Abstract
Context:
Poor glycemic control in individuals with type 1 diabetes (T1D) is associated with both micro- and macrovascular complications, but good glycemic control does not fully prevent the risk of these complications.
Objective:
The objective of the study was to determine whether T1D with good glycemic control have persistent abnormalities of metabolites and pathways that exist in T1D with poor glycemic control.
Design:
We compared plasma metabolites in T1D with poor (glycated hemoglobin ≥ 8.5%, T1D[−] and good (glycated hemoglobin < 6.5%, T1D[+]) glycemic control with nondiabetic controls (ND).
Setting:
The study was conducted at the clinical research unit.
Patients or Other Participants:
T1D with poor (n = 14), T1D(−) and good, T1D(+) (n = 15) glycemic control and matched (for age, sex, and body mass index) ND participants were included in the study.
Intervention(s):
There were no intervention.
Main Outcome Measure(s):
Comparison of qualitative and quantitative profiling of metabolome was performed.
Results:
In T1D(−), 347 known metabolites belonging to 38 metabolic pathways involved in cholesterol, vitamin D, tRNA, amino acids (AAs), bile acids, urea, tricarboxylic acid cycle, immune response, and eicosanoids were different from ND. In T1D(+),154 known metabolites belonging to 26 pathways including glycolysis, gluconeogenesis, bile acids, tRNA biosynthesis, AAs, branch-chain AAs, retinol, and vitamin D metabolism remained altered from ND. Targeted measurements of AA metabolites, trichloroacetic acid, and free fatty acids showed directional changes similar to the untargeted metabolomics approach.
Conclusions:
Comprehensive metabolomic profiling identified extensive metabolomic abnormalities in T1D with poor glycemic control. Chronic good glycemic control failed to normalize many of these perturbations, suggesting a potential role for these persistent abnormalities in many complications in T1D.
Type 1 diabetes (T1D) is characterized by absolute insulin deficiency and insulin treatment to achieve euglycemia results in substantial glucose variability (1, 2). Currently, glycated hemoglobin (HbA1c) is universally used as the measure of glycemic control over a period of about 3 months. However, glucose data in T1D are not normally distributed, and thus, HbA1c does not convey accurate information about hyper- and hypoglycemia (3, 4). Significant glucose variability continues to be a problem, even with the most advanced options of insulin delivery for diabetes such as the low-glucose insulin suspend system (5). Improved HbA1c significantly reduces the risk of target organ complications, but especially macrovascular complications continue to occur, albeit at reduced rates (4). The risk of complications is also reduced by other factors like weight reduction, treatment of high blood pressure, and dyslipidemia. Even accounting for these risk factors, a residual risk continues with increased mortality and decreased lifespan in T1D (6). Here we measured plasma metabolites to determine whether good glycemic control normalizes all metabolic abnormalities and, if not, which metabolites and metabolomic pathways remain abnormal. Such information may pave the way for future research to determine whether remaining metabolic abnormalities may explain the increase in mortality in T1D on insulin treatment.
Metabolomics involves the simultaneous comprehensive measurement of multiple small molecules or metabolites in biological fluids representing a fingerprint of cellular metabolism. Metabolites are the end result of variability in genomic, transcriptomic, and proteomic processes in a biological system (7–11). Insulin is the predominant hormone that regulates cellular fuel metabolism. Insulin deficiency in T1D causes perturbations in plasma metabolites, linking several canonical pathways and biochemical processes (12–14). Because glucose has been traditionally used as a biomarker of insulin action in vivo, all diabetic complications are assessed in the context of glycemic control. Metabolites in plasma represent wider impact of insulin on cellular metabolism and therefore more likely to represent metabolic dysregulation related to diabetes. Plasma metabolome may therefore include more important biomarkers of diabetic control than glucose and potentially correlate better with complications. Such an approach may reveal possible pathways involved in the pathogenesis of complications in T1D that could yield targets for the development of novel therapies. Metabolomic analysis can be applied at two levels to assess metabolic control in diabetes; first, an untargeted approach to identify the metabolites and pathways that are altered by insulin treatment followed by a quantitative approach targeting specific pathways. Untargeted metabolomics is the comprehensive profiling of all measurable metabolite components including chemically unknown metabolites in a biological sample. We have previously reported differential regulations of plasma metabolites by acute insulin withdrawal for 8 hours in T1D using a targeted (12) and an untargeted approach (13).
Thus, the main goals of the current study were to identify plasma metabolites and metabolic pathways that are altered in T1D individuals during chronic poor glycemic control, T1D(−), compared with nondiabetic controls (NDs) and to determine whether good glycemic control in T1D, T1D(+), could correct these abnormalities in comparison with NDs. In the current study, we used ultraperformance liquid chromatography coupled with time-of-flight mass spectrometry (UPLC-ToF MS)-based untargeted metabolomic analysis of fasting plasma to identify the metabolic signature of metabolites and molecular pathways in study groups. Targeted quantitative analysis of selected metabolite panels was performed by Liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry platforms.
Materials and Methods
The Mayo Clinic Institutional Review Board approved this study.
Participants
Fifteen T1D participants with good glycemic control (HbA1c ≤ 6.5%, T1D[+]) and 14 T1D with poor glycemic control (HbA1c ≥ 8.5%, T1D[−]) were studied. Each T1D participant was matched to a ND participant of similar age, body mass index (BMI), and sex. Thus, we studied a total 58 participant. Informed consent approved by Mayo Clinic Institutional Review Board was obtained from all participates. Volunteer eligibility was confirmed during an initial screening visit by obtaining a detailed medical history, physical examination, safety and screening blood tests, and urinalysis.
Study protocol
Participants were admitted to the Clinical Research Unit at St Mary's Hospital (Rochester, Minnesota) the evening before the study and spent overnight in the Clinical Research Unit. The participants were given a standard meal on the evening of the admission after which they fasted overnight. Participants with T1D were treated with insulin as per their usual individual programs. At 5:00 am after an overnight fast, baseline blood samples were collected from study participants. Plasma samples were stored at −80°C until analysis.
Untargeted metabolomic analysis
One hundred microliters of plasma sample were analyzed by UPLC-ToF MS both in positive and negative electrospray ionization modes in the mass range of mass to charge ratio (m/z) 100–1200. The metabolite extraction and instrument settings were performed as described previously with minor modifications (13, 15). Metabolite peak intensities and differential regulation of metabolites between groups were determined as described (13, 15). Each sample was normalized to the median of the baseline and log2 transformed. Default settings were used with the exception of signal to noise ratio threshold, mass limit (0.0025 U), and time limit (9 sec). Putative identification of each metabolite was done based on accurate mass (m/z) against the Metlin database using a detection window of 7 ppm or less. The identified metabolites are annotated as Chemical Abstracts Service, Kyoto Encyclopedia of Genes and Genomes, Human Metabolome Project database, and LIPID MAPS identifiers. The differentially expressed metabolites are analyzed for pathway enrichment using MetaCore (Genego) (13, 15).
Targeted analysis
Quantitative measurements of free fatty acid (FFAs), vitamin D, and 45 amino acid metabolites were performed by tandem mass spectrometry as described previously (12, 14, 16). Plasma levels of tricarboxylic acid (TCA) cycle metabolites were measured after the derivatization with N-methyl-N-(t-butyldimethylsilyl)-trifluoroacetamide + 1% t-butyldimethylchlorosilane by gas chromatography/mass spectrometry under electron impact and selected ion monitoring conditions (17). All quantitative measurements using gas chromatography/mass spectrometry and LC-MS/MS were done against 12-point calibration curves that underwent the same derivatization with internal standard.
Statistical analysis
Metabolites detected in at least 50% or greater of the samples in any of the study groups were selected for differential expression analyses (13). After normalization, an unpaired Student's t test analysis was performed to compare the differentially expressed metabolites between two study groups for qualitative measurements. To address the false discovery rates from multiple comparisons, bootstrapping analysis (0.05) was applied on each pair of analysis and was estimated by Q values (18, 19). Unsupervised principal components analysis (PCA) and hierarchical clustering analysis were performed to reduce the dimensionality of the qualitative data and to reveal clustering between the study groups: T1D(−) vs ND and T1D(+) vs ND (Figure 1).
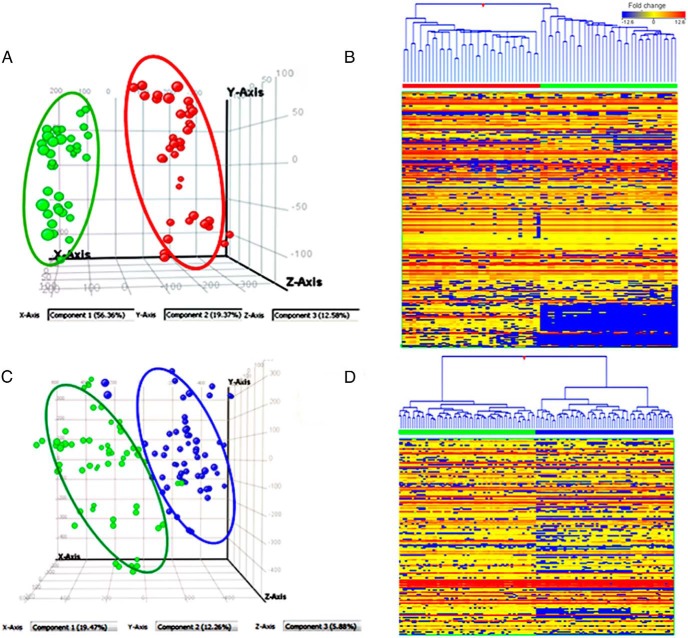
Figure 1.
A, PCA showing group separation between poor glycemic control T1D, T1D(−) in red ( ), and matched ND in green (
), and matched ND in green ( ). B, Heat map of T1D(−) in red bar and ND in green bar on top panel. Heat map shows poor glycemic control (HbA1c of > 8.5%) in T1D causes perturbations in more than 347 known metabolites including many amino acids, organic acids, nucleotides, lipids including eicosanoids, vitamin D, and unknown metabolites. C, PCA of good glycemic control T1D, T1D(+) (●), and matched ND (●) based on their metabolic differences. D, Heat map of T1D(+) in blue bar vs ND in green bar on top panel. Heat map displays that more than 234 metabolites are altered despite good glycemic control (HbA1c of < 6.5%) in T1D. Each row represents a metabolite, and each column depicts a subject. The up- and down-regulation in fold change are denoted as red and blue, respectively, and yellow depicts no significant change.
). B, Heat map of T1D(−) in red bar and ND in green bar on top panel. Heat map shows poor glycemic control (HbA1c of > 8.5%) in T1D causes perturbations in more than 347 known metabolites including many amino acids, organic acids, nucleotides, lipids including eicosanoids, vitamin D, and unknown metabolites. C, PCA of good glycemic control T1D, T1D(+) (●), and matched ND (●) based on their metabolic differences. D, Heat map of T1D(+) in blue bar vs ND in green bar on top panel. Heat map displays that more than 234 metabolites are altered despite good glycemic control (HbA1c of < 6.5%) in T1D. Each row represents a metabolite, and each column depicts a subject. The up- and down-regulation in fold change are denoted as red and blue, respectively, and yellow depicts no significant change.
We used standard summary statistics (mean ± SD) to compare the targeted variables between study groups. All the subjects were combined, and a pairwise partial Pearson correlation controlling for age, BMI, and sex was performed using 48 targeted metabolites, glucose, and HbA1c measurements of each individual. Metabolite levels were log2 transformed for the calculation of partial correlations, and missing values were excluded for analysis. All partial Pearson correlations were calculated using R version 3.0 and the ppcor package.
Results
Clinical characteristic of study participants (Table 1)
Table 1.
Baseline Characteristics of Study Participants
| Traits | T1D(−) (n = 14) | ND (n = 14) | T1D(+) (n = 15) | ND (n = 15) |
|---|---|---|---|---|
| Age | 33 ± 13.2 | 33.7 ± 12.2 | 34.1 ± 0.5 | 34.5 ± 12.3 |
| Sex, M/F | 7/7 | 7/7 | 7/8 | 7/8 |
| BMI | 24.41 ± 3.3 | 24.6 ± 2.6 | 25.9 ± 3.1 | 25.6 ± 3.5 |
| HbA1c, % | 10.04 ± 1.27a | 5.2 ± 0.4 | 6.3 ± 0.5a | 5.4 ± 0.3 |
| Glucose, mg/dL | 208.23 ± 79.5a | 95.2 ± 5.8 | 165.6 ± 58.5b | 93.5 ± 7.8 |
| LDL-cholesterol, mg/dL | 137.07 ± 15.8 | 147.1 ± 45.6 | 124.1 ± 58.8 | 144.1 ± 32.2 |
| Triglycerides, mg/dL | 71.9 ± 19.5 | 111.6 ± 57 | 92.7 ± 61.6 | 102.5 ± 89.5 |
| HDL-cholesterol, mg/dL | 44.6 ± 6.8 | 42.3 ± 12.3 | 42.5 ± 15.1 | 41.7 ± 10.2 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < .001, comparisons are between T1D(− vs ND and T1D(+) vs ND.
P < .05, comparisons are between T1D(− vs ND and T1D(+) vs ND.
Of the 58 participants, ethnic distribution for the study was mostly Caucasian with one each being Asian Indian and Hispanic. As expected, the Hb1Ac and fasting glucose levels in both T1D(−) and T1D(+) were significantly higher than their matched ND controls (P ≤ .001).
Comparison of metabolomic profiling
The UPLC-ToF MS-based untargeted metabolomics profiling platform could detect 1277 metabolite features in plasma of which 422 metabolites are identified (33% of list of metabolites) based on putative identification by accurate mass against the Metlin database (data not shown). The list of identified compounds includes metabolites, drug-derived compounds, peptides, food additives, and their metabolic by-products. For data overview, PCA and hierarchical clustering analysis were used as an unsupervised statistical method to study the metabolomic differences between ND and T1D(−) and (T1D+) (Figure 1). T1D(−) and ND and T1D(+) and ND were distinctly separated in the PCA and heat map based on their inherent metabolic differences as shown in Figure 1, A and C, and Figure 1, B and D, respectively.
T1D(−) vs ND
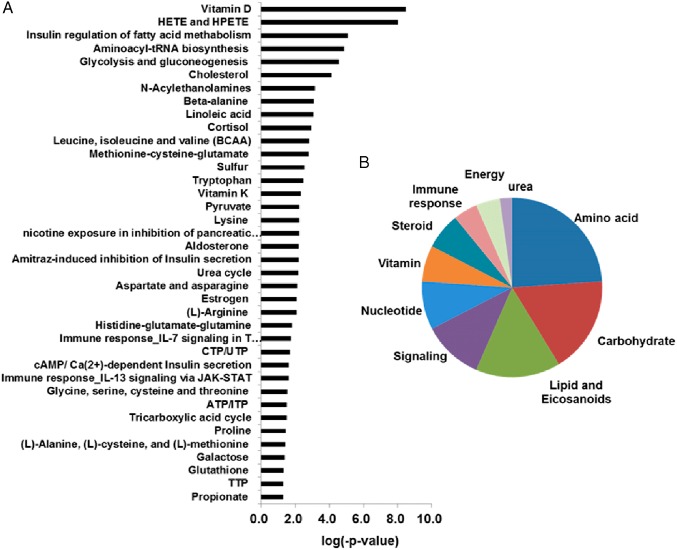
Poor glycemic control in T1D showed significant alterations of 713 metabolites, of which 347 known (P < .05; FC ± 1.20) and 366 unknown (P < .05; FC ± 2.0) metabolites (Figure 1B; Supplemental Table 1). The metabolite classes that were found to be significantly altered between T1D(−) and ND include carbohydrates; amino acid metabolites; branch-chain amino acids (BCAAs); nucleotides; bile acids; ketoacids; and short-chain fatty acids including eicosanoids, ceramides, vitamin D metabolites, corticosteroids, and other steroids. Pathway analysis based on the identified metabolites showed perturbations in more than 38 canonical pathways (Figure 2A) of which 29% involved amino acid metabolism followed by 21% carbohydrate, 18% lipid, 13% cell surface signaling, and 11% nucleotide metabolism (Figure 2B).
Figure 2.
A, Altered canonical pathways in T1D(−) in comparison with ND. B, Percentage of enrichment in pathways in cellular metabolism of T1D(−). The significance of the pathways was evaluated using P values and a false discovery rate of less than 0.10.
T1D(−) showed an expected elevation of carbohydrate metabolites: glucose, glucosamine, lactaldehyde, methylglyoxal, lactate, acetate, and acetoacetate. Glycolytic metabolites such as pyruvate, dihydroxyacetone phosphate, and TCA cycle metabolites were decreased. 1,5-Anhydroglucitol (1,5-AG), which is a marker of short-term glycemic control and shows negative association with diabetes, was found to be significantly lower in T1D(−) (20).
Amino acid metabolism and tRNA biosynthesis pathway were also associated with poor glycemic control. Whereas BCAA, lysine, proline, serine, N,N dimethyl histidine, and methionine were elevated with higher HbA1c, alanine and homoarginine decreased in T1D(−).
The plasma levels of short-chain fatty acids such as acetic, acetoacetic acid, propionic and butyric acid, ketoacids like α-hydroxybutyrate, adipic acid, and 2-hydroxyadipic acid were elevated in T1D(−). 2-Oxovaleric acid or α-ketoisovaleric acid, which is a precursor to leucine and valine synthesis, and 2-oxo-caproicacid were elevated (15), whereas α-hydroxyisovalerate and 2-methyl-3-ketovaleric acid were decreased. 2-Hydroxyisovaleric acid has been identified in the urine of patients with lactic and ketoacidosis. 2-Hydroxyisovaleric acid originates mainly from ketogenesis and from the metabolism of BCAAs (21).
Lipids with longer-chain fatty acids were increased but primarily the FFAs and lipids with shorter-chain fatty acids (5-dodecenoate [12:1n7], heptanoate [7:0], and pelargonate [9:0]) are decreased in T1D(−) relative to ND. This contrasting pattern of association may reflect alterations in triglyceride lipolysis, which could either contribute to or be a result of the dysregulation of glucose metabolism.
Lipid inflammatory mediators like eicosanoid prostaglandins (prostaglandin [PG] F2) and leukotrienes including 12-hydroxyeicosatetraenoic acid and (8Z,11Z,14Z)-5,6-dihydroxyicosa-8,11,14-trienoic acid increased significantly, likely as an indication of an increased inflammatory response. Vitamin D and cortisone metabolism were also severely affected by poor glycemic control, resulting in elevated levels of corticosteroids, 5a-tetrahydrocortisol, and cortisone acetate. Quantitative measurements of total 25-hydroxyvitamin D, 1,25-hydroxyvitamin D, and 24,25-hydroxyvitamin D showed lower levels in T1D(−) than ND but without statistical significance (Table 2).
Table 2.
Quantitative Measurements of Selected Metabolites in Plasma
| Metabolites | Class | T1D(−) ± SD | ND ± SD | P Valuea | T1D(+) ± SD | ND ± SD | P Valueb | |
|---|---|---|---|---|---|---|---|---|
| 1 | Linolenic | NEFA | 9.5 ± 9.9 | 10.4 ± 10.8 | .82 | 15.1 ± 8.9 | 11.3 ± 10 | .28 |
| 2 | Myristic | NEFA | 8.6 ± 3.5 | 11.3 ± 7 | .21 | 12.1 ± 6.7 | 10 ± 4.2 | .31 |
| 3 | Palmitoleic | NEFA | 14.5 ± 6.8 | 18.2 ± 10 | .26 | 26 ± 15.4 | 22.9 ± 14.8 | .57 |
| 4 | Arachidonic | NEFA | 4.1 ± 2.2 | 3.8 ± 1.6 | .71 | 4.1 ± 1 | 4.2 ± 1 | .77 |
| 5 | Linoleic | NEFA | 110.9 ± 65.6 | 106.7 ± 73.8 | .87 | 182.9 ± 95.5 | 131.5 ± 86.7 | .13 |
| 6 | Palmitic | NEFA | 99.8 ± 47 | 117.8 ± 61.4 | .39 | 145 ± 63.5 | 116.1 ± 31.1 | .12 |
| 7 | Oleic | NEFA | 147.5 ± 68.8 | 149.5 ± 68 | .94 | 229.6 ± 101 | 163.1 ± 62.5 | .04 |
| 8 | Elaidic | NEFA | 9.1 ± 2.9 | 9.1 ± 3.6 | .98 | 14.9 ± 5.6 | 11.1 ± 3.4 | .03 |
| 9 | Stearic | NEFA | 39.7 ± 18.3 | 36.8 ± 15.2 | .65 | 53.4 ± 18.3 | 39.3 ± 9.5 | .02 |
| 10 | Citrate | TCA | 133.7 ± 18.1 | 144.9 ± 22.3 | .15 | 149.9 ± 29.9 | 153.6 ± 28.8 | .73 |
| 11 | Cis-aconitate | TCA | 8.2 ± 1.9 | 8 ± 1.8 | .75 | 9.7 ± 1.4 | 9 ± 1.7 | .25 |
| 12 | Isocitrate | TCA | 4.1 ± 2 | 5.6 ± 1 | .02 | 4.9 ± 2.1 | 5.7 ± 1.3 | .26 |
| 13 | Ketoglutarate | TCA | 10.9 ± 2 | 10.3 ± 1.5 | .42 | 12.3 ± 3.3 | 12.1 ± 2.6 | .83 |
| 14 | Succinate | TCA | 3.7 ± 1.1 | 3.8 ± 1 | .88 | 3.9 ± 0.8 | 4 ± 1.2 | .73 |
| 15 | Fummarate | TCA | 1.7 ± 0.4 | 1.9 ± 0.5 | .32 | 2 ± 0.6 | 2.1 ± 0.7 | .71 |
| 16 | Malate | TCA | 3.8 ± 0.9 | 4.2 ± 1.7 | .46 | 4.5 ± 1 | 4.1 ± 1.3 | .33 |
| 17 | Ammonia | 160.2 ± 185.1 | 121 ± 61.7 | .48 | 91 ± 22.1 | 88.9 ± 27.3 | .83 | |
| 18 | Histidine | AA | 129.3 ± 16.2 | 154 ± 33.5 | .11 | 125.4 ± 25.8 | 124.4 ± 21.8 | .92 |
| 19 | 1-Methylhistidine | AA | 13.3 ± 8.1 | 16.5 ± 8.2 | .41 | 15.4 ± 8.1 | 12 ± 11.6 | .38 |
| 20 | 3-Methylhistidine | AA | 4.5 ± 1.2 | 5.5 ± 1.9 | .20 | 5.2 ± 1.7 | 4.8 ± 1.7 | .59 |
| 21 | Asparagine | AA | 55.2 ± 12.8 | 62.1 ± 12.3 | .26 | 57.2 ± 11.8 | 56.8 ± 10.3 | .92 |
| 22 | Arginine | AA | 92.8 ± 15.6 | 71.7 ± 12.4 | .00 | 73.5 ± 26.2 | 74.5 ± 15.2 | .90 |
| 23 | Taurine | AA | 40.2 ± 11.5 | 36.7 ± 7.8 | .43 | 38.1 ± 4.6 | 38.7 ± 9.5 | .86 |
| 24 | Serine | AA | 121 ± 22.3 | 101.4 ± 17.4 | .04 | 123.5 ± 32.1 | 100.5 ± 25.7 | .05 |
| 25 | Glutamine | AA | 312 ± 84.6 | 311.5 ± 40 | .99 | 342.3 ± 63.9 | 299.5 ± 63.8 | .09 |
| 26 | Ethanolamine | AA | 9.9 ± 2.2 | 9 ± 1.5 | .28 | 9 ± 1.6 | 8.9 ± 1.5 | .79 |
| 27 | Glycine | AA | 302.1 ± 96.6 | 216.1 ± 43.5 | .01 | 301.2 ± 77.8 | 224.9 ± 59.4 | .01 |
| 28 | Aspartic acid | AA | 10.7 ± 2 | 9 ± 2 | .08 | 9.6 ± 3.4 | 9.9 ± 4.8 | .86 |
| 29 | Sarcosine | AA | 1.4 ± 0.5 | 1.2 ± 0.5 | .25 | 1.3 ± 0.3 | 1.2 ± 0.5 | .54 |
| 30 | Citrulline | AA | 39.3 ± 10.6 | 27.4 ± 5.4 | .00 | 30.9 ± 8.2 | 28.3 ± 4.8 | .30 |
| 31 | Glutamic acid | AA | 109.8 ± 42.4 | 85.8 ± 12.1 | .07 | 89.8 ± 35.3 | 92.6 ± 27.9 | .82 |
| 32 | Threonine | AA | 131.5 ± 38.2 | 143.1 ± 33.3 | .48 | 143.7 ± 34.4 | 139.9 ± 37.4 | .78 |
| 33 | Alanine | AA | 331 ± 135.2 | 344.7 ± 161.2 | .67 | 324.5 ± 99.8 | 336.6 ± 85.6 | .72 |
| 34 | α-Aminoadipic-acid | AA | 0.8 ± 0.3 | 0.7 ± 0.2 | .78 | 0.7 ± 0.2 | 0.8 ± 0.3 | .73 |
| 35 | β-Aminoisobutyric-acid | AA | 1.3 ± 0.6 | 1.4 ± 0.4 | .59 | 1.5 ± 0.6 | 1.3 ± 0.5 | .37 |
| 36 | Proline | AA | 189.6 ± 51.2 | 178.5 ± 49.9 | .64 | 216.8 ± 71.4 | 193.7 ± 69.3 | .39 |
| 37 | Hydroxylysine-2 | AA | 1 ± 0.6 | 0.5 ± 0.1 | .03 | 0.7 ± 0.2 | 0.6 ± 0.3 | .22 |
| 38 | α-Amino-N-butyric-acid | AA | 26.5 ± 10.7 | 17.2 ± 5.6 | .02 | 22.4 ± 8.2 | 16.9 ± 4 | .03 |
| 39 | Ornithine | AA | 90.1 ± 34.8 | 67.4 ± 32.6 | .17 | 86.2 ± 31.6 | 70.4 ± 35.4 | .23 |
| 40 | Lysine | AA | 184.4 ± 37.1 | 158.8 ± 27.3 | .09 | 177.1 ± 31.4 | 158.5 ± 20 | .07 |
| 41 | Cystine | AA | 22.5 ± 16 | 19.2 ± 10.1 | .57 | 27.8 ± 13.5 | 24.7 ± 14.4 | .57 |
| 42 | Tyrosine | AA | 56.3 ± 12.3 | 52.3 ± 7.5 | .36 | 60.4 ± 14.7 | 52.8 ± 11.5 | .14 |
| 43 | Methionine | AA | 25 ± 4.8 | 23.8 ± 2.7 | .47 | 27.1 ± 5.5 | 23.8 ± 2.6 | .05 |
| 44 | Valine | AA | 264.1 ± 72.6 | 241.7 ± 50.5 | .42 | 246.9 ± 44 | 219.4 ± 42.5 | .11 |
| 45 | Isoleucine | AA | 84.9 ± 23.3 | 75.7 ± 24.2 | .43 | 82.9 ± 20.4 | 68.7 ± 14.5 | .04 |
| 46 | Leucine | AA | 140.8 ± 37.8 | 128.3 ± 31.4 | .43 | 137.7 ± 28.8 | 117.5 ± 25 | .06 |
| 47 | Phenylalanine | AA | 54.9 ± 6.2 | 54.9 ± 6.3 | 1.00 | 54.9 ± 8.6 | 54.1 ± 8.5 | .81 |
| 48 | Tryptophan | AA | 51.8 ± 9.2 | 50.9 ± 4.9 | .78 | 52.1 ± 9.9 | 51.3 ± 8.1 | .81 |
| 49 | 25-Hydroxyvitamin D, ng/mL | Vitamin D | 25.31 ± 1.8 | 23.23 ± 2.3 | .53 | 26.15 ± 2.2 | 25.8 ± 1.9 | .88 |
| 50 | 1,25-Dihydroxyvitamin D, pg/mL | Vitamin D | 39.71 ± 2.4 | 35.77 ± 3.1 | .20 | 40.15 ± 3.6 | 37.71 ± 3.4 | .56 |
| 51 | 24,25-Dihydroxyvitamin D, ng/mL | Vitamin D | 2.69 ± 0.3 | 2.33 ± 0.4 | .52 | 2.68 ± 0.3 | 2.73 ± 0.3 | .92 |
Abbreviations: AA, amino acid; NEFA, nonesterified acid.
We quantified 51 selected metabolites in amino acid, TCA, FFAs, and vitamin D metabolism (Table 2). The directional changes in metabolites by the untargeted approach were in general agreement with quantitative measurements, although many changes in individual metabolites were not statistically significant. Additionally, a quantitative platform detected elevated levels of many amino acids such as arginine (P < .001), glycine (P < .01), hydroxylysine (P < .03), amino butyric acid, and citrulline (P < .02) in T1D(−), which were not detected by untargeted profiling.
T1D(+) vs ND
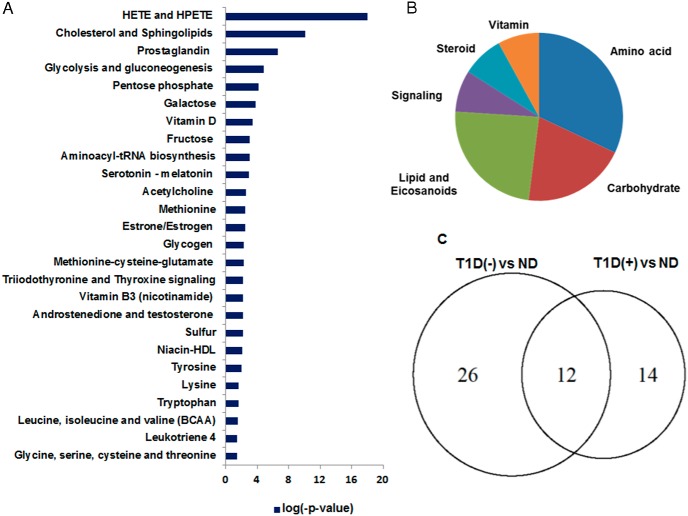
A distinct group clustering was observed between T1D(+) and ND based on their metabolic differences (Figure 1C). A total of 287 metabolites remained altered in comparison with ND, of which 154 are known (Figure 1D and Supplemental Table 2). Among the identified metabolites, BCAAs, carbohydrates, amino acid metabolites, ketoacids, and eicosanoids were not restored despite good glycemic control. We found 154 known metabolites are connected to 26 canonical pathways (P < .05) that were differentially regulated in T1D(+) (Figure 3A). The pathway enrichment of metabolites occurred in the following order: 32% amino acid, 20% carbohydrate, 24% lipid, 8% cell signaling, and 8% vitamin hormone/steroid metabolism (Figure 3B). T1D(+) did not correct many pathways in carbohydrate metabolism including galactose glycolysis and gluconeogenesis, amino acids involving glycine, serine, cysteine and threonine, lysine, tryptophan, methionine-cysteine-glutamate, BCAAs, aminoacyl-tRNA biosynthesis, sulfur, and lipid and eicosanoid (PGE, 12-hydroxyeicosatetraenoic acid [12-HETE], and 5-hydroperoxyeicosatetraenoic acid) metabolism (Figure 3).
Figure 3.
A, Altered canonical pathways in T1D(+) in comparison with ND. B, Percentage of enrichment in pathways in T1D(+). C, A comparison of canonical pathways betweenT1D(−) and T1D(+) using a Venn diagram. Twelve metabolic pathways were not corrected despite good glycemic control, and 14 new pathways were found to be altered in T1D(+) in comparison with ND. The significance of the pathways was evaluated using P values and a false discovery rate less than 0.10.
Quantification of long-chain FFAs (oleic, elaidic, and stearic acids) and amino acids (glycine, serine, and α-aminoisobutyric acid) showed increased levels in T1D(+) (Table 2).
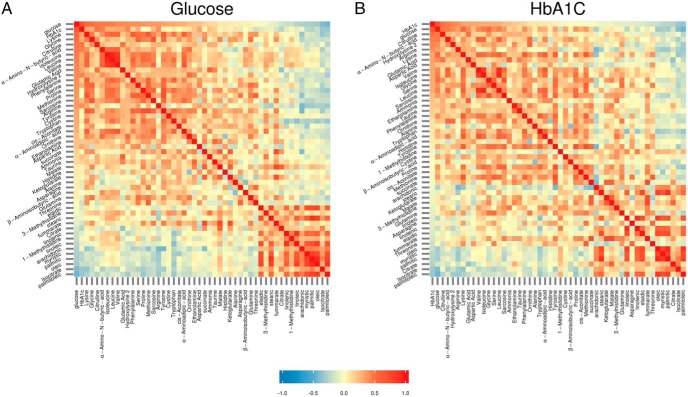
Correlations of plasma metabolites with HbA1c and fasting plasma glucose
The correlation of targeted baseline concentrations of metabolites were assessed with glucose and HbA1c used as indicators for acute and chronic glycemic control in T1D, respectively. As expected, the correlation of metabolites and correlation coefficients with HbA1c (Figure 4A) showed similar correlation as with glucose (Figure 4B). A change in the value of one variable (metabolite) will predict a change in the same direction in the second variable. Whereas a coefficient of +1 indicates a perfect positive correlation, a coefficient of −1 indicates a perfect negative correlation. A coefficient of zero indicates there is no discernable relationship between fluctuations of the variables (metabolites). Thus, the range of correlation coefficient of metabolite is from +1.0 (shown in red) to −1.0 (shown in blue), with a value of 0 (shown in yellow) (Figure 4). Whereas glucose and HbA1c were positively correlated with hydroxylysine, lysine, glycine, citrulline, BCAAs, γ-amino butyric acid, hydroxyproline (R > 0.3, P < .05), palmitoleic, arachidonic, citrate, and isocitrate showed a negative correlation (R < −0.3).
Figure 4.
Heat maps of partial Pearson correlation matrices for glucose, HbA1c, and plasma metabolite levels controlling for age, BMI, and sex in T1D and control patients. Metabolite levels were transformed using log2. A, Metabolites are ordered by partial correlation with glucose. B, Metabolites are ordered by partial correlation with HbA1c.
Discussion
T1D is associated with substantial morbidity and mortality from target organ complications despite better glycemic control made possible by vast improvement in glucose monitoring, insulin delivery, innovation in insulin preparations, and better vascular risk factor management. The current study determined the plasma metabolites and metabolic pathways in T1D with poor and good glycemic control. We observed that although many of the altered metabolites and pathways during poor glycemic control are normalized by good glycemic control, many abnormalities persist on chronic good control that maintained HbA1c of 6.3% ± 0.5%. We identified 347 plasma metabolites (Supplemental Table 1) involved in 38 canonical pathways that are differentially expressed in T1D with HbA1c 10.04% ± 1.27% in comparison with ND (5.2% ± 0.4%). Of these 347 metabolites, the identification and annotations of selected 83 metabolites were confirmed with standards (Supplemental Table 3). The identification of the remaining 264 metabolites was putative based on accurate mass (m/z) against the Metlin database. Poor glycemic control-associated perturbations include the following: 1) reduced 1,5-AG, a marker of glycemic control; 2) increased allantoin and gluconic acid, indicators of oxidative stress; 3) increased BCAAs; 4) increased glucogenic amino acids such as glycine, serine, and arginine; 5) reduced TCA cycle metabolites and unsaturated fatty acids; and 6) perturbations in ketoacids, urea cycle, nucleotides, bile acid intermediates, glucocorticoids, eicosanoids, and vitamin D metabolites.
We also found that T1D(+) continue to have 154 metabolites and pathways that remained altered in comparison with ND. Overall, 26 canonical pathways were abnormal in T1D(+) with 14 pathways (glycolysis and gluconeogenesis, galactose, glycine, serine, cysteine and threonine, lysine, tryptophan, sulfur, methionine-cysteine-glutamate, BCAAs, cholesterol, aminoacyl-tRNA biosynthesis, HETE and 5-hydroperoxyeicosatetraenoic acid, and vitamin D) shared between T1D(−) and T1D(+) and 12 pathways unique to T1D(+). The pathways that are corrected by good glycemic control include nucleotide metabolism, lysine, histidine-glutamate-glutamine, propionate, urea cycle, cortisol, and aldosterone metabolism. Here we also report the association or correlations of selected metabolites with HbA1c and current glycemic status indicated by plasma glucose abnormal in these T1D subjects with good glycemic control.
Carbohydrates
In addition to an elevation of glucose in T1D(−), other carbohydrates such as myoinositol, glucosamine, lactaldehyde, methylglyoxal, and lactate are elevated, whereas a decrease in glycolytic metabolites such as pyruvate, dihydroxyacetone phosphate, and 1,5-AG were noted. Plasma 1,5-AG is a naturally occurring dietary polyol that has been identified as a more sensitive indicator of short-term glycemic control than HbA1c (20). 1,5-AG level was found to be very low in T1D(−) compared to T1D(+) although T1D(+) showed lower level than ND. Other pathways involving glyoxylate and dicarboxylate, fructose, mannose and TCA cycle metabolites were metabolites found to be altered in T1D with good glycemic control.
Nucleotides
The majority of metabolites involved in purine and pyrimidine metabolism were down-regulated in T1D(−). Perturbations of metabolites in purine metabolism like adenine, inosine monophosphate, hypoxanthine, urate, aminoimidazole ribotide, 1-(5′-phosphoribosyl)-5-amino-4-imidazolecarboxamide, imidazolone sulfate, urea, and those in pyrimidine metabolism such as uracil, uridine, cytosine, and deoxy-CTP were normalized by good glycemic control and systemic insulin treatment, consistent with a previous report (22). Decreased nucleotide metabolites in T1D(−) could reflect impaired repair and/or regenerative capacity consistent with the altered wound-healing process in T1D. In diabetic patients, normalizing dysfunctional purine metabolism has been shown to accelerate diabetic wound healing (22, 23).
Amino acids
Elevated levels of the amino acids in T1D(−) and T1D(+) might be due to increased proteolysis as previously reported (24). Our untargeted metabolite measurements showed most of the glucogenic and ketogenic amino acids are increased, whereas alanine and threonine are decreased. Additionally, concentrations of several amino acids such as BCAAs, glycine, serine, methionine, and α-NH2-butyric acid remain elevated, even with good glycemic control. We observed a significant association of citrulline, lysine, hydroxylysine, glycine, serine, α-amino butyric acid, and BCAAs both with HbA1c and glucose. As expected, glucose showed the strongest association with HbA1c and vice versa along with BCAAs, glycine, and lysine. Hydroxylysine is derived from lysine hydroxylation, a collagen component, and α-NH2-adipic acid (AAA) is an intermediate of lysine degradation. Recently AAA is reported to be an early indicator of type 2 diabetes (9, 25). We found no significant difference in the concentrations of AAA levels in both T1D(−/+). A significant association of metabolites including hydroxylysine, hydroxyproline, and AAA was observed with glucose than HbA1c, whereas aspartic acid showed greater associations with HbA1c. Amino acids like glycine and serine are not amenable to good glycemic control, whereas arginine, hydroxylysine, and citrulline are corrected by good glycemic control. Citrulline and ornithine are nonproteinogenic amino acids that are involved in the urea cycle. Ammonia and these urea cycle metabolites were found to be elevated in T1D(−) and indicate either increased amino acids catabolism and/or decreased urea cycle.
Lipids
T1D is associated with high or normal plasma lipid profile and high-density lipoprotein cholesterol levels. Despite having a normal lipid profile, T1D individuals are at an increased risk for cardiovascular disease and premature mortality (1, 2). We observed lower levels of triglycerides, low-density lipoprotein cholesterol, and FFAs in T1D(−). Oleic acid (C18:1ω9), stearic acid (C18), and elaidic acid (trans-C18:1ω9) were significantly elevated in T1D(+). Insulin is a well-known inhibitor of lipolysis (26), and studies performed in T1D individuals have demonstrated that they require significantly higher insulin to suppress lipolysis (27). It remains to be determined whether altered plasma fatty acids in T1D(+) in comparison with ND represent altered fatty acid metabolism.
Eicosanoids
Altered regulation of hydroperoxyeicosatetraenoic acid and its hydroxylated form HETE were observed in both T1D cohorts. It is possible that complications of diabetes involving inflammation and endothelial dysfunction may be mediated by alterations in the prostaglandin pathways (13, 28–30). Eicosanoids function as autocrine and paracrine mediators and are oxygenated hydrophobic derivatives of 20-carbon polyunsaturated essential fatty acids, predominantly arachidonic acid in humans. Arachidonic acid is metabolized by cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 enzymes into eicosanoids, which are involved in diverse diseases, including T1D and type 2 diabetes. AA metabolites of the COX pathway, especially PGE2, appear to be significant factors in cellular dysfunction and destruction, participating in the pathogenesis of diabetes and its complications (28). PGE2, the most important COX product found in islets, reduces insulin secretion and insulin sensitivity during cytokine-induced inflammation. There is direct evidence that 12(S)-HETE, a 12-LOX product, promotes β-cell destruction (31). Animal and human studies have shown that the LOX pathway contributes to insulin resistance and diabetic complications (31–33).
A previous study reported high levels of plasma PGE2 and PGF2 and low levels of serum dihomo-γ-linolenic acid and AA in association with increased platelet aggregation in diabetic children (34). A recent report showed that insulin deficiency affected the arachidonic metabolism pathway. Arachidonic acid may be converted to PGs, leukotrienes, and lipoxins, of which PGEs and leukotrienes are potent proinflammatory lipid mediators and are also linked to hepatic steatosis (28, 30).
The lipoxin pathway has also been found to be affected by insulin deficiency and the inhibitory action of lipoxins, and superoxide production in the neutrophil may result in a diminished inflammatory response in T1D (35).
The current study also noted a substantial up-regulation of aldosterone biosynthesis and metabolic pathways. Aldosterone excess has been shown to be a major cause of cardiovascular complications in many insulin-resistant conditions (36) and may contribute to vascular complications in T1D.
Vitamin D
We found that metabolites associated with the vitamin D pathway are abnormal both in T1D(−) and T1D(+). Quantitative measurements of clinically relevant vitamin D metabolites such as total 25-hydroxyvitamin D, 1,25-hydroxyvitamin D, and 24,25-hydroxyvitamin D showed no significant differences between T1D(−/+) vs ND (Table 2). However, the untargeted approach detected 15 other vitamin D metabolites. The role of vitamin D in the pathogenesis of T1D (37) and in the immune system has been evaluated, with possible implications for antigen-presenting cell function and lymphocyte function (38). There may be redundancy in the system because the vitamin D receptor knockout mice did not show an immune deficiency phenotype (37, 38). There are no data about the importance of vitamin D in the pathogenesis of complications. However, the risks of metabolic bone disease in T1D are significantly elevated (37–40). The current data suggested involvement of other vitamin D-derived metabolites beyond the traditional vitamin D metabolites, and thus, the role of vitamin D metabolites in T1D require further investigation.
One of the limitations of the current study is its cross-sectional nature and a relatively small number of participants. A longitudinal study could evaluate the reproducibility of these findings including the correlation between HbA1c and various metabolites. A larger number of subjects would also serve to validate our findings. An untargeted profiling approach detected many potentially valuable observations in the current study that advance our understanding of the pathophysiology of diabetic complications and need to be investigated further in larger prospective studies.
Conclusion
In conclusion, we report that 347 known metabolites are abnormal in T1D with poor glycemic control, and although many of these metabolites are normalized by good glycemic control, 154 known metabolites belonging to 26 different pathways remain altered despite good glycemic control. These alterations in metabolites despite tight glycemic control based on current guidelines may represent biomarkers of continuing complications in T1D. Alternatively, the route of insulin administration and elevated peripheral insulin levels may contribute to the observed metabolomics abnormalities. Future studies should consider adjunct therapies to insulin treatment or alternative routes of insulin delivery to normalize these metabolites and their potential to have greater impact in preventing diabetic complications.
Acknowledgments
We acknowledge the nursing and laboratory staff of the Clinical Research Unit and Mayo Clinic Metabolomics Resource Core.
This work was supported by National Institutes of Health Grants R01 DK41973, U24 DK100469, and UL1 TR000135 as well as the David Murdock Dole Professorship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- amino acid
- AAA
- α-NH2-adipic acid
- 1,5-AG
- 1,5-anhydroglucitol
- BCAA
- branch-chain amino acid
- BMI
- body mass index
- COX
- cyclooxygenase
- FFA
- free fatty acid
- HbA1c
- glycated hemoglobin
- HETE
- hydroxyeicosatetraenoic acid
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LOX
- lipoxygenase
- m/z
- mass to charge ratio
- ND
- nondiabetic control
- PCA
- principal components analysis
- PG
- prostaglandin
- TCA
- tricarboxylic acid cycle
- T1D
- type 1 diabetes
- T1D(+)
- individuals during good glycemic control
- T1D(−)
- T1D individuals during chronic poor glycemic control
- UPLC-ToF MS
- ultraperformance liquid chromatography coupled with time-of-flight mass spectrometry.
References
- 1. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;69:224–232. [DOI] [PubMed] [Google Scholar]
- 2. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. [DOI] [PubMed] [Google Scholar]
- 3. Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171–182. [DOI] [PubMed] [Google Scholar]
- 4. Lachin JM, Orchard TJ, Nathan DM. Group ftDER. Update on cardiovascular outcomes at 30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care. 2014;37:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. [DOI] [PubMed] [Google Scholar]
- 7. Suhre K. Metabolic profiling in diabetes. J Endocrinol. 2014;221:R75–R85. [DOI] [PubMed] [Google Scholar]
- 8. Roberts LD, Koulman A, Griffin JL. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol. 2014;2:65–75. [DOI] [PubMed] [Google Scholar]
- 9. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2013;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. 2009 Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes. 2011;58:2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One. 2010;5:e10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutta T, Chai HS, Ward LE, et al. Concordance of changes in metabolic pathways based on plasma metabolomics and skeletal muscle transcriptomics in type 1 diabetes. Diabetes. 2012;61:1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Irving BA, Weymiller AJ, Syed H, Karakelides H, Soop M, Carter RE, Nair KS. Amino acid metabolites and insulin resistance in human. Diabetes. 2011;60(suppl 1):A464. [Google Scholar]
- 15. Trushina E, Dutta T, Persson XM, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. PLoS One. 2013;8:e63644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Persson X-MT, Błachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51:2761–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koek MM, Muilwijk B, van der Werf MJ, Hankemeier T. Microbial metabolomics with gas chromatography/mass spectrometry. Anal Chem. 2006;78:1272–1281. [DOI] [PubMed] [Google Scholar]
- 18. Storey JD. A direct approach to false discovery rates. J R Stat Soc B. 2002;64:479–498. [Google Scholar]
- 19. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8:9–19. [DOI] [PubMed] [Google Scholar]
- 21. Liebich HM, Forst C. Hydroxycarboxylic and oxocarboxylic acids in urine—products from branched-chain amino-acid degradation and from ketogenesis. J Chromatogr. 1984;309:225–242. [DOI] [PubMed] [Google Scholar]
- 22. Weber G, Lui MS, Jayaram HN, et al. Regulation of purine and pyrimidine metabolism by insulin and by resistance to tiazofurin. Adv Enzyme Regul. 1985;23:81–99. [DOI] [PubMed] [Google Scholar]
- 23. Weinstein AL, Lalezarzadeh FD, Soares MA, Saadeh PB, Ceradini DJ. Normalizing dysfunctional purine metabolism accelerates diabetic wound healing. Wound Repair Regen. 2015;23(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nair KS, Ford GC, Ekberg K, Fernqvistforbes E, Wahren J. Protein dynamics in whole-body and in splanchnic and leg tissues in type 1 diabetic-patients. J Clin Invest. 1995;95:2926–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang TJ, Ngo D, Psychogios N, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123:4309–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meek SE, Nair KS, Jensen MD. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48:10–14. [DOI] [PubMed] [Google Scholar]
- 27. Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38:1595–1601. [DOI] [PubMed] [Google Scholar]
- 28. Luo P, Wang M-H. Eicosanoids, β-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 2011;95:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Metz S, VanRollins M, Strife R, Fujimoto W, Robertson RP. Lipoxygenase pathway in islet endocrine cells. Oxidative metabolism of arachidonic acid promotes insulin release. J Clin Invest. 1983;71:1191–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson RP, Chen M. A role for prostaglandin E in defective insulin secretion and carbohydrate intolerance in diabetes mellitus. J Clin Invest. 1977;60:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ehses JA, Donath MY. Targeting 12-lipoxygenase as a novel strategy to combat the effects of inflammation on beta cells in diabetes. Diabetologia. 2015;58:425–428. [DOI] [PubMed] [Google Scholar]
- 32. Weaver JR, Holman TR, Imai Y, et al. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet β cell dysfunction. Mol Cell Endocrinol. 2012;358:88–95. [DOI] [PubMed] [Google Scholar]
- 33. Li X, Zhao G, Ma B, et al. 20-Hydroxyeicosatetraenoic acid impairs endothelial insulin signaling by inducing phosphorylation of the insulin receptor substrate-1 at Ser616. PLoS One. 2014;9:e95841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orešič M, Simell S, Sysi-Aho M, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med. 2008;205:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khan SA, Ali A, Zahran SA, Damanhouri G, Azhar E, Qadri I. Unraveling the complex relationship triad between lipids, obesity, and inflammation. Mediators Inflamm. 2014;2014:502749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Androulakis II, Kaltsas GA, Kollias GE, et al. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J Clin Endocrinol Metab. 2014;99:2754–2762. [DOI] [PubMed] [Google Scholar]
- 37. Sivitz WI, Chertow BS, Baranetsky NC, Clark SA, Waite A, Deluca HF. The effects of vitamin-D deficiency and repletion on rat insulin-secretion. Calcified Tissue Int. 1983;35:703–703. [DOI] [PubMed] [Google Scholar]
- 38. Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. [DOI] [PubMed] [Google Scholar]
- 39. Lombardi F, Franzese A, Iafusco D, et al. Bone involvement in clusters of autoimmune diseases: just a complication? Bone. 2010;46:551–555. [DOI] [PubMed] [Google Scholar]
- 40. Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab. 2011;96:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]