Abstract
Context:
Somavaratan (VRS-317) is a long-acting form of recombinant human GH under development for children and adults with GH deficiency (GHD).
Objectives:
To determine the optimal somavaratan dose regimen to normalize IGF-1 in pediatric GHD and to evaluate safety and efficacy of somavaratan over 6 months.
Design:
Open-label, multicenter, single ascending dose study followed by 6-month randomized comparison of 3 dosing regimens.
Setting:
Twenty-five United States pediatric endocrinology centers.
Patients:
Naive-to-treatment, prepubertal children with GHD (n = 68).
Intervention(s):
Patients received single sc doses of somavaratan (0.8, 1.2, 1.8, 2.7, 4.0, or 6.0 mg/kg) during the 30-day dose-finding phase, then were randomized to somavaratan 1.15 mg/kg weekly, 2.5 mg/kg twice monthly, or 5.0 mg/kg monthly for 6 months.
Main Outcome Measures:
Safety, pharmacokinetics, pharmacodynamics, 6-month height velocity (HV).
Results:
Somavaratan pharmacokinetics was linearly proportional to dose; dose-dependent increases in the magnitude and duration of IGF-1 responses enabled weekly, twice-monthly or monthly dosing. A single dose of somavaratan sustained IGF-1 responses for up to 1 month. No somavaratan or IGF-1 accumulation occurred with repeat dosing. Mean annualized HVs for somavaratan administered monthly, twice monthly, or weekly (7.86 ± 2.5, 8.61 ± 2.7, and 7.58 ± 2.5 cm/y, respectively) were similar between groups. Adverse events were mostly mild and transient.
Conclusions:
Somavaratan demonstrated clinically meaningful improvements in HV and IGF-1 in prepubertal children with GHD, with no significant differences between monthly, twice-monthly, or weekly dosing.
Successful development of a long-acting recombinant human GH (rhGH) will reduce the frequency of administration, compared with daily rhGH, potentially increasing overall compliance and resulting in improved long-term treatment outcomes (1–5). Somavaratan (VRS-317) is a fusion protein (Molecular Weight 119 kDa) produced in Escherichia coli. The pharmacologically active portion is rhGH and the pharmacologically inactive portions are long chains of natural hydrophilic amino acids (XTEN) (6, 7). Although in vitro potency is reduced by approximately 12-fold compared with rhGH, in vivo potency is enhanced by its delayed clearance and by the resultant prolonged exposure at the target tissues (7). It was demonstrated that, in adults with GH deficiency (GHD), somavaratan has the potential for up to once-monthly dosing. In GHD adults, the elimination half-life of somavaratan was 30–60 times longer than rhGH, and a dose proportional increase in the somavaratan total exposure and IGF-1 responses were observed with persistent IGF-1 responses for 1 month after a single sc dose (8). It is known that rhGH dose requirements differ greatly between adults and children. Adults with GHD receive 2- to 12-μg rhGH/kg · d (9–11), whereas prepubertal GHD children are treated with 22–50 μg/kg · d (12–14). Accordingly, a somavaratan dose-finding trial was conducted for naive-to-treatment, prepubertal GHD children. A somavaratan dose to normalize IGF-1 levels over a month was established in a single ascending dose format and was then followed by a 6-month assessment of safety and efficacy when the selected dose equivalents were administered as monthly, twice-monthly, or weekly regimens.
Patients and Methods
Study design
This phase 1b/2a study consisted of a 30-day single ascending dose phase, followed by a 6-month, randomized, open-label safety and efficacy phase to compare treatment effects of 3 somavaratan dosing regimens. The study was conducted in 25 pediatric endocrinology clinics in the United States. Before any study activity, parents or guardians provided written informed consent and patients provided signed assent, where required. ClinicalTrials.gov identifier is NCT01718041.
Patients
Patients had GHD as confirmed by short stature (height SD score [HT-SDS] ≤−2.0), 2 or more GH stimulation tests (maximal GH level ≤10.0 ng/mL using stimulation agents currently used in each Investigator's practice), IGF-1 SDS less than or equal to −1.0, and a delayed bone age. Patients were excluded if they had previous use of agents that affect growth, presence of significant chronic illness, syndromes, chromosomal aneuploidy, significant gene mutations (other than those that cause GHD), confirmed diagnosis of a named syndrome (eg, Turners, Prader-Willi, Russell Silver, etc), active malignancy, or medication use that could confound the detection of treatment effects. Before receiving study drug, all patients not receiving glucocorticoid replacement therapy underwent adrenal status testing. Patients with known additional pituitary hormone deficiencies received a minimum of 4 weeks of effective treatment before study drug administration.
Study protocol
In the single ascending dose phase of the study, patients were allocated using age balancing (stratified above and below the anticipated median age of 7.5 y at screening) to receive somavaratan (0.8, 1.2, 1.8, 2.7, 4.0, or 6.0 mg/kg) as a single sc injection on day 1, with 8 patients treated per dose level. All safety data were reviewed against protocol-specified stopping criteria before the next higher dosing arm was enrolled. All patients had pharmacokinetics/pharmacodynamics (PK/PD) measurements determined on days 1 (predose), 4 (±1 d), 8, 15, 22, and 30. Antibody assessments were conducted on days 1 (predose), 15, 30, and 60. A 1-compartment model was used to derive PK parameters for somavaratan and to permit simulations of somavaratan concentrations that would occur under repeat-dosing conditions. A PK/PD correlational model was used to relate the average change in IGF-1 SDS to the total somavaratan exposure (area under the plasma concentration vs time curve [AUC]) over the dosing interval. Dose selection for phase 2a was performed by determining the dose that would achieve the appropriate simulated drug exposure to effect a 1.0–1.2 SDS increase in average IGF-1 SDS over the dosing interval and to move the baseline IGF-1 SDS (−1.7) into the normal range, which has been shown historically to result in catch-up growth (see reference 17 below).
Safety and efficacy assessments
After dose selection from the phase 1b stage of the study, 64 patients (44 from phase 1a plus 20 newly enrolled patients) were randomized and age balanced into 3 treatment groups for the phase 2a stage: 1.15 mg/kg weekly, 2.5 mg/kg twice monthly, or 5.0 mg/kg monthly. In this phase 2a stage, all patients were scheduled to receive a total of 30-mg/kg somavaratan over 6 months to permit detection of any treatment group effect. All doses were administered and recorded by a health care professional. Safety and efficacy data were collected before treatment (d 1) and at 1, 3, and 6 months. Bone age was determined for eligibility purposes at screening and for efficacy purposes in phase 2a predose and after 6 months treatment. All radiographs were interpreted by a central reader using the Fels method (LifeSpan). All data were captured in an electronic data capture system (OmniComm). All electronic data capture data entries were verified against source documents by monitors from ResearchPoint Global. Adverse events were collected at each visit and graded using the Common Terminology Criteria for Adverse Events (v4.0). During phase 2a, samples for PK/PD were collected on days 1 (predose), 4 (±1 d), 30, and 90, on 4 (±1) days after the last dose, and on day 180. Samples for antisomavaratan antibodies were collected at baseline and day 90 and 180.
Assays
IGF-1 was determined by liquid chromatography-mass spectroscopy at Quest Diagnostics. IGF-binding protein 3 concentrations and routine safety laboratory results were also determined at Quest. Somavaratan plasma concentration and antisomavaratan antibody serum titers were performed with validated assays at Intertek Pharmaceutical Services. The antibody assessment followed a standard tiered approach of screening, confirmation, and titration according to published guidance (15). The specificity of a confirmed antisomavaratan antibody response to GH and XTEN was also confirmed by immunodepletion with somavaratan or rhGH.
PK/PD analysis
Somavaratan PK parameters were estimated with 1-compartment techniques using WinNonLin professional v5.3 (Pharsight Corp). The AUC for IGF-1 was calculated using the linear trapezoid method. For phase 2a dose selection, correlational models were developed to relate the drug exposure (somavaratan AUC) to change in IGF-1 SDS from baseline over weekly, twice-monthly, and monthly intervals. In phase 2a, the average and increase in average for IGF-1 SDS was calculated as the mean of all samples over 6 months.
Statistical analysis
The safety population consisted of all 48 patients in phase 1b and all 64 patients in phase 2b (44 continuing patients from phase 1b plus 20 newly enrolled patients). The phase 2a efficacy population consisted of 64 patients. The PK/PD population consisted of all patients in each phase. Some PK/PD parameters are also reported for the per protocol group who had complete collections of all planned PK/PD samples.
Descriptive statistics and multivariate analyses were conducted according to a statistical analysis plan developed before database lock. Efficacy and IGF-1 responses were analyzed with analysis of covariance (ANCOVA) with the response parameter as the dependent variable and age and dose regimen and baseline parameter values as independent variables. An exploratory analysis was conducted with this model to determine whether IGF-1 responses were correlated to growth responses. Potential effects of antisomavaratan antibody status were assessed with the height velocity (HV) ANCOVA model.
Results
Patient disposition and characteristics
In phase 1b, 48 patients were enrolled into the 6 dosing groups (8 per group) and 48 patients completed the 30-day study. In phase 2a, 44 of the phase 1b patients elected to continue and 20 additional patients were enrolled. Three phase 2 completers opted to start daily rhGH in lieu of waiting for the start of phase 3 and one moved to a location where no study site was available. In phase 2a, a total of 64 patients were randomized and 63 completed the 6-month treatment period. At screening, all patients were prepubertal with Tanner stage 1 breast development in girls and testicular volumes less than 4.0 mL in boys. The characteristics of the patients are listed in Table 1. There were no clinically meaningful between-group differences in screening characteristics in either study phase. For the phase 2a weekly, twice-monthly, and monthly dosing groups, comparable numbers of patients had previously participated in phase 1b (n = 14, 14, and 16, respectively) with comparable mean phase 1b somavaratan doses (2.7, 2.9, and 3.0 mg/kg, respectively).
Table 1.
Baseline Patient Characteristics at Entry of Study Phase
| Parameter | Phase 1b Dose Group |
Phase 2a Dose Group |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.8 mg/kg (n = 8) | 1.2 mg/kg (n = 8) | 1.8 mg/kg (n = 8) | 2.7 mg/kg (n = 8) | 4.0 mg/kg (n = 8) | 6.0 mg/kg (n = 8) | 1.15 mg/kg Weekly (n = 21) | 2.5 mg/kg Twice Monthly (n = 20) | 5.0 mg/kg Monthly (n = 23) | |
| Mean age, years (SD) | 7.1 (1.8) | 7.0 (2.2) | 7.6 (2.1) | 7.6 (2.7) | 7.0 (2.8) | 6.7 (1.9) | 7.5 (2.3) | 8.0 (2.4) | 8.0 (2.5) |
| Sex | |||||||||
| Male | 3 | 5 | 7 | 6 | 4 | 2 | 10 | 13 | 14 |
| Female | 5 | 3 | 1 | 2 | 4 | 6 | 11 | 7 | 9 |
| Mean bone age, years (SD) | 6.1 (1.5) | 4.6 (2.3) | 5.0 (2.9) | 5.3 (2.0) | 4.5 (2.5) | 4.4 (1.5) | 6.1 (2.5) | 6.6 (2.3) | 6.4 (2.6) |
| Mean HT-SDS (SD) | −2.5 (0.7) | −2.9 (0.6) | −2.9 (0.5) | −2.6 (0.3) | −2.6 (0.8) | −2.8 (0.3) | −2.7 (0.7) | −2.5 (0.4) | −2.3 (0.5) |
| Mean GHmax, ng/mL (SD) | NA | NA | NA | NA | NA | NA | 5.7 (2.0) | 4.9 (2.8) | 5.5 (2.8) |
| Mean IGF-1 SDS (SD) | −1.6 (0.7) | −1.9 (0.6) | −1.8 (0.9) | −1.4 (0.4) | −1.9 (0.9) | −1.6 (0.6) | −1.5 (0.9) | −2.0 (0.8) | −1.7 (0.6) |
GHmax, the maximal GH concentration in 2 GH stimulation tests; HT-SDS, height standard deviation score; IGF-I SDS, insulin growth factor-I standard deviation score; NA, not available; SD, standard deviation. Note: Baseline characteristics represent those at the start of each phase of the study.
Pharmacokinetics
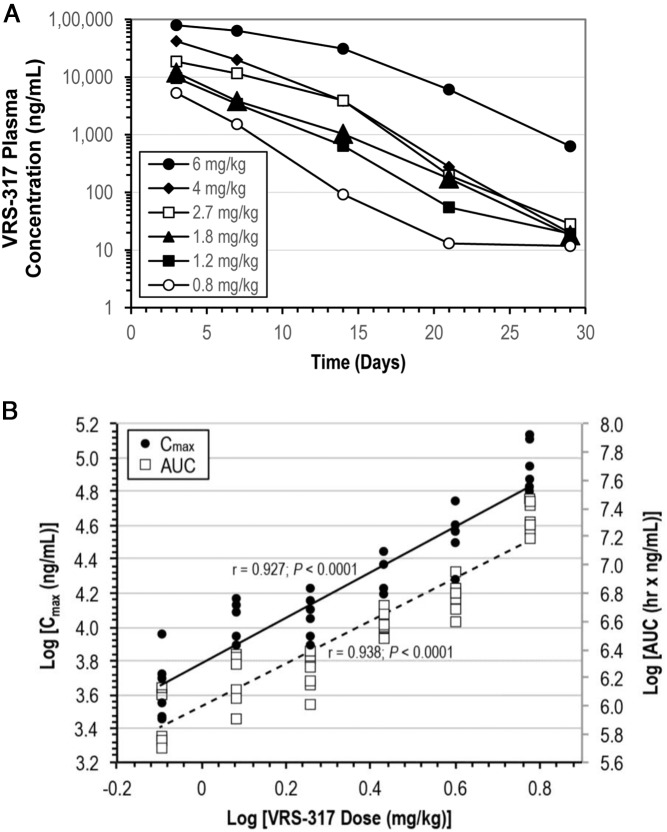
The phase 1b PK data identified somavaratan doses associated with quantifiable plasma somavaratan concentrations persisting for up to 1 month after a single sc dose administration (Figure 1A). The model that best described the PK was a 1-compartment elimination model with first order input. Log transformed PK parameters (Cmax, AUC) were linearly related to log-transformed somavaratan dose (Figure 1B). No gender or age effect was noted on PK parameters. During multiple dosing in phase 2a, analysis of successive PK peaks and troughs showed minimal drug accumulation during the first month for weekly administration and no other significant accumulation for any dose regimen throughout 6 months of dosing (data not shown).
Figure 1.
Somavaratan pharmacokinetic parameters. A, Plasma somavaratan concentrations (ng/mL) after a single sc dose administration (phase 1b). Data shown for 3 days after dose includes patients with samples taken 2 and 4 days after dose. The molecular weight of somavaratan is 119 kDa, with rhGH contributing 22 kDa and the remaining mass contributed by the XTEN construct. B, Linear correlation of log-transformed somavaratan pharmacokinetic parameters (Cmax, AUC) to log-transformed phase 1b somavaratan doses. Cmax and AUC were estimated using a single compartmental model.
Pharmacodynamics
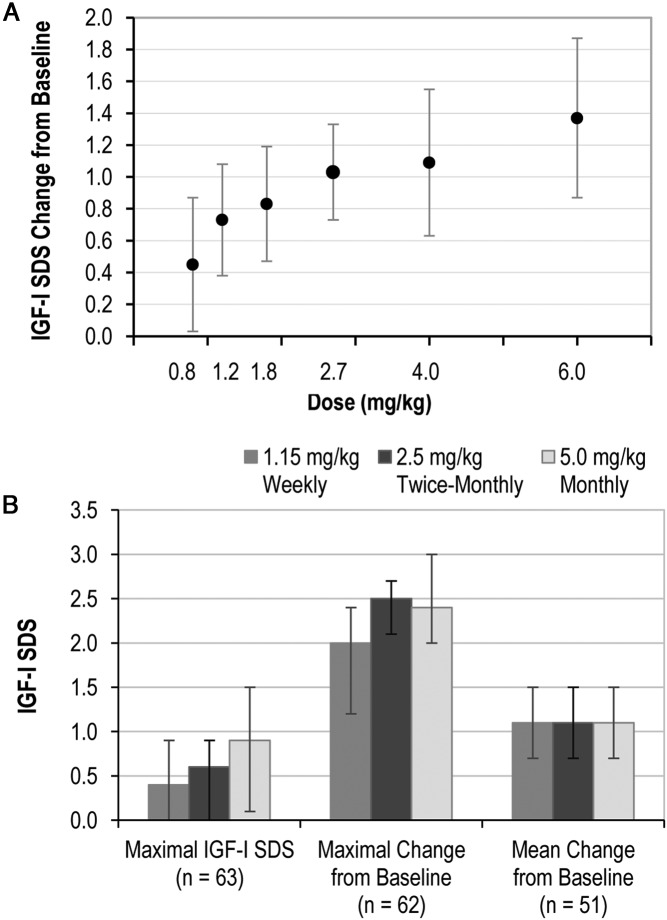
Total IGF-1 concentrations and SD scores were the primary PD parameters. In phase 1b, the mean maximal IGF-1 SDS increased with increasing dose and ranged between 0.5 and 1.0 for the top 3 dosing groups. All patients had relative IGF-1 deficiency at baseline (IGF-1 SDS <−1.0) and the increase from baseline in the 30-day average IGF-1 SDS was proportional to dose (Figure 2A). Only 2 patients had a transient IGF-1 level above the reference range (IGF-1 SDS > 2.0), and no patients had an IGF-1 SDS more than or equal to 3.0. In phase 2a, the mean and interquartile range values for maximal IGF-1 SDS, maximal IGF-1 SDS change from baseline and average IGF-1 SDS change from baseline are shown in Figure 2B. The mean maximal IGF-1 SDS showed a dose frequency effect with the lowest values in the weekly dosing group and highest in the monthly group. There were no dose frequency effects on other phase 2a PD parameters. Importantly, a substantial monthly average in IGF-1 SDS change from baseline was demonstrated for weekly, twice-monthly, and monthly dosing. Six patients, all in the monthly dosing group, had transient IGF-1 SDS more than 2.0, and no patients had an IGF-1 SDS more than 3.0. After the first month and during 6 months of somavaratan treatment, there were no meaningful differences in successive IGF-1 SDS peaks or troughs, indicating no accumulation of somavaratan and IGF-1 and no decrease in IGF-1 responses with repeat dosing at these intervals (data not shown).
Figure 2.
IGF-1 SDS. A, Dose proportional increase in the average IGF-1 SDS change from baseline in phase 1b. The change in IGF-1 SDS was computed as the numerical average of samples drawn at days 4, 8, 15, 22, and 30. B, Phase 2a IGF-1 responses: maximal IGF-1 SDS, maximal change from baseline, and mean change from baseline. Values are means, and bars represent interquartile ranges. Changes were computed from the average values on days 4, 8, 15, 22, and 30. Sample sizes represent patients who had complete sample collections and no missing samples.
Efficacy
The mean ± SD change in height from predose to completion of 6 months of treatment in phase 2a is reported as annualized HV and was 7.86 ± 2.46 cm/y for the monthly group, 8.61 ± 2.67 cm/y for the twice-monthly group, and 7.58 ± 2.53 cm/y for the weekly group. In the HV ANCOVA, responses were greatest in younger children and those with lower baseline IGF-1 SDS (model r2 = 0.22, P = .0044). Treatment group, baseline HT-SDS, GH stimulation test results and gender were not significant factors for HV. The corresponding mean ± SD 6-month changes in HT-SDS were 0.28 ± 0.22 (monthly), 0.33 ± 0.23 (twice monthly), and 0.24 ± 0.22 (weekly). By ANCOVA, changes were greatest in those with lowest baseline IGF-1 SDS, and no treatment group effect was observed. The mean ± SD 6-month changes in bone age (in y) were 0.40 ± 0.42 (monthly), 0.39 ± 0.37 (twice monthly), and 0.39 ± 0.23 (weekly). No treatment group or other effects on bone age were noted in ANCOVA. These changes, and those for weight and body mass index, are summarized in Table 2. Little change was noted in pubertal status of these prepubertal patients. No individual changes in pubic hair stage or mean testicular volume were observed; however, 2 female patients in the weekly group proceeded to Tanner stage 2 breast development.
Table 2.
Mean Changes in Efficacy Parameters After 6 Months of Somavaratan Treatment
| Parameter | 5.0 mg/kg Monthly (n = 23) | 2.5 mg/kg Twice Monthly (n = 20) | 1.15 mg/kg Weekly (n = 21) |
|---|---|---|---|
| Mean annualized HV, cm/y (SD)a | 7.86 (2.5) | 8.61 (2.7) | 7.58 (2.5) |
| Change in HT-SDS (SD) | 0.28 (0.22) | 0.33 (0.23) | 0.24 (0.22) |
| Mean change in BMI, kg/m2 (SD) | 0.96 (0.72) | 0.84 (0.73) | 1.02 (0.72) |
| Mean change in bone age, years (SD) | 0.40 (0.42) | 0.39 (0.37) | 0.39 (0.23) |
BMI, body mass index; HT-SDS, height standard deviation score; HV, height velocity; SD, standard deviation.
Values are for the intention-to-treat population. For the 1 patient in the twice-monthly dose group who did not complete the 6-month study, annualized HV was imputed from the last observation of change in HT-SDS.
Safety
There were no related serious adverse events or unexpected adverse events. Related adverse events were primarily mild (grade 1) and transient and of the type expected when rhGH is initiated in GHD children naive to rhGH treatment. With more than 1300 injections administered in the phase 2a stage, injection site pain was reported in approximately half of all patients and was mild and transient (<30 min). Nodule formation or lipoatrophy was not observed at injection sites. The adverse events for phase 1b and 2a are shown in Table 3. One patient withdrew from the twice-monthly group in phase 2a after experiencing urticaria.
Table 3.
Treatment-Related Adverse Events
| Phase 1b (n = 48); n (%) | Phase 2a (n = 64); n (%) | |
|---|---|---|
| Any adverse event | 23 (48) | 34 (53) |
| Injection site pain | 16 (33) | 31 (48) |
| Headache | 5 (10) | 2 (3) |
| Arthralgia | 4 (8) | 2 (3) |
| Injection site discomfort | 2 (4) | 0 |
| Pain in extremity | 2 (4) | 2 (3) |
| Injection site erythema | 2 (4) | 6 (9) |
| Dizziness | 1 (2) | 1 (2) |
| Increased appetite | 1 (2) | 0 |
| Malaise | 1 (2) | 0 |
| Myalgia | 1 (2) | 0 |
| Hunger | 1 (2) | 0 |
| Maculopapular and urticarial rash | 0 | 2 (3) |
| Injection site hematoma | 0 | 1 (2) |
| Injection site reaction | 0 | 1 (2) |
| Presyncope | 0 | 1 (2) |
| Back pain | 0 | 1 (2) |
| Bone pain | 0 | 1 (2) |
| Muscle spasms | 0 | 1 (2) |
| Increased blood glucose | 0 | 1 (2) |
| Decreased free T4 | 0 | 1 (2) |
Antisomavaratan antibodies were detected in a screening assay and their specificity confirmed by inhibition by adding somavaratan and rhGH. In a planned post hoc analysis, the antibody status (positive at any time vs negative at all times) was added as a covariate in the HV ANCOVA. No significant effect of antibody status on HV was observed.
Discussion
Somavaratan is an investigational rhGH fusion protein in clinical development and is designed to reduce the frequency of administration of daily rhGH to up to once-monthly somavaratan in children and adults with GHD. We present the results of the first randomized trial in GHD children. Somavaratan was designed to achieve up to once-monthly dosing with the anticipation that a reduced burden of dosing schedules might alleviate challenges of treatment adherence with daily rhGH (1, 2, 16) and avoid the diminished efficacy associated with decreased adherence (5). Somavaratan is rhGH with XTEN sequences of hydrophilic, naturally occurring amino acids bound to the N and C termini (6). Somavaratan was designed to have reduced renal clearance through an increase in hydrodynamic size and reduced clearance mediated by the GH receptor through reduced binding affinity (7). Somavaratan plasma half-life in GHD adults was 131 hours, a 30- to 60-fold increase over sc-injected rhGH, thus making possible somavaratan administration at up to monthly intervals (8).
In children, weight-based rhGH dose requirements are greater than in adults (9). Accordingly, the somavaratan pediatric GHD development program began with a single ascending dose study (phase 1b), with dose ranging beginning at the highest dose proven safe and effective in GHD adults (8). The PK/PD properties in GHD children were similar to those observed in GHD adults. PK parameters (Cmax, AUC) were directly proportional to dose with no gender effect. Somavaratan exposures and IGF-1 responses persisted for up to 1 month and were dose dependent. The 30-day average for IGF-1 responses was also proportional to dose. This PK/PD relationship enabled the creation of a correlational model indicating a direct relationship between somavaratan total exposure (AUC) and average increase in IGF-1 SDS. The PK dose linearity of somavaratan enabled testing of effects of dose frequency on IGF-1 SDS increases, as well as HV after 6 months of treatment. All patients in the 3 phase 2a dose groups were scheduled to receive a total somavaratan dose of 30 mg/kg over 6 months, and the study was balanced across treatment groups to enable evaluation of dose frequency. With the exception of a higher mean maximal somavaratan concentration and mean maximal IGF-1 SDS, no dose frequency effects of somavaratan were noted on PK, PD, safety, or growth response variables. Somavaratan concentrations were observed to persist for the duration of weekly, twice-monthly, and monthly intervals and were associated with substantial increases of IGF-1 within anticipated levels (17, 18). At observed somavaratan concentrations, a minimal number of IGF-1 SDS transiently exceeded 2.0 (n = 6 during repeat dosing in phase 2a), and none exceeded 3.0.
Safety events were mild and transient and consistent with adverse events reported in labels for rhGH products (19, 20). No related serious adverse events or unexpected adverse events were observed. In particular, the dose administrations were well tolerated. There were no cases of injection site lipoatrophy or nodule formation. Routine funduscopy was performed at all visits and no signs of increased intracranial pressure (eg, papilledema) were noted. Antisomavaratan antibodies had no impact on safety, PK/PD, or efficacy.
The pattern of catch up growth with rhGH treatment in children, as determined by HV and change in height standard deviation scores, has an inverse relationship to age and a direct relationship to dose (17, 21, 22). Midparental target height and markers of disease severity have also been significant predictors of growth response (12, 22, 23). Similar age dependencies have been observed for other rhGH-treated patient groups, including small for gestational age (24), idiopathic short stature (25) and for rhGH- or rhIGF-1-treated children with short stature, low IGF-1, and normal stimulated GH treated (17, 26). The age-dependence of HV with somavaratan was as anticipated. Before puberty, younger children grow more quickly than older children (27, 28). In accord with these findings, growth responses to somavaratan are inversely related to age and further related to a marker of disease severity, in this case, the baseline IGF-1 SDS. Lower baseline IGF-1 SDS predicts a greater treatment response.
Age-related mean growth velocities are less in children with moderate rather than severe GHD (12). During phase 1b, the median baseline HT-SDS ranged from −2.3 for the 4.0-mg/kg group to −2.8 for the 1.2-, 1.8-, and 6.0-mg/kg groups, and the median baseline IGF-1 SDS ranged from −1.3 for the 0.8-mg/kg cohort to −1.8 for the 1.2-mg/kg cohort. During phase 2a, the maximal GH stimulation test ranged from a mean of 4.9 ng/mL for the 2.5-mg/kg twice-monthly cohort to 5.5 for the 5.0-mg/kg monthly cohort. All of these were indicative of a moderate degree of GHD. Nevertheless, mean annualized HVs on somavaratan (∼7.5–8.5 cm/y) were substantially above mean pretreatment HVs (4.52 cm/y, collected from 38 patients), with no significant differences between dosing regimens.
Overall, somavaratan demonstrated clinically meaningful improvements in HV and IGF-1 in prepubertal children with GHD, with mild and transient adverse events.
Acknowledgments
We thank the patients and their caregivers; Leslie Jones and Morgan Seaman of ResearchPoint Global and Naureen Sheikh, PhD, of Versartis, Inc, for their support in clinical operations; and Ingrid Koo, PhD, for editorial assistance in the preparation of the manuscript.
This work was supported by Versartis, Inc.
Disclosure Summary: E.H. and G.M.B. are employees of Versartis, Inc; W.V.M., H.J.N., G.B.K., B.S.M., and D.R. were investigators for the trial; J.A.M. is a pharmacology consultant to Versartis, Inc; J.L.C. is a consultant to Versartis, Inc and was an employee of Versartis, Inc at the time of the study. D.N. and J.A.M. authors have nothing to disclose.
Footnotes
- ANCOVA
- analysis of covariance
- AUC
- area under the plasma concentration vs time curve
- GHD
- GH deficiency
- HT-SDS
- height SD score
- HV
- height velocity
- PK/PD
- pharmacokinetics/pharmacodynamics
- rhGH
- recombinant human GH.
References
- 1. Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14:143–154. [DOI] [PubMed] [Google Scholar]
- 2. Desrosiers P, O'Brien F, Blethen S. Patient outcomes in the GHMonitor: the effect of delivery device on compliance and growth. Ped Endocr Rev. 2005;2(suppl 3):327–331. [PubMed] [Google Scholar]
- 3. Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93:147–148. [DOI] [PubMed] [Google Scholar]
- 4. Noble SE, Leyland K, Findlay CA, et al. School based screening for hypothyroidism in Down's syndrome by dried blood spot TSH measurement. Arch Dis Child. 2000;82:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cutfield SW, Derraik JG, Reed PW, Hofman PL, Jefferies C, Cutfield WS. Early markers of glycaemic control in children with type 1 diabetes mellitus. PloS One. 2011;6:e25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schellenberger V, Wang CW, Geething NC, et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotechnol. 2009;27:1186–1190. [DOI] [PubMed] [Google Scholar]
- 7. Cleland JL, Geething NC, Moore JA, et al. A novel long-acting human growth hormone fusion protein (vrs-317): enhanced in vivo potency and half-life. J Pharm Sci. 2012;101:2744–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuen KC, Conway GS, Popovic V, et al. A long-acting human growth hormone with delayed clearance (VRS-317): results of a double-blind, placebo-controlled, single ascending dose study in growth hormone deficient adults. J Clin Endocrinol Metab. 2013;98:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vance ML, Mauras N. Growth hormone therapy in adults and children. New Engl J Med. 1999;341:1206–1216. [DOI] [PubMed] [Google Scholar]
- 10. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609. [DOI] [PubMed] [Google Scholar]
- 11. Chihara K, Kato Y, Kohno H, et al. Efficacy and safety of growth hormone (GH) in the treatment of adult Japanese patients with GH deficiency: a randomised, placebo-controlled study. Growth Horm IGF Res. 2006;16:132–142. [DOI] [PubMed] [Google Scholar]
- 12. Ranke MB, Lindberg A, KIGS International Board. Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95:1229–1237. [DOI] [PubMed] [Google Scholar]
- 13. de Lind van Wijngaarden RF, Siemensma EP, Festen DA, et al. Efficacy and safety of long-term continuous growth hormone treatment in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2009;94:4205–4215. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka T. Growth hormone treatment in Japan: past, present, and future. Pediatr Endocrinol Rev. 2012;10(suppl 1):89–97. [PubMed] [Google Scholar]
- 15. Mire-Sluis AR, Barrett YC, Devanarayan V, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289:1–16. [DOI] [PubMed] [Google Scholar]
- 16. Smith SL, Hindmarsh PC, Brook CG. Compliance with growth hormone treatment–are they getting it? Arch Dis Child. 1993;68:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen P, Bright GM, Rogol AD, Kappelgaard AM, Rosenfeld RG, American Norditropin Clinical Trials Group. Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002;87:90–98. [DOI] [PubMed] [Google Scholar]
- 18. Cohen P, Rogol AD, Weng W, et al. Efficacy of IGF-based growth hormone (GH) dosing in nonGH-deficient (nonGHD) short stature children with low IGF-I is not related to basal IGF-I levels. Clin Endocrinol. 2013;78:405–414. [DOI] [PubMed] [Google Scholar]
- 19. Novo Nordisk. Norditropin (somatropin). Package insert. Plainsboro, NJ: Novo Nordisk; Revised January, 2015. [Google Scholar]
- 20. Pfizer. GENOTROPIN (somatropin). Package insert. New York, NY: Pfizer; Revised May, 2015. Available at http://labeling.pfizer.com/ShowLabeling.aspx?id=577 Accessed November 9, 2015. [Google Scholar]
- 21. Blethen SL, Compton P, Lippe BM, Rosenfeld RG, August GP, Johanson A. Factors predicting the response to growth hormone (GH) therapy in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 1993;76:574–579. [DOI] [PubMed] [Google Scholar]
- 22. Ranke MB, Lindberg A, Chatelain P, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999;84:1174–1183. [DOI] [PubMed] [Google Scholar]
- 23. Ranke MB, Lindberg A, Albertsson-Wikland K, Wilton P, Price DA, Reiter EO. Increased response, but lower responsiveness, to growth hormone (GH) in very young children (aged 0–3 years) with idiopathic GH Deficiency: analysis of data from KIGS. J Clin Endocrinol Metab. 2005;90:1966–1971. [DOI] [PubMed] [Google Scholar]
- 24. Ranke MB, Lindberg A, Cowell CT, et al. Prediction of response to growth hormone treatment in short children born small for gestational age: analysis of data from KIGS (Pharmacia International Growth Database). J Clin Endocrinol Metab. 2003;88:125–131. [DOI] [PubMed] [Google Scholar]
- 25. Ranke MB, Lindberg A, Price DA, et al. Age at growth hormone therapy start and first-year responsiveness to growth hormone are major determinants of height outcome in idiopathic short stature. Hormone Res. 2007;68:53–62. [DOI] [PubMed] [Google Scholar]
- 26. Midyett LK, Rogol AD, Van Meter QL, Frane J, Bright GM, MS301 Study Group. Recombinant insulin-like growth factor (IGF)-I treatment in short children with low IGF-I levels: first-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2010;95:611–619. [DOI] [PubMed] [Google Scholar]
- 27. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. [DOI] [PubMed] [Google Scholar]
- 28. Kelly A, Winer KK, Kalkwarf H, et al. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99:2104–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]