Abstract
Context:
Patients with 25-hydroxyvitamin D deficiency (25OHD <20 ng/ml) and primary hyperparathyroidism (PHPT) have more severe disease reflected by higher serum PTH levels compared to those with vitamin D levels in the insufficient (20–29 ng/ml) or replete range (≥30 ng/ml).
Objective:
To study the effect of low vitamin D in PHPT on volumetric bone mineral density (vBMD), bone microarchitecture, and bone strength.
Design, Setting, and Participants:
This is a cross-sectional analysis of 99 PHPT patients with and without 25OHD insufficiency and deficiency from a university hospital.
Outcome Measures:
Bone microarchitecture and strength were assessed with high-resolution peripheral quantitative computed tomography (HRpQCT), microfinite element analysis, and individual trabecula segmentation.
Results:
In this cohort, 25OHD levels were deficient in 18.1%, insufficient in 35.4% and replete in 46.5%. Those with lower 25OHD levels had higher PTH (P < .0001), were younger (P = .001) and tended to weigh more (P = .053). There were no age-, weight- and sex-adjusted between-group differences (<20 vs 20–29 vs ≥30 ng/ml) in any HRpQCT, microfinite element analysis, or individual trabecula segmentation indices. Because few participants had 25OHD below 20 ng/ml, we also compared those with 25OHD below 30 vs at least 30 ng/ml and found only a trend toward lower adjusted cortical vBMD (3.1%, P = .08) and higher cortical porosity (least squares mean ± SEM 7.5 ± 0.3 vs 6.6 ± 0.3%, P = .07) at the tibia but not the radius. Stiffness did not differ at either site. In multiple regression analysis, 25OHD accounted for only three of the 49.2% known variance in cortical vBMD; 25OHD was not significant in the model for cortical porosity at the tibia.
Conclusion:
Low 25OHD levels are associated with higher PTH levels in PHPT, but contrary to our hypothesis, these differences did not significantly affect vBMD or microarchitecture, nor did they result in lower stiffness. Low vitamin D in PHPT using current 25OHD thresholds for insufficiency and deficiency did not significantly affect skeletal integrity as assessed by HRpQCT.
Vitamin D deficiency and insufficiency are common in primary hyperparathyroidism (PHPT), although the prevalence of very low levels of vitamin D is decreasing in some locales (1–3). We recently reported that vitamin D deficiency (25-hydroxyvitamin D [25OHD] <20 ng/ml) in PHPT is associated with more severe hyperparathyroidism as reflected by higher serum PTH levels (4). However, we did not find differences in the clinical presentation of PHPT, such as the frequency of kidney stones or fractures, by vitamin D status.
It is unclear whether vitamin D deficiency or insufficiency, and the associated heightened PTH levels, differentially affect cortical and trabecular bone compartments or alter volumetric bone density, bone microarchitecture, or strength in patients with PHPT. Recently, we reported modestly lower areal bone mineral density (BMD) by dual-energy x-ray absorptiometry (DXA) at the predominantly cortical one-third radius site in women with PHPT and vitamin D insufficiency (25OHD 20–29 ng/ml) (4). In contrast, we found no differences in areal BMD by vitamin D status at the more cancellous lumbar spine or hip sites.
The limited available microarchitectural data regarding vitamin D deficiency in PHPT come from a subset of patients in our prior natural history study who underwent percutaneous iliac crest bone biopsy and had simultaneous 25OHD levels available (5). Histomorphometric analysis demonstrated reduced cortical width in PHPT patients with vitamin D deficiency (25OHD <20 ng/ml), whereas trabecular indices were preserved. There are no data available in this regard using newer noninvasive skeletal imaging modalities such as high-resolution peripheral quantitative computed tomography (HRpQCT).
The purpose of this study was to determine if lower vitamin D levels in PHPT were associated with lower volumetric BMD (vBMD), worse microarchitectural parameters and lower bone strength at the radius and tibia using HRpQCT, microfinite element analysis (μFEA) and individual trabecula segmentation (ITS). Though the definitions of vitamin D insufficiency and deficiency are controversial even in the general population, our recently published data in PHPT suggests that PTH rises in those with 25OHD levels below 20 ng/ml (4). Therefore, for purposes of this analysis, we used commonly employed vitamin D thresholds that are recognized by the Endocrine Society Clinical Practice guidelines and other bone and mineral organizations (insufficiency was defined as 25OHD 20–29 ng/ml and deficiency: <20 ng/ml) (6).
Materials and Methods
This is a cross-sectional study comparing vBMD and bone microarchitecture by HRpQCT, estimated stiffness by μFEA, and trabecular plate/rod parameters by ITS in PHPT patients with and without vitamin D deficiency and insufficiency. All patients gave written, informed consent. This study was approved by the Institutional Review Board of Columbia University Medical Center. Participants represent those studied in our previous report on the effects vitamin D deficiency and insufficiency in PHPT (4) in whom HRpQCT images were available (n = 99 of 100). Recruitment methods and enrollment criteria have been described (4). Briefly, participants had PHPT, diagnosed by both hypercalcemia (calcium >10.2 mg/dl) and an elevated or inappropriately normal PTH level on more than one occasion before enrollment. None had thiazide-induced hyperparathyroidism or familial hypocalciuric hypercalcemia (excluded on the basis of family history and 24-hour urine calcium). Exclusion criteria included bisphosphonate use within 2 years; current use of cinacalcet or denosumab; current or previous use of prednisone 7.5 mg for more than 6 months; current or past use of carbamazepine, phenytoin, or phenobarbital for more than 3 months; malignancy within 5 years other than nonmelanomatous skin cancer; granulomatous diseases, HIV, serum creatinine level of at least 1.5 mg/dl; liver disease; gastrointestinal diseases known to affect calcium metabolism such as Crohn's disease, celiac disease, or gastric bypass; and pregnancy.
Clinical and biochemical evaluation
Demographic data, medical history and medication use were obtained from participants. Calcium and vitamin D intake and sun exposure were assessed by validated questionnaire (7, 8). We used the standard National Institutes of Health classification system for self-identified race/ethnicity, which categorizes individuals into five different racial groups (American Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, Black, and White) and two ethnicities (Hispanic/non-Hispanic). Fasting samples for serum calcium, phosphate, and creatinine were measured by an automated chemistry analyzer. PTH was measured by immunoradiometric assay for intact PTH (Scantibodies Laboratory, normal range 14–66 pg/ml; intra- and interassay coefficient of variation [CV], 3.2–4.8% and 3.6–6.8%-, respectively; limit of detection, 1.2 pg/ml). Serum 25OHD was measured by liquid chromatography/tandem mass spectrometry (CV, 7.0–7.7% for D3 and 4.5–8.3% for D2; limit of detection, 4 ng/ml). Serum 1,25-dihydroxyvitamin D (1,25(OH)2D) was measured by liquid chromatography/tandem mass spectrometry (intra- and interassay CV, 6%–8% and 9%–10%; limit of detection, 8 pg/ml). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (9).
HRpQCT
All participants were scanned with the same HRpQCT (XtremeCT, Scanco Medical) scanner at Columbia University Medical Center. The nondominant distal radius and left distal tibia were scanned unless there was a previous fracture at the desired site, in which case we scanned the opposite limb. A highly trained single operator acquired and analyzed all scans according to the manufacturer's standard in vivo protocol. We scanned all participants using 60 kVp effective energy, 900 μA current, and 100 ms integration time to acquire 110 slices at an 82 μm nominal isotropic resolution. Scans were manually scored for motion on a scale of 1 (no motion) to 5 (significant blurring of the periosteal surface, discontinuities in the cortical shell, or streaking in the soft tissue). No images had a score of 4 or 5, thus all scans were included. We used the manufacturer's standard method to filter and binarize the HRpQCT images (10). To segment the cortical and trabecular regions, we used a previously validated, automatic segmentation algorithm independently on all scans (11). We assessed all standard HRpQCT morphological microstructure outcomes including total, trabecular and cortical vBMD (mg hydroxyapatite [HA]/cm3), inner and outer trabecular vBMD, trabecular number (Tb.N, 1/mm), thickness (Tb.Th, mm), separation (Tb.Sp, mm), and heterogeneity (mm) (12). Inner trabecular density was defined as the inner 60% and outer-trabecular density was defined as the outer 40%. These measurements were previously validated against microcomputed tomography and, in adult populations, have in vivo short-term reproducibility of CV below 4.5% (13, 14). Using the automatic segmentation method, we measured total area, cortical porosity (%) calculated as the number of void voxels within the cortex (15) and directly measured cortical thickness (mm).
Bone strength estimates
We estimated bone strength from the HRpQCT images using FEA based on a voxel conversion approach (16). We simulated uniaxial compression on each radius and tibia model up to 1% strain using a homogeneous Young's modulus of 6829 MPa and Poisson's ratio of 0.3 (13). We used a custom FEA solver (FAIM, version 6.0; Numerics88) on a desktop workstation (Linux Ubuntu 12.10, 2 × 6-core Intel Xenon, 64 GB RAM) to solve the models as previously described in other studies (15). We estimated whole bone stiffness (MPa) and failure load (N).
ITS analysis
The whole trabecular bone compartment of each HRpQCT image was separated from the whole bone based on an automatic segmentation technique (15). Briefly, digital topological analysis–based skeletonization (17) was applied to transform a trabecular bone image into a representation composed of surfaces and curves skeleton while preserving the topology (ie, connectivity, tunnels, and cavities) (18, 19) as well as the rod and plate morphology of the trabecular microarchitecture. Then, digital topological classification was applied in which each skeletal voxel was uniquely classified as either a surface or a curve type (20). Using an iterative reconstruction method, each voxel of the original image was classified as belonging to either an individual plate or rod. Based on the three-dimensional evaluations of each trabecular plate and rod, bone volume (BV) and number of plates and rods were evaluated by plate BV (pBV) and rod BV (rBV) total volume (TV) (pBV/TV and rBV/TV) as well as plate and rod number densities (1/mm). Plate-to-rod ratio, a parameter of plate vs rod characteristics of trabecular bone, was defined as pBV divided by rBV. The average size of plates and rods was quantified by plate and rod thickness (mm). Intactness of the trabecular network was characterized by rod-rod junction density (1/mm3) and plate-rod junction density (1/mm3), calculated as the total junctions between trabecular rod-rod or plate-rod normalized by the bulk volume. The orientation of the trabecular bone network is characterized by axial BV/TV defined as axially aligned BV divided by the bulk volume. Detailed methods of the complete volumetric decomposition technique and ITS-based measurements can be found in our recent publications (14, 21, 22).
Statistical analysis
Associations of 25OHD, PTH, and 1,25(OH)2D with skeletal indices were assessed with Spearman correlation. Between-group differences in demographic and skeletal indices were evaluated with ANOVA, χ2, or Fisher's exact test as appropriate. Values are expressed as least squares means ± SEM or percentages. SEM was used to facilitate comparisons among groups. Analysis of covariance models were used to assess between-group differences in HRpQCT, μFEA, and ITS parameters adjusting for covariates (age and weight or age, weight, and sex). We also stratified by sex and repeated analyses adjusting for age and weight. Vitamin D levels were normally distributed. Stepwise multiple regression was used to evaluate independent predictors (age, sex, height, weight, race/ethnicity, calcium, PTH, 25OHD, 1,25(OH)2D, and eGFR) of cortical vBMD and cortical porosity at the tibia. These outcomes were assessed with regression because of their borderline significant associations with vitamin D. The stepwise selection process criterion for entry to the model was a univariate P < .3 and the criterion for retention in the model was a multivariate P < .10. For all analyses, a two-tailed P < .05 was considered to indicate statistical significance. Statistical analysis was performed using SAS, version 9.4.
Results
In the cohort as a whole, participants were predominantly female (79%) and had evidence of mild PHPT (mean ± SEM: serum calcium, 10.7 ± 0.1 mg/dl; PTH, 85 ± 4.8 pg/ml). Mean duration of PHPT was 4.3 ± 6 years. Mean 25OHD in all participants was 29 ± 1 ng/ml. Vitamin D deficiency (25OHD <20 ng/ml) and insufficiency (20–29 ng/ml) were present in 18.1% and 35.4% of participants respectively, whereas 46.5% were vitamin D sufficient (≥30 ng/ml). In the whole cohort, lower 25OHD was linearly correlated with several demographic factors including younger age (r = 0.34, P = .0006) as well as higher weight (r = −0.23, P = .02) and body mass index (r = −0.23, P = .02). There was a tendency for men to have lower vitamin D levels compared to women (26 ± 7 vs 30 ± 11 ng/ml, P = .09), but frequency of supplement use did not differ by sex (60 vs 65%, P = .79).
To begin to assess associations between 25OHD and skeletal health, we first evaluated univariate linear correlations among 25OHD as a continuous variable with HRpQCT, μFEA, and ITS parameters. There were no linear associations of any HRpQCT, μFEA, and ITS parameters with 25OHD at the radius, whereas at the tibia, 25OHD was negatively correlated with tibial failure load (r = −0.25, P = .01) and stiffness (r = −0.25, P = .01) only. Higher PTH levels were associated with fewer axially aligned plates (r = −0.22, P = .03) and lower pBV/TV (r = −0.20, P = .04) at the tibia, and at the radius with fewer axially aligned plates (r = −0.22, P = .03) and lower inner trabecular density (r = −0.28, P = .006), but there were no other associated variables at either skeletal site. 1,25(OH)2D was negatively associated with cortical porosity (r = −0.21, P = .05) at the radius, but with none of the other skeletal indices at either site. There was no association between PHPT disease duration and 25OHD level (r = 0.02, P = .83). Longer disease duration was associated with lower plate BV/TV (r = −0.20, P = .046) and rod number (r = −0.31, P = .002) at the radius but not with any other HRpQCT variables at either site.
In addition to the associations between 25OHD levels (as a continuous measure) and skeletal indices, we were interested in the utility of commonly used clinical cut points for vitamin D status (25OHD <20 vs 20–29 vs ≥30 ng/ml). As shown in Table 1, those with lower vitamin D were younger (P = .001) and were less likely to be consuming vitamin D supplements (P < .0001). There were no between-group differences in sex, race/ethnicity, height, body mass index, tobacco use, sun exposure, or duration of PHPT (Table 1). There was a trend toward higher weight in those with lower vitamin D, but it did not reach statistical significance (P = .053). As shown in Table 2, those with lower vitamin D levels had higher serum PTH (P < .0001) and lower phosphate levels (P = .0007), but there were no between-group differences in serum calcium or eGFR.
Table 1.
Demographic and Lifestyle Characteristics by Vitamin D Status
| 25OHD <20 ng/ml n = 18 | 25OHD 20–29 ng/ml n = 35 | 25OHD ≥30 ng/ml n = 46 | P Value | |
|---|---|---|---|---|
| Age (y) | 56.7 ± 2.7 | 58.5 ± 2.0 | 66.8 ± 1.7a,b | .001 |
| Female (n/%) | 14/77.8% | 25/71.4% | 40/87.0% | .22 |
| % of women postmenopausal | 10/71.4% | 20/80.0% | 40/100% | .002 |
| Age at menopause (y) | 47.6 ± 2.2 | 49.0 ± 1.54 | 50.9 ± 1.1 | .33 |
| Height (in.) | 65.1 ± 0.8 | 65.3 ± 0.6 | 64.0 ± 0.5 | .22 |
| Weight (lb) | 181.4 ± 9.9 | 174.2 ± 7.1 | 156.5 ± 6.2 | .053 |
| BMI (kg/m2) | 29.6 ± 1.4 | 28.7 ± 1.0 | 26.8 ± 0.9 | .16 |
| White (%) | 13/86.7 | 30/85.7 | 42/93.3 | .42 |
| Hispanic (%) | 5/27.8% | 5/14.3% | 7/15.2% | .42 |
| % taking vitamin D supplements | 4/22.2% | 21/60.0% | 39/84.8% | <.0001 |
| Daily supplement use (IU/day) | 668 ± 711 | 1056 ± 310 | 2059 ± 228b | .02 |
| Current tobacco use (%) | 1/5.6% | 1/2.9% | 4/8.7 | .83 |
| Sun exposure score | 8.3 ± 1.4 | 8.5 ± 1.0 | 9.1 ± 0.9 | .84 |
| PHPT duration (y) | 3.1 ± 1.4 | 3.6 ± 1.0 | 5.2 ± 0.9 | .33 |
Values represent group mean ± sem or percentages.
P < .01 vs 25OHD <20 ng/ml.
P < .05 vs 25OHD 20–29 ng/ml.
Table 2.
Biochemical Characteristics by Vitamin D Status
| Normal Range | 25OHD <20 ng/ml | 25OHD 20–29 ng/ml | 25OHD ≥30 ng/ml | P Value | |
|---|---|---|---|---|---|
| 25-hydroxyvitamin D (ng/ml) | 30–100 | 13.7 ± 1.1 | 25.5 ± 0.8 | 37.9 ± 0.7 | NA |
| Serum calcium (mg/dl) | 8.6–10.2 | 10.8 ± 0.1 | 10.7 ± 0.1 | 10.6 ± 0.1 | .33 |
| Serum PTH (pg/ml) | 14–66 | 127 ± 10 | 81 ± 8a | 72 ± 7b | <.0001 |
| Phosphate (mg/dl) | 2.7–4.5 | 2.8 ± 0.1 | 3.0 ± 0.1 | 3.2 ± 0.1c | .0007 |
| 1,25-dihydroxyvitamin D (ng/ml) | 18–72 | 78 ± 6 | 73 ± 4 | 63 ± 4 | .06 |
| eGFR (ml/min) | ≥60 | 92 ± 6 | 86 ± 4 | 79 ± 4 | .18 |
Values represent group mean ± sem.
Abbreviation: NA, not available.
P < .01 vs 25OHD <20 ng/ml.
P < .0001 vs 25OHD <20 ng/ml.
P < .001 vs 25OHD <20 ng/ml.
By HRpQCT (Table 3), there were no between-group differences by vitamin D status (25OHD <20 vs 20–29 vs ≥30 ng/ml) in any vBMD, microarchitecture, or mechanical competence indices before or after adjustment for covariates (age and weight or age, weight, and sex) at the radius. There was only a trend toward lower Tb.N at the radius in those with lower vitamin D after adjustment for age and weight (P = .06) or age, weight, and sex (P = .07). At the tibia (Table 3), there was a trend toward greater total vBMD and cortical thickness in those with lower vitamin D, greater whole bone stiffness (P = .02), and higher failure load (P = .02). These differences at the tibia were attenuated and no longer significant after adjustment for age and weight or age, weight, and sex (Table 3). There were no differences in any ITS parameters before or after controlling for age and weight or age, weight, and sex at either skeletal site (data not shown).
Table 3.
HRpQCT Parameters by Vitamin D Status at the Radius and Tibia
| 25OHD <20 ng/ml | 25OHD 20–29 ng/ml | 25OHD ≥30 ng/ml | P Value | P Value Adjusted for Age and Weight | P Value Adjusted for Age, Weight, and Sex | |
|---|---|---|---|---|---|---|
| Radius | ||||||
| Total area (mm2) | 259 ± 16 | 286 ± 12 | 255 ± 10 | .12 | .24 | .21 |
| Total vBMD (mg HA/cm3) | 282 ± 17 | 277 ± 12 | 267 ± 11 | .72 | .63 | .65 |
| Cortical vBMD (mg HA/cm3) | 842 ± 22 | 806 ± 16 | 812 ± 14 | .38 | .12 | .17 |
| Direct cortical thickness (mm) | 0.944 ± 0.052 | 0.876 ± 0.039 | 0.872 ± 0.032 | .48 | .41 | .56 |
| Cortical porosity (%) | 2.1 ± 0.3 | 2.3 ± 0.2 | 2.5 ± 0.2 | .43 | .96 | .99 |
| Trabecular vBMD (mg HA/cm3) | 118 ± 10 | 135 ± 7 | 121 ± 6 | .25 | .21 | .26 |
| Dinn (mg HA/cm3) | 74 ± 10 | 98 ± 7 | 83 ± 6 | .11 | .08 | .11 |
| Dmeta (mg HA/cm3) | 182 ± 10 | 192 ± 7 | 181 ± 6 | .44 | .39 | .44 |
| Tb.N (1/mm) | 1.65 ± 0.10 | 1.86 ± 0.07 | 1.76 ± 0.06 | .20 | .06 | .07 |
| Tb.Th (mm) | 0.058 ± 0.003 | 0.059 ± 0.002 | 0.056 ± 0.002 | .46 | .95 | .99 |
| Tb.Sp (mm) | 0.575 ± 0.049 | 0.517 ± 0.035 | 0.561 ± 0.031 | .55 | .36 | .39 |
| Tb.Sp sd (mm) | 0.300 ± 0.054 | 0.257 ± 0.039 | 0.311 ± 0.034 | .57 | .58 | .61 |
| Whole bone stiffness (kN/mm) | 33.5 ± 2.7 | 33.9 ± 2.0 | 29.5 ± 1.7 | .19 | .54 | .50 |
| Failure load (kN) | 1.6 ± 1.3 | 1.6 ± 0.9 | 1.4 ± 0.8 | .16 | .50 | .47 |
| Tibia | ||||||
| Total area (mm2) | 765 ± 34 | 764 ± 25 | 714 ± 21 | .22 | .82 | .98 |
| Total vBMD (mg HA/cm3) | 271 ± 16 | 252 ± 11 | 231 ± 10 | .08 | .53 | .50 |
| Cortical vBMD (mg HA/cm3) | 782 ± 21 | 774 ± 15 | 759 ± 13 | .59 | .20 | .22 |
| Direct cortical thickness (mm) | 1.24 ± 0.06 | 1.23 ± 0.04 | 1.11 ± 0.04 | .053 | .92 | .65 |
| Cortical porosity (%) | 6.8 ± 0.6 | 7.1 ± 0.5 | 7.1 ± 0.4 | .92 | .18 | .19 |
| Trabecular vBMD (mg HA/cm3) | 141 ± 9 | 153 ± 6 | 140 ± 5 | .25 | .37 | .49 |
| Dinn (mg HA/cm3) | 95 ± 10 | 107 ± 7 | 95 ± 6 | .39 | .47 | .57 |
| Dmeta (mg HA/cm3) | 208 ± 8 | 222 ± 6 | 209 ± 5 | .20 | .25 | .37 |
| Tb.N (1/mm) | 1.86 ± 0.10 | 1.83 ± 0.07 | 1.78 ± 0.06 | .77 | .70 | .61 |
| Tb.Th (mm) | 0.065 ± 0.003 | 0.070 ± 0.002 | 0.066 ± 0.002 | .25 | .19 | .23 |
| Tb.Sp (mm) | 0.518 ± 0.038 | 0.503 ± 0.03 | 0.528 ± 0.024 | .80 | .65 | .67 |
| Tb.Sp sd (mm) | 0.285 ± 0.052 | 0.255 ± 0.037 | 0.288 ± 0.032 | .79 | .67 | .69 |
| Whole bone stiffness (kN/mm) | 105.4 ± 6.3 | 109.9 ± 4.7 | 93.0 ± 3.8a | .02 | .40 | .45 |
| Failure load (kN) | 4.9 ± 2.9 | 5.1 ± 2.1 | 4.4 ± 1.8a | .02 | .49 | .55 |
Abbreviations: Dinn, inner trabecular vBMD; Dmeta, outer trabecular vBMD; Tb.Sp, trabecular separation.
P < 0.05 vs 25OHD 20–29; values represent group mean ± sem.
Because the vast majority of participants were women, we assessed this group separately. There were no between-group differences in any vBMD, microarchitecture, bone strength, or ITS variables by vitamin D status before or after adjusting for age and weight at either the radius or tibia (data not shown). Additionally, in the subgroup of women only, there were no interactions between age (in tertiles) and vitamin D status (<20, 20–29, ≥30 ng/ml) for any HRpQCT, ITS, or bone strength variable at either site. Similarly, there was no interaction between menopausal status and vitamin D status for any HRpQCT, ITS, or bone strength variable at either site (data not shown).
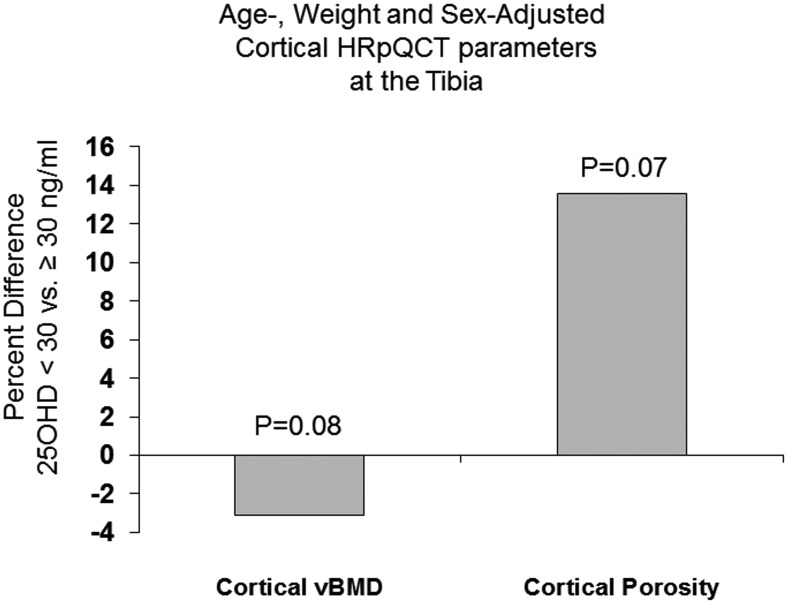
Because of the relatively smaller sample size in the 25OHD <20 ng/ml category, we also assessed HRpQCT, μFEA, and ITS differences grouping together all those (including both men and women) with suboptimal vitamin D levels (<30 ng/ml). Adjusting for age and weight, those with 25OHD lower than 30 ng/ml vs at least 30 ng/ml had a tendency toward lower cortical vBMD (757 ± 9 vs 782 ± 10, mg HA/cm3, P = .07) and higher cortical porosity (7.5 ± 0.3 vs 6.6 ± 0.3%, P = .06) at the tibia that did not reach statistical significance. There were no differences at the radius. Results were similar after additionally adjusting for sex: cortical vBMD was marginally lower (3.1%, P = .08) and cortical porosity (7.5 ± 0.3 vs 6.6 ± 0.3%, P = .07) marginally higher at the tibia in the group with vitamin D <30 ng/ml (Figure 1). There were no other between-group differences at either skeletal site. Representative HRpQCT images are shown in Figure 2.
Figure 1.
Percent difference in cortical vBMD and porosity adjusted for age, weight, and sex between those with 25OHD <30 and ≥30 ng/ml (whole cohort including men and women).
Figure 2.
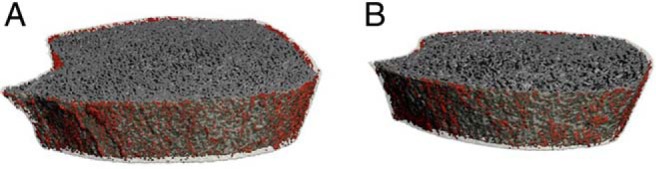
Representative three-dimensional HRpQCT images of the tibia in (A) a patient with low vitamin (25-hydroxyvitamin D, 15 ng/dl) and cortical porosity of 9.5% and (B) a vitamin D sufficient patient (25-hydroxyvitamin D, 38 ng/ml) with a cortical porosity of 6.5%. The bone's cortex has been made transparent and pores are shown in red.
Multiple regression analysis
In multiple regression modeling (Table 4) in which age, sex, height, weight, race/ethnicity, calcium, PTH, 25OHD, 1,25(OH)2D, and eGFR were considered as potential continuous predictors, three variables (age, sex, and 25OHD) were significant in the model of cortical vBMD at the tibia. Each year increase in age was associated with 5.4 mg HA/cm3 lower vBMD; male sex was associated with 40.2 mg HA/cm3 higher vBMD, whereas each nanogram per milliliter increase in 25OHD was associated with 1.6 mg HA/cm3 higher vBMD. The model accounted for 49.2% of the variance in vBMD, but 25OHD accounted for only 3% of the known variance. In a separate model for cortical porosity at the tibia (data not shown), only age (P < .001) and calcium level (P = .0008) were significant in the model, whereas 25OHD and 1,25(OH)2D were not.
Table 4.
Multiple Regression Model of Cortical vBMD by HRpQCT at the Tibia
| Variable | Parameter Estimate | SE | Partial r2 | P Value | Model r2 |
|---|---|---|---|---|---|
| Age (per 1-y increase) | −5.39 | 0.59 | 0.429 | <.001 | 0.492 |
| Male sex | 40.24 | 17.92 | 0.036 | .03 | |
| 25OHD (per 1 ng/ml increase) | 1.61 | 0.75 | 0.028 | .03 |
Discussion
In this study we assessed the association between low serum 25OHD levels, vBMD, bone microarchitecture, and bone strength in patients with PHPT. To our knowledge, this is the first study to use HRpQCT, μFEA, and ITS to assess the effects of vitamin D status upon the skeleton in PHPT. Vitamin D insufficiency and deficiency were present in about one-half our cohort with mild PHPT. We hypothesized that those with low vitamin D levels would have reduced cortical vBMD and thickness, increased cortical porosity, and reduced strength because of heightened PTH elevations. These hypotheses were not confirmed. Instead, we found that those with lower 25OHD levels tended to have better unadjusted indices of skeletal health (cortical thickness, total vBMD, higher stiffness at the tibia) despite having more severe PHPT (higher PTH). This finding is likely explained by the tendency of lower 25OHD levels to be associated with younger age, greater weight, and male sex. After controlling for these demographic factors, we found only a nonsignificant trend toward lower cortical vBMD and higher cortical porosity in those with vitamin D <30 ng/ml at the tibia. To ensure that these factors were not masking an effect of vitamin D, we also assessed if there was an interaction between age and vitamin D as well as menopausal status and vitamin D in women; we found none.
Although these findings are not inconsistent with our DXA results indicating a tendency toward lower T-scores at the cortical 1/3 radius in women with vitamin D insufficiency (4), our data suggest that the current 25OHD thresholds do not appear to have a major effect upon skeletal health in PHPT and that the effects of demographic factors (such as younger age) in those who were vitamin D deficient tended to predominate. Even when considering the extremes of vitamin D status (<20 vs ≥30 ng/ml), we did not find differences in HRpQCT parameters. Further, when we assessed vitamin D as a continuous variable (in multiple regression modeling), 25OHD contributed minimally to the known variance in cortical parameters.
Although we initially hypothesized vitamin D deficiency and resulting heightened PTH elevations would preferentially affect the cortical compartment, recent data using new imaging modalities such as HRpQCT and trabecular bone score suggest that the hyperparathyroid process has detrimental effects upon trabecular as well as cortical bone (23). This led us to carefully investigate this possibility in patients with vitamin D deficiency by assessing not only standard trabecular HRpQCT parameters, but also trabecular plate and rod morphology by ITS. Despite thorough examination of this possibility, we did not find vitamin D deficiency to be associated with worse trabecular structure.
That vitamin D had so little effect upon indices of skeletal health in PHPT, assessed using advancing imaging techniques, was unexpected and in contrast with our hypotheses. Likewise, active vitamin D was also not strongly associated with microarchitecture in univariate analyses and was not significant in multiple regression models. These generally negative findings, however, are congruent with a growing body of emerging data, in both the general population and PHPT, which suggest vitamin D deficiency using current thresholds (<20 and <30 ng/ml) as well as vitamin D treatment has a minimal effect on skeletal health (4, 24–26). The reasons why vitamin D had so little effect are not clear and were not investigated by this study. It is possible that an anabolic effect of the higher PTH level could have compensated for the negative skeletal effects of vitamin D deficiency; however, if this were the case, we might expect to see better trabecular microarchitecture and worse cortical parameters in those with higher PTH and lower vitamin D. We saw no evidence of such an effect. Unfortunately, we cannot completely dissociate the effects of elevated PTH vs vitamin D deficiency with the current study design.
Although this is the first study using HRpQCT to assess the impact of low 25OHD levels on microarchitecture in PHPT, limited prior histomorphometric data from a subset of patients in our prior natural history study of PHPT demonstrated reduced cortical width but no difference in cortical porosity in those with 25OHD lower than 20 vs at least 20 ng/ml (5). In that study, trabecular parameters such as BV/TV, trabecular number, and separation were actually more favorable in those with 25OHD below 20 ng/ml vs at least 20 ng/ml consistent with an anabolic effect of PTH. The disparate results could be related to either differences in the degree of vitamin D deficiency (25OHD was lower in prior studies) or elevations in PTH (PTH was higher in prior studies) or skeletal site (iliac crest vs tibia). Reports in non-PHPT patients have shown similar discrepancies between HRpQCT and iliac crest bone biopsy data (27). Even in the current study, findings vary by skeletal site, possibly because of differences in weight-bearing. Cortical differences in those with low 25OHD levels and higher PTH tended to be present at the tibia but not the radius. Interestingly, the finding of a predominant effect of low vitamin D at the weight-bearing tibia suggests a different mechanism from the effect of PHPT itself, in which HRpQCT differences between PHPT and non-PHPT are more prominent at the radius (23). Not unexpectedly, we did observe similar univariate associations between higher PTH levels and reduced plate-rod connectivity, fewer axially aligned plates, and lower pBV/TV at the tibia as in other studies (23).
Our study has several limitations. Most notably, few participants had very low 25OHD levels, which may have impaired our ability to detect between-group differences. Our recent work suggests vitamin D deficiency is now less common in PHPT than in the past, likely related to increasing use of vitamin D supplementation (3). The findings of this study therefore cannot be generalized to populations with more widespread or severe vitamin D deficiency and may not be applicable to PHPT patients in other countries where vitamin D supplementation is uncommon. With the current sample size, however, we had 80% power to detect 9.4% differences among the three groups in cortical vBMD at the radius with a two-tailed experiment-wise α of 0.05%. Because of sample size limitations, we conducted both categorical and continuous analyses. Although we cannot rule out smaller differences, our results are consistent with other studies in the general population and in PHPT using other modalities (DXA, markers of bone turnover) (4, 24–26). It is also possible that a single 25OHD level may not be reflective of chronic exposure, particularly when many participants were taking vitamin D supplements. Additionally, duration of vitamin D deficiency was unknown. Further, the limited resolution of the HRpQCT instrument did not allow us to visualize micropores. Last, bioavailable vitamin D levels are not available for this cohort. Despite these limitations, the study has important strengths, including the relatively large group of PHPT patients; the comprehensive assessment of skeletal health using newer techniques such as HRpQCT, FEA, and ITS as well as more traditional methods; and the use of liquid chromatography/tandem mass spectroscopy to measure vitamin D (4). In addition, we assessed the utility of commonly employed clinical vitamin D thresholds to assess the effects of vitamin D upon the skeleton.
In summary, our findings suggest that vitamin D deficiency and insufficiency, using current clinical thresholds, do not significantly affect volumetric bone density, bone microarchitecture, or strength in PHPT. We cannot rule out the possibility that more severe vitamin D deficiency may have a greater impact on the skeleton, nor do these data permit a prediction regarding the skeletal response to vitamin D repletion. Nonetheless, in this PHPT cohort in whom low serum 25OHD levels were associated with worse hyperparathyroidism, vitamin D insufficiency, and deficiency did not appear to have a major impact on skeletal integrity. We believe the negative results are reassuring and helpful in guiding management of PHPT patients with regard to vitamin D levels. Our work suggests 25OHD levels of at least 20 ng/ml in PHPT may be considered adequate based on PTH, bone microarchitecture, and bone strength outcomes.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK084986, K24 DK074457, and R01 AR051376 as well as the Joseph Weintraub Family Foundation.
Author Contributions: M.D.W. participated in study conception/design, data acquisition, data analysis, interpretation of data, and drafting of the manuscript. –K.K.N. participated in data acquisition, data analysis, interpretation of data, and critical revision of the paper. B.Z., E.C., J.W., J.A.L., A.K., C.Z., and X.E.G. participated in data acquisition and critical revision of the paper. S.J.S. participated in study conception/design, data acquisition, data analysis, interpretation of data, and critical revision of the paper.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- CV
- coefficient of variation
- DXA
- dual-energy x-ray absorptiometry
- eGFR
- estimated glomerular filtration rate
- μFEA
- microfinite element analysis
- HA
- hydroxyapatite
- HRpQCT
- high-resolution peripheral quantitative computed tomography
- ITS
- individual trabecula segmentation
- 25OHD
- 25-hydroxyvitamin D deficiency
- 1,25(OH)2D
- serum 1,25-dihydroxyvitamin D
- pBV
- plate bone volume
- PHPT
- primary hyperparathyroidism
- rBV
- rod bone volume
- Tb.N
- trabecular number
- Tb.Th
- trabecular thickness
- TV
- tissue volume
- vBMD
- volumetric bone mineral density.
References
- 1. Boudou P, Ibrahim F, Cormier C, Sarfati E, Souberbielle JC. A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J Endocrinol Invest. 2006;29:511–515. [DOI] [PubMed] [Google Scholar]
- 2. Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L. Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol (Oxf). 2005;63:506–513. [DOI] [PubMed] [Google Scholar]
- 3. Walker MD, Cong E, Lee JA, et al. Low vitamin D levels have become less common in primary hyperparathyroidism. Osteoporos Int. 2015;26:2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker MD, Cong E, Lee JA, et al. Vitamin D in primary hyperparathyroidism: effects on clinical, biochemical, and densitometric presentation. J Clin Endocrinol Metab. 2015;100:3443–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stein EM, Dempster DW, Udesky J, et al. Vitamin D deficiency influences histomorphometric features of bone in primary hyperparathyroidism. Bone. 2011;48:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 7. Hertzler A, Frary R. A dietary rapid assessment method (RAM). Top Clin Nutr. 1994;9:76–85. [Google Scholar]
- 8. Stein EM, Strain G, Sinha N, et al. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf). 2009;71:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 10. Laib A, Häuselmann HJ, Rüegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–337. [PubMed] [Google Scholar]
- 11. Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41:505–515. [DOI] [PubMed] [Google Scholar]
- 12. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515. [DOI] [PubMed] [Google Scholar]
- 13. MacNeil JA, Boyd SK. Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2008;30:792–799. [DOI] [PubMed] [Google Scholar]
- 14. Zhou B, Zhang Z, Wang J, et al. In vivo precision of digital topological skeletonization based individual trabecula segmentation (ITS) analysis of trabecular microstructure at the distal radius and tibia by HR-pQCT. Patt Recog Lett. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25:882–890. [DOI] [PubMed] [Google Scholar]
- 16. Muller R, Ruegsegger P. Three-dimensional finite element modelling of non-invasively assessed trabecular bone structures. Med Eng Phys. 1995;17:126–133. [DOI] [PubMed] [Google Scholar]
- 17. Saha PK, Chaudhuri BB, Majumder DD. A new shape preserving parallel thinning algorithm for 3D digital images. Pattern Recogn. 1997;30:1939–1955. [Google Scholar]
- 18. Saha PK, Chaudhuri BB. Detection of 3-D simple points for topology preserving. IEEE Trans Pattern Anal Mach Intell. 1994;16:1028–1032. [Google Scholar]
- 19. Saha PK, Chaudhuri BB, Chanda B, Dutta Majumder D. Topology preservation in 3D digital space. Pattern Recogn. 1994;27:295–300. [Google Scholar]
- 20. Saha PK, Chaudhuri BB. 3D digital topology under binary transformation with applications. Comput Vis Image Underst. 1996;63:418–429. [Google Scholar]
- 21. Liu XS, Sajda P, Saha PK, et al. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res. 2008;23:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu XS, Sajda P, Saha PK, Wehrli FW, Guo XE. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res. 2006;21:1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stein EM, Silva BC, Boutroy S, et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res. 2013;28:1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyd SK, Burt LA, Sevick LK, Hanley DA. The relationship between serum 25(OH)D and bone density and microarchitecture as measured by HR-pQCT. Osteoporos Int. 2015;26:2375–2380. [DOI] [PubMed] [Google Scholar]
- 25. Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women's Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94:1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383:146–155. [DOI] [PubMed] [Google Scholar]
- 27. Cohen A, Dempster DW, Muller R, et al. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int. 2010;21:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]