPARα [P = .002], ZIC1 [P = .004], LHX8 [P = .03], PRDM16 [P = .0008], PAT2 [P = .008], P2RX5 [P = .02]), beige fat (TMEM26 [P = .004], CD137 [P = .008]), and other genes (DIO2 [P = .002], leptin [P = .003], HSP60 [P = .0004]) was further decreased in abdominal sc fat comparing HIV subjects with vs without excess DCAT. Down-regulation of Dicer in the abdominal sc fat correlated with the down-regulation of all brown and beige fat genes (all P ≤ .01).
Conclusion:
Our results demonstrate dysfunctional sc adipose tissue marked by reduced Dicer in relationship to the down-regulation of brown and beige fat-related genes in lipodystrophic HIV patients and may provide a novel mechanism for metabolic dysregulation. A strategy to increase browning of white adipose tissue may improve cardiometabolic health in HIV.
Long-term antiretroviral therapy (ART) is the current treatment for HIV infection and substantially improves patient survival and quality of life. However, chronic ART use has been linked to increased cardiometabolic risk, partly due to its effects on adipose tissue dysfunction and fat redistribution characterized by peripheral lipoatrophy, visceral adipose tissue accumulation, and lipomatous accumulation in the dorsocervical area (1). Although mechanisms for this lipodystrophic pattern are not fully understood, recent studies suggest phenotypic changes in the dorsocervical adipose tissue (DCAT) consistent with increased expression of brown adipose tissue (BAT) markers such as uncoupling protein-1 UCP1 (2, 3), type 2 iodothyronine deiodinase DIO2 (4), and Zinc finger of the cerebellum ZIC1 (3). Notably, the dorsocervical fat pad in HIV-lipodystrophy lacks both fully functional brown adipocytes (5) and inducible brown-like cells known as beige adipocytes (3, 6) and cannot be identified on positron emission tomography imaging like classical BAT, suggesting a partial phenotypic transition from white to BAT putatively linked to adaptive thermogenesis (4).
The endoribonuclease Dicer is a critical component of the microRNA pathway, which has a significant role in the differentiation and function of white and brown fat (7, 8). We previously demonstrated that mice with a knockout of Dicer in adipose tissue acquire a lipodystrophic phenotype of decreased white adipose tissue (WAT) mass, whitening of interscapular BAT, insulin resistance, adipose tissue inflammation, dyslipidemia, and other features resembling changes found in human models of acquired lipodystrophy, such as HIV (8). These data suggest a critical role for adipose tissue Dicer and microRNA in modulating brown and white fat identity, potentially contributing to dysfunctional adipose tissue and metabolic abnormalities in HIV lipodystrophy.
The purpose of the current study was to extend our prior findings, in which we examined gene expression in lipomatous dorsocervical fat in HIV patients (8). We now investigate whether brown and beige fat gene expressions are down-regulated in nonlipomatous abdominal sc adipose tissue as a function of reduced Dicer expression among HIV subjects. To that end, we hypothesized dysfunctional sc adipose tissue with reduced Dicer and related BAT gene expression in HIV patients with a lipodystrophy phenotype. We examined expression of these genes in abdominal sc fat of well-characterized HIV subjects with and without lipodystrophic changes in fat distribution, manifested by dorsocervical fat accumulation and confirmed by neck magnetic resonance imaging (MRI), compared with non-HIV subjects. Additionally, we examined the relationships between these markers in the abdominal sc fat and metabolic indices.
Subjects and Methods
Study subjects
Eighteen HIV and nine non-HIV male subjects were newly recruited for the study and assessed prospectively. All subjects were between 18 and 60 years of age with a BMI of 18–35.0 kg/m2. HIV subjects were required to be on a stable ART regimen longer than 12 months. HIV subjects were assessed for lipodystrophic changes in fat on physical examination by the investigator based on the presence or absence of excess dorsocervical fat accumulation, which was subsequently confirmed by MRI examination (see MRI methods below). Exclusion criteria were hemoglobin less than 10.0 g/dL; a known history of diabetes and the use of antidiabetic medications; abnormal thyroid function; use of glucocorticoids, GH, or other anabolic therapies within 3 months of participation; current substance abuse; a history of opportunistic infection within 6 weeks of participation; or active or serious chronic medical conditions other than HIV. The study was approved by the Partners Human Research Committee, and subjects provided written informed consent.
Assessment of body composition
Waist circumference was determined from circumferential measurements of the waist at the level of the top of the iliac crest. Dual-energy X-ray absorptiometry (Hologic) was used to assess fat and lean mass body composition. MRI of the neck was performed on a Siemens 3T Trio magnetic resonance system using phased array neck and body matrix coils. A volumetric three-dimensional Dixon gradient-echo multiecho pulse sequence with six echo times (repetition time = 20 msec, echo time = 2.46, 6.15, 9.84, 12.3, 14.76, 17.22 msec, flip angle = 5, slice thickness = 3 mm, field of view = 42 cm, matrix = 256 × 256) was used. Sagittal images were reconstructed to identify the level of C7 vertebral body. Axial images at the level of C7 were reconstructed and used for measurement of DCAT area. Vertical reference lines were placed along the lateral border of the vertebral body of C7 and projected over DCAT, providing standardized lateral boundaries for the fat depot. DCAT was demarcated anteriorly by the paraspinal muscles and posteriorly by the dorsocervical skin. Area measurements within these boundaries were expressed in centimeters squared. In addition, MRI scans were acquired using an axial T1-weighted fat suppressed pulse sequence obtained at the level of L4 vertebral body for the determination of visceral and sc fat areas using commercial software (Vitrak; Merge e/Film).
Abdominal fat biopsy and PCR analysis
Sampling of sc abdominal fat was performed under local anesthesia with 1% lidocaine, using a 4-mm diameter punch biopsy. Specimens were snap frozen in a dry ice/ethanol bath and immediately transferred to −80°C. Total cellular RNA was extracted from tissue using an RNeasy minikit (QIAGEN). Quantity and purity were assessed by UV absorbance at 260 and 280 nm. cDNA was prepared from 6 ng/μL of RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems). Six microliters (36 ng) of cDNA were used in a 20-μL PCR using TaqMan gene expression assays with a FAM dye label for the following genes: TBP, Dicer, UCP-1, DIO2, PGC-1α, ZIC1, LHX8, PRDM16, PAT2, P2RX5, TMEM26, CD137, DIO2, and leptin. Quantitative RT-PCR assays were run in duplicates and quantitated in the ABI Prism 7700 sequence detection system (Applied Biosystems). Gene expression was performed by a single investigator blinded to group status. The values were normalized to the expression of TATA-binding protein (TBP), and results were expressed as ratios in arbitrary units (AU). The cycle threshold values of TBP, a housekeeping gene, did not differ by HIV status (37.48 [36.45, 38.24] vs 37.11 [33.61, 37.65] in non-HIV vs HIV, P = .20) or in the three group analyses (37.48 [36.45, 38.24] vs 37.45 [35.60, 38.44] vs 33.69 [30.32, 37.41] in non-HIV vs HIV nonlipodystropic vs HIV lipodystrophic, P > .05). Furthermore, no relationships were found between TBP and BMI (ρ = −0.11, P = .57) and TBP and percentage of total body fat (ρ = −0.20, P = .31). For most genes, mRNA expression was detectable and could be quantified. In a small subset of samples for UCP1, LIM homeobox protein 8 (LHX8), proton/amino acid cotransporter-2 (PAT2), PR domain containing 16 (PRDM16), and transmembrane protein 26 (TMEM26), mRNA expression was below standard PCR limits, and thus, we imputed the half-minimum value as previously described (9). For miR-365-5p abundance estimation, Thermo-Fisher miRNA Taqman assay 007917_mat was used following the manufacturer's protocol. The noncoding RNA RNU6B (assay 001093) was used for normalization of microRNA quantitative PCR results.
Biochemical and metabolic assessment
Subjects presented after an overnight fast for blood sampling. Insulin was measured using the Access immunoassay system (Beckman Coulter). CD4+ T cell counts were assessed by flow cytometry. HIV viral load was determined by ultrasensitive RT-PCR (Roche COBAS amplicor). Resting energy expenditure (REE) was measured for 20 minutes by indirect calorimetry (Deltatrac; Sensormedics) after 20 minutes of rest.
Statistical analysis
Normality of distribution was determined using the Shapiro-Wilk test. Data are presented as mean ± SEM or median (interquartile range), depending on the normality of the distribution. Categorical variables are reported as proportions. Primary comparisons were made between non-HIV and HIV subjects using the Student's t test for normally distributed continuous variables, the Wilcoxon rank sum test for nonnormally distributed data, and the χ2 test for categorical variables. Furthermore, comparisons were made among HIV subjects based on the presence or absence of significant, clinically apparent dorsocervical fat accumulation, confirmed by MRI of the neck.
For gene expression, we compared all three groups, non-HIV, HIV nonlipodystrophic, and HIV lipodystrophic after first performing overall comparison among the group by the Kruskal-Wallis test. Individual group comparisons were made if the overall comparison was significant and permitted multiple subgroup comparisons. Power calculation indicated an 80% probability to detect a difference at a two-sided 0.05 significance level to detect a 1.2 SD difference between the HIV and non-HIV groups. Detectable differences for the sample size (n = 27) and power of this study were determined for selected metabolic variables (Supplemental Table 1). Linear regression was evaluated using Spearman's correlation coefficient among all subjects and within the non-HIV and HIV groups separately. Regression modeling was performed to determine the interaction between HIV status, Dicer, and brown and beige fat genes using an interaction threshold of α < .10. A nonparametric posttest for linear trend was performed across all three groups. Statistical significance was defined as P < .05. All statistical analyses were performed using SAS JMP (version 9.0).
Results
Baseline demographic and clinical characteristics
HIV and non-HIV subjects were of similar age and race. The HIV population had a long duration of HIV infection and ART use (21 ± 2 y and 16 ± 2 y, respectively) and demonstrated good virological control and CD4 count (582 ± 66 cells/μL). All HIV subjects were receiving therapy with nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and 56% with protease inhibitors (PIs), the duration of which is shown in Table 1. BMI and percentage of total body fat did not differ between groups. HIV subjects were defined as having a lipodystrophic phenotype based on clinical assessment of dorsocervical fat accumulation, which was subsequently confirmed by neck MRI showing increased DCAT in HIV vs. non-HIV as well as highly significant differences among those HIV subjects with and without clinically apparent dorsocervical fat accumulation (Table 1). Those HIV subjects characterized by lipodystrophic changes in fat distribution demonstrated a longer duration of ART use, which corresponded with a tendency towards longer duration of HIV infection (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics Among Non-HIV-Infected Subjects and HIV-Infected Subjects (n = 27)
| Non-HIV-Infected (n = 9) | HIV-Infected (n = 18) | P Value, Non-HIV vs HIV | HIV-Infected Nonlipodystrophic (n = 9) | HIV-Infected Lipodystrophic (n = 9) | P value, Nonlipodystrophic vs Lipodystrophic | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y | 55 ± 2 | 54 ± 2 | .78 | 52 ± 3 | 56 ± 2 | .24 |
| Race, % | .66 | .48 | ||||
| Caucasian | 78 | 72 | 67 | 78 | ||
| African American | 22 | 22 | 22 | 22 | ||
| Other | 0 | 6 | 11 | 0 | ||
| HIV parameters | ||||||
| Duration HIV, y | N/A | 21 ± 2 | N/A | 18 ± 3 | 24 ± 2 | .07 |
| Duration ART use, y | N/A | 16 ± 2 | N/A | 11 ± 2 | 20 ± 2 | .007 |
| Duration PI use, y | N/A | 13 ± 2 | N/A | 9 ± 2 | 16 ± 3 | .08 |
| Current PI use, % | N/A | 56 | N/A | 38 | 75 | .03 |
| Duration NRTI use, y | N/A | 13 ± 2 | N/A | 11 ± 2 | 16 ± 1 | .08 |
| Current NRTI use, % | N/A | 100 | N/A | 100 | 100 | 1.00 |
| Duration NNRTI, y | N/A | 10 ± 2 | N/A | 9 ± 4 | 10 ± 1 | .95 |
| CD4 cell count, n/mm3 | N/A | 582 ± 66 | N/A | 681 ± 90 | 482 ± 90 | .14 |
| Undetectable viral load, % | N/A | 72 | N/A | 78 | 67 | .60 |
| HIV RNA viral load, copies/mL, log10 | N/A | 1.28 (1.28, 1.33) | N/A | 1.28 (1.28, 1.30) | 1.28 (1.28, 1.41) | .65 |
| Body composition parameters | ||||||
| BMI, kg/m2 | 29.8 ± 1.3 | 29.7 ± 1.0 | .92 | 29.5 ± 1.5 | 29.9 ± 1.3 | .85 |
| Total body fat, % | 29.2 ± 1.6 | 31.3 ± 1.1 | .30 | 31.6 ± 1.7 | 31.0 ± 1.4 | .78 |
| WHR | 0.99 ± 0.01 | 1.02 ± 0.02 | .25 | 1.03 ± 0.03 | 1.01 ± 0.03 | .62 |
| Waist circumference, cm | 103.4 ± 3.9 | 108.0 ± 2.8 | .35 | 108.0 ± 4.4 | 108.1 ± 3.7 | .99 |
| VAT area, cm2 | 216.7 ± 35.2 | 244.6 ± 23.5 | .52 | 229.2 ± 33.2 | 260.1 ± 34.3 | .53 |
| SAT area, cm2 | 281.0 ± 35.7 | 304.4 ± 27.0 | .61 | 308.1 ± 30.8 | 300.7 ± 46.4 | .90 |
| DCAT area, cm2 | 6.6 ± 0.8 | 9.8 ± 1.0 | .02 | 7.1 ± 0.7 | 12.5 ± 1.3 | .003 |
| Metabolic parameters | ||||||
| REE, kcal/d | 1618 (1467, 1715) | 1536 (1378, 1728) | .64 | 1575 (1398, 1784) | 1496 (1314, 1747) | .72 |
| REE/FFM, kcal/d · kg | 26.1 (25.4, 29.3) | 25.8 (24.3, 27.2) | .23 | 25.6 (25.0, 28.3) | 25.9 (22.1, 26.6) | .25 |
| Fasting glucose, mg/dL | 88 ± 3 | 84 ± 2 | .26 | 87 ± 2 | 81 ± 2 | .11 |
| Fasting insulin, μIU/mL | 8.0 ( 4.0, 9.6) | 9.1 (5.2, 11.0) | .40 | 7.7 (5.8, 10.0) | 9.9 (4.0, 11.3) | .60 |
| Triglycerides, mg/dL | 122 ± 24 | 145 ± 15 | .43 | 141 ± 18 | 149 ± 24 | .81 |
Abbreviations: BMI, body mass index; FFM, fat-free mass; N/A, nonapplicable; NNRTI, nonnucleoside reverse transcriptase inhibitor; VAT, visceral adipose tissue; WHR, waist to hip ratio. Normally distributed data are reported as mean ± SEM; nonnormally distributed data are reported as median (interquartile range).
Gene expression of Dicer, other related adipose tissue genes in abdominal sc fat among HIV and non-HIV subjects, and by lipodystrophy status within the HIV group
Dicer expression was significantly decreased among HIV vs non-HIV subjects (4.88 [1.91,11.93] vs 17.69 [10.72, 47.91], P = .01) (Figure 1A), as was the expression of other BAT-related genes, including peroxisome proliferator-activated receptor coactivator-α (PGC1α; P = .004), ZIC1 (P = .007), and PRDM16 (P = .03) in the abdominal sc fat (Table 2). In addition, expression of LHX8 (P = .06) and leptin (P = .08) were lower in HIV; however, the differences did not reach significance (Table 2). Furthermore, there was a decreased expression of metabolically relevant genes DIO2 (P = .03) and heat shock protein 60 (HSP60; P = .004) among the HIV population compared with the non-HIV control group.
Figure 1.
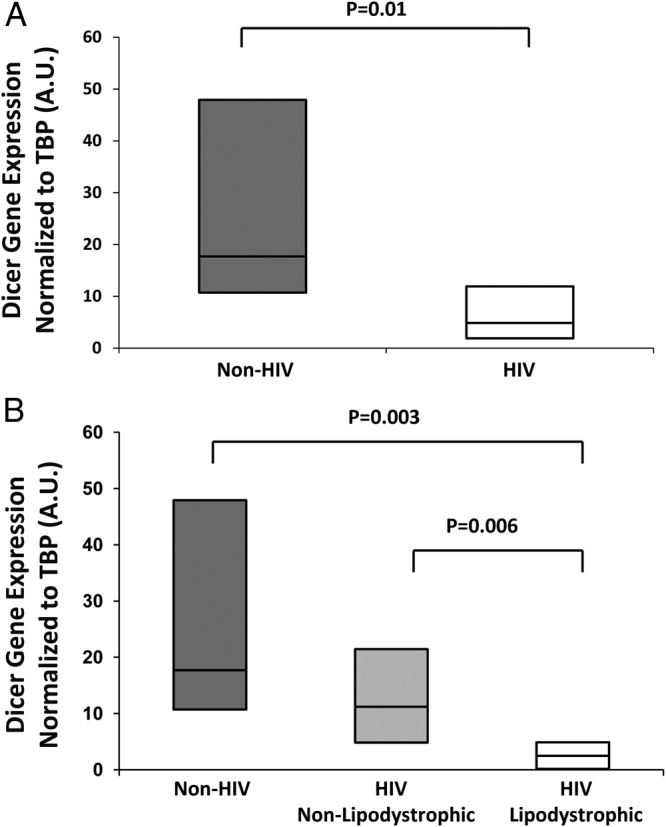
Dicer mRNA expression normalized to TBP (TATA binding protein) in abdominal SAT among non-HIV and HIV subjects (A) and non-HIV and HIV subjects (B) with and without lipodystrophic changes in fat distribution. mRNA results are expressed as ratios in arbitrary units. Box plot represents the 25th and 75th percentile and line within the box represents the median.
Table 2.
Gene Expression Data in the Abdominal Subcutaneous Fat Among Non-HIV-Infected Subjects and HIV-Infected Subjects (n = 27)
| Non-HIV-Infected (n = 9) | HIV-Infected (n = 18) | P Value, Non-HIV vs HIV | Non-HIV-Infected (n = 9) | HIV-Infected Nonlipodystrophic (n = 9) | HIV-Infected Lipodystrophic (n = 9) | Overall P Valuea | P Value, Non-HIV vs Nonlipodystrophic HIV | P Value, Non-HIV vs HIV Lipodystrophic | P Value, HIV Nonlipodystrophic vs HIV Lipodystrophic | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dicer | 17.69 (10.72, 47.91) | 4.88 (1.91, 11.93) | .01 | 17.69 (10.72, 47.91) | 11.20 (4.83, 21.45) | 2.49 (0.02, 4.88) | .002 | .22 | .003 | .006 |
| Brown adipose tissue markers | ||||||||||
| UCP1 | 0.17 (0.08, 0.28) | 0.14 (0.01, 0.29) | .52 | 0.17 (0.08, 0.28) | 0.16 (0.07, 1.45) | 0.01 (0.00, 0.20) | .16 | N/A | N/A | N/A |
| PGC1α | 31.12 (7.14, 56.02) | 0.24 (0.02, 11.88) | .004 | 31.12 (7.14, 56.02) | 11.71 (0.56, 33.12) | 0.03 (0.00, 0.14) | .0003 | .16 | .0006 | .002 |
| ZIC1 | 64.45 (39.43, 185.94) | 6.48 (0.53, 42.63) | .007 | 64.45 (39.43, 185.94) | 37.14 (15.14, 138.59) | 1.02 (0.06, 2.78) | .0005 | .25 | .0006 | .004 |
| LHX8 | 0.23 (0.08, 0.47) | 0.11 (0.01, 0.17) | .06 | 0.23 (0.08, 0.47) | 0.13 (0.06, 0.38) | 0.01 (0.00, 0.13) | .01 | .54 | .009 | .03 |
| PRDM16 | 1.57 (0.23, 2.27) | 0.20 (0.02, 0.56) | .03 | 1.57 (0.23, 2.27) | 0.51 (0.30, 1.45) | 0.02 (0.00, 0.16) | .0007 | .54 | .002 | .0008 |
| PAT2 | 0.31 (0.18, 0.49) | 0.28 (0.04, 0.49) | .33 | 0.31 (0.18, 0.49) | 0.39 (0.28, 0.94) | 0.04 (0.00, 0.27) | .01 | .54 | .02 | .008 |
| P2RX5 | 0.35 (0.17, 1.15) | 0.33 (0.11, 2.18) | .80 | 0.35 (0.17, 1.15) | 1.99 (0.20, 4.50) | 0.14 (0.00, 0.51) | .04 | .29 | .12 | .02 |
| Beige adipose tissue markers | ||||||||||
| TMEM26 | 0.78 (0.50, 1.33) | 0.79 (0.20, 2.59) | 1.00 | 0.78 (0.50, 1.33) | 2.54 (0.79, 3.68) | 0.24 (0.01, 0.75) | .005 | .06 | .07 | .004 |
| CD137 | 5.17 (2.53, 8.61) | 2.00 (0.15, 5.71) | .11 | 5.17 (2.53, 8.61) | 4.11 (2.13, 37.84) | 0.17 (0.01, 2.00) | .006 | 1.00 | .006 | .008 |
| Other metabolic markers | ||||||||||
| DIO2 | 75.06 (33.38, 120.85) | 9.54 (0.22, 54.54) | .03 | 75.06 (33.38, 120.85) | 53.63 (12.36, 153.74) | 0.26 (0.03, 3.53) | .0007 | .60 | .001 | .002 |
| Leptin | 77.98 (20.18, 112.04) | 3.55 (0.12, 87.90) | .08 | 77.98 (20.18, 112.04) | 68.36 (13.16, 296.16) | 0.12 (0.01, 3.55) | .001 | .86 | .001 | .003 |
| HSP60 | 325.16 (219.2, 643.9) | 48.33 (1.75, 179.90) | .004 | 325.16 (219.2, 643.9) | 164.28 (79.79, 851.49) | 2.09 (0.22, 4.59) | .0001 | .19 | .0004 | .0004 |
Abbreviation: N/A, not applicable. Nonnormally distributed data are reported as median (interquartile range). Gene expression values are normalized to TBP, and results are expressed as ratios in arbitrary units.
Overall P value was obtained by a Kruskal-Wallis test.
Moreover, among HIV subjects with lipodystrophic changes in fat marked by excess DCAT compared with those without, we found significantly reduced abdominal sc fat expression of Dicer (2.49 [0.02, 4.88] vs 11.20 [4.83, 21.45], P = .006), PGC1α (P = .002), ZIC1 (P = .004), LHX8 (P = .03), PRDM16 (P = .0008), PAT2 (P = .008), purinergic receptor P2X (P2RX5; P = .02), TMEM26 (P = .004), CD137 (P = .008), DIO2 (P = .002), leptin (P = .003), and HSP60 (P = .0004) (Table 2 and Figure 1B). A nonparametric posttest for linear trend confirmed the significance of differences between groups for most parameters (Supplemental Table 2).
An exploratory analysis of miR-365 was performed, requiring exclusion of one data point with a value beyond 5 SD of the mean. miR-365 expression appeared to be somewhat lower in HIV compared with non-HIV controls (41.8 [13.3, 49.4] vs 49.5 [28.6, 87.2], P = .11) and followed a stepwise decrease similar to Dicer across the three groups; however, these differences did not reach statistical significance (HIV lipodystrophic: 25.8 [18.1, 69.2], HIV nonlipodystrophic: 43.1 [11.6, 48.6], non-HIV controls: 49.5 [28.6, 87.2], P = .22 for three group comparison).
Relationships among Dicer expression and other related adipose tissue genes in abdominal sc fat, DCAT accumulation, and energy expenditure
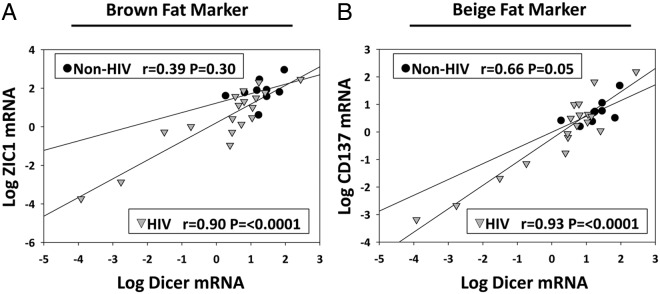
Among all subjects, the down-regulation of Dicer in the abdominal sc fat significantly correlated with the down-regulationvk of UCP1, PGC1α, ZIC1, LHX8, PRDM16, PAT2, P2RX5, TMEM26, CD137, DIO2, leptin, and HSP60 in this depot (all P ≤ .01), and these relationships remained highly significant in analyses limited to the HIV group (Table 3 and Figure 2, A and B). In regression analyses, there was a significant interaction of HIV status and Dicer in relation to ZIC1, PRDM16, and DIO2, whereas there was no interaction of HIV status and Dicer on CD137 or other genes (Supplemental Table 3). Excess DCAT measured by MRI negatively correlated with the expression of Dicer, PGC1α, ZIC1, LHX8, PRDM16, PAT2, P2RX5, TMEM26, DIO2, leptin, and HSP60 in abdominal sc fat in the entire group (all P ≤ .04) as well as in analyses limited to the HIV population (Table 4). Resting energy expenditure normalized to fat-free mass correlated with the up-regulation of UCP1, LXH8, PAT2, TMEM26, and HSP60 (all P ≤ .04) and potentially to Dicer expression (ρ = 0.35, P = .08) in the abdominal sc fat in the entire group.
Table 3.
Correlations Between Dicer and Adipose Tissue Gene Expression in the Abdominal Subcutaneous Fat
| All Subjects (n = 27) |
Non-HIV-Infected Subjects (n = 9) |
HIV-Infected Subjects (n = 18) |
||||
|---|---|---|---|---|---|---|
| ρ | P Value | ρ | P Value | ρ | P Value | |
| Brown adipose tissue markers | ||||||
| UCP1 | 0.46 | .01 | 0.63 | .07 | 0.48 | .04 |
| PGC1α | 0.78 | <.0001 | 0.15 | .70 | 0.79 | .0001 |
| ZIC1 | 0.81 | <.0001 | 0.42 | .26 | 0.86 | <.0001 |
| LHX8 | 0.56 | .002 | −0.08 | .83 | 0.67 | .003 |
| PRDM16 | 0.77 | <.0001 | 0.67 | .05 | 0.84 | <.0001 |
| PAT2 | 0.65 | .0002 | 0.35 | .36 | 0.79 | <.0001 |
| P2RX5 | 0.60 | .0009 | 0.60 | .09 | 0.74 | .0004 |
| Beige adipose tissue markers | ||||||
| TMEM26 | 0.59 | .001 | 0.72 | .03 | 0.72 | .0007 |
| CD137 | 0.76 | <.0001 | 0.78 | .01 | 0.82 | <.0001 |
| Other metabolic markers | ||||||
| DIO2 | 0.86 | <.0001 | 0.83 | .005 | 0.80 | <.0001 |
| Leptin | 0.77 | <.0001 | 0.35 | .36 | 0.91 | <.0001 |
| HSP60 | 0.71 | <.0001 | −0.02 | .97 | 0.79 | .0001 |
Relationships were determined by Spearman's correlation coefficient.
Figure 2.
Relationship between Dicer and ZIC1 (a brown fat marker) mRNA expression (A) and CD137 (a beige fat marker) mRNA expression (B) in the abdominal SAT. Gene expression values are normalized to TBP, and mRNA results are expressed as ratios in arbitrary units. Data were analyzed using Spearman's correlation coefficient and are represented here as linear regression using Pearson's correlation coefficient after logarithmic transformation for the purposes of illustrating the relationship. Separate analyses demonstrated a significant interaction between HIV status and Dicer in relationship to ZIC1, whereas there was no significant interaction between HIV status and Dicer in relationship to CD137. Circles represent data points from non-HIV subjects and triangles represent data points from HIV subjects.
Table 4.
Correlations Between DCAT Area and Adipose Tissue Gene Expression in the Abdominal Subcutaneous Fat
| All Subjects (n = 27) |
Non-HIV-Infected Subjects (n = 9) |
HIV-Infected Subjects (n = 18) |
||||
|---|---|---|---|---|---|---|
| ρ | P Value | ρ | P Value | ρ | P Value | |
| Dicer | −0.42 | .03 | −0.24 | .57 | −0.50 | .03 |
| Brown adipose tissue markers | ||||||
| UCP1 | −0.34 | .09 | −0.48 | .23 | −0.36 | .14 |
| PGC1α | −0.41 | .04 | 0.43 | .29 | −0.59 | .01 |
| ZIC1 | −0.53 | .005 | −0.55 | .16 | −0.49 | .04 |
| LHX8 | −0.55 | .004 | −0.40 | .32 | −0.57 | .01 |
| PRDM16 | −0.57 | .003 | −0.19 | .65 | −0.71 | .0009 |
| PAT2 | −0.62 | .0007 | −0.48 | .23 | −0.71 | .0009 |
| P2RX5 | −0.56 | .003 | −0.50 | .20 | −0.66 | .003 |
| Beige adipose tissue markers | ||||||
| TMEM26 | −0.55 | .004 | −0.45 | .26 | −0.63 | .005 |
| CD137 | −0.31 | .13 | 0.17 | .69 | −0.39 | .11 |
| Other metabolic markers | ||||||
| DIO2 | −0.46 | .02 | 0.00 | 1.00 | −0.54 | .02 |
| Leptin | −0.58 | .002 | −0.45 | .26 | −0.60 | .009 |
| HSP60 | −0.56 | .003 | −0.10 | .82 | −0.63 | .005 |
Relationships were determined by Spearman's correlation coefficient.
Relationship of insulin to Dicer expression and other related adipose tissue genes in the abdominal sc fat among HIV subjects with dorsocervical fat accumulation
Increasing serum insulin levels were significantly and negatively correlated with the down-regulation of Dicer (ρ = −0.70, P = .04), LHX8 (ρ = −0.67, P = .05), PRDM16 (ρ = −0.75, P = .02), PAT2 (ρ = −0.67, P = .05), P2RX5 (ρ = −0.82, P = .007), TMEM26 (ρ = −0.75, P = .02), CD137 (ρ = −0.72, P = .03), and leptin (ρ = −0.77, P = .02) in the abdominal sc fat among lipodystrophic HIV subjects with excess DCAT accumulation. In addition, among the HIV group with excess DCAT, reduced abdominal sc fat expression of UCP1 (ρ = −0.62, P = .08), ZIC1 (ρ = −0.60, P = .09), DIO2 (ρ = −0.60, P = .09), and HSP60 (ρ = −0.65, P = .06) were potentially related to increased insulin levels. No significant relationships between insulin and expression of adipose tissue genes were demonstrated among HIV subjects without excess DCAT, and relationships to insulin were not generally significant among the non-HIV subjects.
Discussion
Limited data are available regarding mechanisms involved in systemic adipose tissue dysfunction in HIV lipodystrophy. In this study, we examined the expression of Dicer and brown and beige fat genes in abdominal sc adipose tissue of HIV subjects with and without changes in fat distribution clinically characterized by excess dorsocervical fat, as confirmed by neck MRI, compared with non-HIV subjects. Among HIV subjects, expression of Dicer in the abdominal sc fat depot was inversely associated with excess DCAT accumulation. Moreover, our data demonstrate a broad down-regulation of brown and beige fat markers in relationship to reduced Dicer expression in nonlipomatous sc fat of HIV subjects with dorsocervical fat accumulation. Overall, our results strongly suggest dysfunctional sc adipose tissue in HIV patients with a common clinical phenotype. Down-regulation of Dicer in abdominal sc fat of lipodystrophic HIV patients may limit the capacity for adipose browning and may represent a novel mechanism for metabolic dysregulation in this population.
Dicer is an ribonuclease III endoribonuclease that mediates RNA interference by cleaving double-stranded RNA into multiple nucleotide double-stranded microRNA fragments (7, 10). Dicer has an important function in microRNA processing, and its down-regulation can adversely affect many microRNAs that maintain cell identity and play important roles in brown and white fat differentiation (10–15). The significantly reduced Dicer expression in the abdominal sc fat in lipodystrophic HIV subjects in the current study follows prior evidence from our group showing development of lipodystrophy with decreased WAT mass and whitening of interscapular BAT in adipose tissue-specific Dicer knockout mice (8). This lipodystrophic phenotype in the Dicer knockout mice was related to features of metabolic dysregulation common to HIV lipodystrophy, including insulin resistance, adipose tissue inflammation, and dyslipidemia (8). Furthermore, our prior studies of adipose tissue from HIV patients demonstrate adipose Dicer mRNA expression was significantly reduced in the dorsocervical fat pad and abdominal sc depot compared with non-HIV subjects (8). Our current study builds upon these findings showing that markers of brown and beige adipose tissue are broadly and significantly reduced in nonlipomatous abdominal sc fat in a human model of an acquired HIV lipodystrophy phenotype, correlating with decreased Dicer expression in this sc depot.
The findings in this study are supported by recent evidence that modulation of specific microRNAs processed by Dicer promote a shift in adipocyte identity: for example, microRNAs miR-193b/365 (12), miR-196a (15), miR-155 (16), miR-133 (13), and miR-455 (10) have been recently implicated in brown adipocyte differentiation by targeting adipogenic regulators. Although the mechanisms for reduced Dicer in HIV lipodystrophy remain uncertain, a direct effect of HIV on suppressing microRNA processing and expression of Dicer has been found in macrophages (17, 18). Indeed, recent studies have suggested that adipose tissue can serve as a viral reservoir for HIV (19, 20), despite virological control with ART. In this regard, in situ effects of the virus in adipose tissue could potentiate inflammation and consequently lead to alterations in gene expression. Importantly, no systemic differences in viral load were observed between groups in the current study. Alternatively, adipocytes treated with NRTIs and PIs undergo significant oxidative stress, leading to apoptosis and increased secretion of adipokines (21); however, the effect of ART on Dicer expression is unknown. In our study, all subjects were using NRTIs but overall duration of NRTI use and current PI use were higher in those with lipodystrophy, suggesting the need to better understand potential effects of these agents on Dicer function.
Brown fat contributes to enhanced energy expenditure and is present in adult humans, mostly in the cervical and supraclavicular area, consisting of clustered classical brown fat cells or inducible beige fat cells emerging in WAT upon stimulation (22, 23). Both cell types contain UCP1, but each have distinct gene expression profiles with subphenotypes potentially arising from unique adipocyte differentiation (24). In our study, decreased Dicer expression in the abdominal sc fat was significantly associated with decreased expression of numerous brown and beige fat genes, which were generally reduced in the HIV group compared with the non-HIV group and further reduced in lipodystrophic vs nonlipodystrophic HIV groups. In addition, the overall BAT gene expression obtained in the current study from the abdominal sc depot is markedly reduced when compared with those levels generally found in classical BAT-enriched depots, including the supraclavicular area (23).
Prior studies have focused on lipomatous accumulations in HIV subjects and showed mixed results regarding their adipose phenotype. Increased UCP1 expression in the dorsocervical fat of HIV subjects with lipohypertrophy has been reported (2, 3, 25); however, others failed to identify UCP1 or increased FDG uptake in DCAT of HIV lipodystrophy patients (4, 5, 26), showing instead the up-regulation of DIO2 (4), lower inflammatory markers, and mitochondrial depletion (5). In a recent study, buffalo-hump lipomas in HIV subjects had a shift from white to brown adipocyte lineage with higher expression of classical BAT markers UCP1 and ZIC1 compared with nonbuffalo-hump lipomas; however, no evidence of a beige phenotype was seen (3). Although the exact adipose identity of the dorsocervical fat pad in HIV remains uncertain, this depot does not contain fully functional BAT or beige cells and likely constitutes a transitional phenotype (3, 4).
Although studies from our group and others have demonstrated that brown fat-like features may be found in the dorsocervical fat pad in HIV lipodystrophy, our current study suggests that the thermogenic capacity in the more abundant nonlipomatous sc fat is severely reduced among HIV patients with dorsocervical adipose tissue accumulation. Furthermore, significant associations between Dicer and brown and beige fat genes suggest that the reduced capacity for browning could be driven by a down-regulation of Dicer and microRNAs important to the modulation of adipose browning. For example, miR-365 is involved in BAT adipogenesis, which in a prior study induced expression of UCP1 in Dicer knockout preadipocytes (8). In an exploratory analysis of microRNA modulation potentially mediated by decreased Dicer, we found that miR-365 was somewhat lower in HIV vs non-HIV infected and followed a stepwise decrease similar to Dicer across the three groups; however, these findings did not reach statistical significance. Moreover, we have also demonstrated reduced leptin expression in the abdominal sc fat of HIV compared with non-HIV subjects, suggesting that the abdominal sc fat compartment is a dysfunctional type of fat with altered expression of white, brown, and beige fat genes. Taken together, alterations in several lineages of adipocytes may have a contributory role to metabolic dysfunction in HIV when lipodystrophy is present. In this regard, Dicer expression and other brown and beige fat genes in the abdominal sc fat were generally lowest in those HIV subjects with excess dorsocervical adipose tissue, a marker of a lipodystrophic phenotype, despite similar BMIs between the groups. Moreover, we demonstrate that both DCAT area and multiple markers of abdominal sc brown and beige fat, which are exclusive variables with no interdependence, are significantly related to Dicer. Therefore, the physiological nature of these data are corroborated by the independent relationships shown in the current study between the following: 1) the down-regulation of Dicer and brown and beige fat markers, 2) the down-regulation of Dicer and increase in DCAT area, and 3) the down-regulation of brown and beige fat genes and increase in DCAT area. These relationships provide strong support for the novel findings observed in this study.
Regarding metabolic indices, decreased markers of BAT (UCP1, LHX8, PAT2) and beige fat (TMEM26) in all subjects correlated significantly with lower REE, suggesting lower capacity for browning may influence REE during nonshivering thermogenesis. We previously observed that lipodystrophic Dicer knockout mice developed a compensatory response to BAT whitening, maintaining body temperature during cold exposure by increased shivering, which contributes to increased REE and helps maintain body temperature (8). Although the specific cause and mechanism by which adipose phenotypical changes occur in HIV lipodystrophy remains unknown, oxidative stress and mitochondrial toxicity from ART may be relevant factors (27). Differentiation of WAT showing features of BAT within the dorsocervical fat pad may be an adaptive mechanism to increase thermogenesis in the setting of mitochondrial defects due to oxidative stress imposed by ART in other select fat depots, such as the abdominal sc fat depot, which we have now demonstrated is limited in its thermogenic capacity. To that end, the dorsocervical fat pad may develop as a compensatory depot in lipodystrophy, a disease state in which dysfunctional sc adipose tissue may precipitate cardiometabolic disease and may provide potential mechanistic insight as to why certain HIV patients are prone to DCAT accumulation. Concordant with our prior observation that Dicer knockout mice had a 4.5-fold increase in circulating insulin levels (8), we now demonstrate that in HIV patients with a lipodystrophic phenotype, reduced expression of sc Dicer, BAT, and beige genes correlated with higher fasting insulin. Indeed, the inverse relationship between Dicer and levels of insulin in this subset of HIV patients may relate to the degree of insulin resistance associated with the down-regulation of Dicer, which is a clinically relevant link consistent with known metabolic complications of the HIV lipodystrophy. Further metabolic consequences of reduced Dicer expression in HIV-lipodystrophy, aside from energy and glucose homeostasis, should be determined.
Although subjects in the study were well phenotyped, including objective characterization of dorsocervical fat accumulation in relationship to sc adipose gene expression, the relatively small size of subgroups and the inclusion of only male subjects are limitations. In addition, more detailed indices from SAT could be obtained to characterize this depot further, including adipocyte morphology, mitochondrial density, oxidative capacity, DNA abundance, and Dicer enzyme activity studies. For gene expression studies, we did not separate adipocytes from stromovascular cells. However, in a prior study decreased Dicer expression in sc WAT, perigonadal WAT, and BAT of fat-specific Dicer knockout mice was entirely due to the adipocyte fraction, with no change in Dicer levels in stromovascular cells (8). Despite these limitations, the study size was sufficient and methods adequate to show robust differences in expression of Dicer and related genes among the groups, including well-matched control subjects. Furthermore, although brown and beige fat genes are generally lower in WAT compared with classical BAT, we were able to identify lower levels of such markers in SAT of HIV-infected individuals compared with non-HIV control subjects. We did not specifically examine the expression of a comprehensive panel of microRNAs recently implicated in brown adipocyte differentiation. Given the complexity in identifying microRNAs and their specific targets in adipogenesis, examining a comprehensive panel of microRNAs should be considered for future studies to establish a link between adipose tissue Dicer expression and alterations of specific microRNAs in HIV lipodystrophy. In addition, recent evidence shows effects of viral proteins to reduce Dicer in cell cultures (28), and future studies should investigate whether such proteins have direct effects to reduce Dicer in sc adipose tissue.
In summary, we show that dysfunction of abdominal, nonlipomatous, sc adipose tissue in HIV patients with a lipodystrophy phenotype marked by dorsocervical fat accumulation, is characterized by reduced expression of Dicer and brown and beige fat genes. Further exploration of the pathophysiological mechanisms and consequences of reduced sc adipose tissue Dicer expression in this population may provide novel targets to improve cardiometabolic health in HIV. In this regard, strategies to increase browning of WAT could ultimately prove useful to improve cardiometabolic health in patients with HIV lipodystrophy.
Acknowledgments
We thank the nursing staff on the Massachusetts General Hospital Clinical Research Center for their dedicated patient care, the volunteers who participated in this study, Carla Roberts-Toler for technical assistance in the laboratory, and Hang Lee, PhD, for statistical consultation.
The study had the clinical trial registration number of NCT01098045.
This work was supported by the National Institutes of Health Grants 1UL1RR025758, 2P30AI060354 (to M.T.), K23DK081604 (to A.M.C.), M01RR01066, 1UL1RR025758, and UL1TR001102 (to the Harvard Catalyst/Harvard Clinical and Translational Science Center) from the National Center for Research Resources and National Center for Advancing Translational Sciences, and Grant P30DK40561, a pilot and feasibility grant to the Nutrition and Obesity Research Center at Harvard University, and the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Summary: A.M.C. is a recipient of sponsored research grants from Chugai Pharmaceutical Co, Ltd, and Molecular Metabolism, LLC, both through the Joslin Diabetes Center, and he has received payment for lecturing about clinical diabetes on behalf of the Joslin Diabetes Center to employees of Sanofi, Genentech, Eli Lilly, Janssen, and Regeneron, all unrelated to this manuscript. C.R.K. serves as a consultant for Catabasis, MedImmune, Merck, Antriabio, and Sunstar Foundation and receives grant funding from MedImmune Regeneron, all unrelated to this manuscript. S.K.G. has received research funding from Bristol-Myers Squibb, Immunex, Gilead, and Theratechnologies and served as a consultant for Navidea Inc, Merck, Bristol-Myers Squibb, Gilead, and NovoNordisk, all unrelated to this manuscript. The other authors have nothing to disclose.
Footnotes
- ART
- antiretroviral therapy
- BAT
- brown adipose tissue
- DCAT
- dorsocervical adipose tissue
- DIO2
- type 2 iodothyronine deiodinase
- HSP60
- heat shock protein 60
- LHX8
- LIM homeobox protein 8
- MRI
- magnetic resonance imaging
- NRTI
- nucleoside/nucleotide reverse transcriptase inhibitor
- PAT2
- proton/amino acid cotransporter-2
- PGC1α
- peroxisome proliferator-activated receptor coactivator-α
- PI
- protease inhibitor
- PRDM16
- PR domain containing 16
- P2RX5
- purinergic receptor P2X
- REE
- resting energy expenditure
- SAT
- sc adipose tissue
- TBP
- TATA-binding protein
- TMEM26
- transmembrane protein 26
- UCP1
- uncoupling protein-1
- WAT
- white adipose tissue
- ZIC1
- Zinc finger of the cerebellum.
References
- 1. Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. [DOI] [PubMed] [Google Scholar]
- 2. Guallar JP, Gallego-Escuredo JM, Domingo JC, et al. Differential gene expression indicates that “buffalo hump” is a distinct adipose tissue disturbance in HIV-1-associated lipodystrophy. AIDS. 2008;22:575–584. [DOI] [PubMed] [Google Scholar]
- 3. Cereijo R, Gallego-Escuredo JM, Moure R, et al. The molecular signature of HIV-1-associated lipomatosis reveals differential involvement of brown and beige/brite adipocyte cell lineages. PLoS One. 2015;10:e0136571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torriani M, Fitch K, Stavrou E, et al. Deiodinase 2 expression is increased in dorsocervical fat of patients with HIV-associated lipohypertrophy syndrome. J Clin Endocrinol Metab. 2012;97:E602–E607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sevastianova K, Sutinen J, Greco D, et al. Comparison of dorsocervical with abdominal subcutaneous adipose tissue in patients with and without antiretroviral therapy-associated lipodystrophy. Diabetes. 2011;60:1894–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mori MA, Raghavan P, Thomou T, et al. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mori M, Thomou T, Boucher J, et al. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J Clin Invest. 2014;124:3339–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cypess AM, Weiner LS, Roberts-Toler C, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang H, Guan M, Townsend KL, et al. MicroRNA-455 regulates brown adipogenesis via a novel HIF1an-AMPK-PGC1α signaling network. EMBO Rep. 2015;16:1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu W, Bi P, Shan T, et al. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013;9:e1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun L, Xie H, Mori MA, et al. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol. 2011;13:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol. 2012;14:1330–1335. [DOI] [PubMed] [Google Scholar]
- 14. Sun L, Trajkovski M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism. 2014;63:272–282. [DOI] [PubMed] [Google Scholar]
- 15. Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. 2012 Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 10:e1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y, Siegel F, Kipschull S, et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun. 2013;4:1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ouellet DL, Plante I, Barat C, Tremblay MJ, Provost P. Emergence of a complex relationship between HIV-1 and the microRNA pathway. Methods Mol Biol. 2009;487:415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coley W, Van Duyne R, Carpio L, et al. Absence of DICER in monocytes and its regulation by HIV-1. J Biol Chem. 2010;285:31930–31943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Damouche A, Lazure T, Avettand-Fènoël V, et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. Silvestri G, ed. PLOS Pathog. 2015;11:e1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Couturier J, Suliburk JW, Brown JM, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS. 2015;29:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lagathu C, Bastard JP, Auclair M, et al. Antiretroviral drugs with adverse effects on adipocyte lipid metabolism and survival alter the expression and secretion of proinflammatory cytokines and adiponectin in vitro. Antivir Ther. 2004;9:911–920. [PubMed] [Google Scholar]
- 22. Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–E31. [DOI] [PubMed] [Google Scholar]
- 23. Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 2014;20:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez de la Concepcion ML, Domingo JC, Domingo P, Giralt M, Villarroya F. Uncoupling protein 1 gene expression implicates brown adipocytes in highly active antiretroviral therapy-associated lipomatosis. AIDS. 2004;18:959–960. [DOI] [PubMed] [Google Scholar]
- 26. Kosmiski L, Sage-El A, Kealey EH, Bessesen DH. Brown fat activity is not apparent in subjects with HIV lipodystrophy and increased resting energy expenditure. Obesity (Silver Spring). 2011;19:2096–2098. [DOI] [PubMed] [Google Scholar]
- 27. Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casey Klockow L, Sharifi HJ, Wen X, et al. The HIV-1 protein Vpr targets the endoribonuclease Dicer for proteasomal degradation to boost macrophage infection. Virology. 2013;444:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]