Abstract
Context:
Experimental data in rodents suggest that the adverse reproductive health effects of bisphenol A (BPA) can be modified by intake of soy phytoestrogens. Whether the same is true in humans is not known.
Objective:
The purpose of this study was to evaluate whether soy consumption modifies the relation between urinary BPA levels and infertility treatment outcomes among women undergoing assisted reproduction.
Setting:
The study was conducted in a fertility center in a teaching hospital.
Design:
We evaluated 239 women enrolled between 2007 and 2012 in the Environment and Reproductive Health (EARTH) Study, a prospective cohort study, who underwent 347 in vitro fertilization (IVF) cycles. Participants completed a baseline questionnaire and provided up to 2 urine samples in each treatment cycle before oocyte retrieval. IVF outcomes were abstracted from electronic medical records. We used generalized linear mixed models with interaction terms to evaluate whether the association between urinary BPA concentrations and IVF outcomes was modified by soy intake.
Main Outcome Measure:
Live birth rates per initiated treatment cycle were measured.
Results:
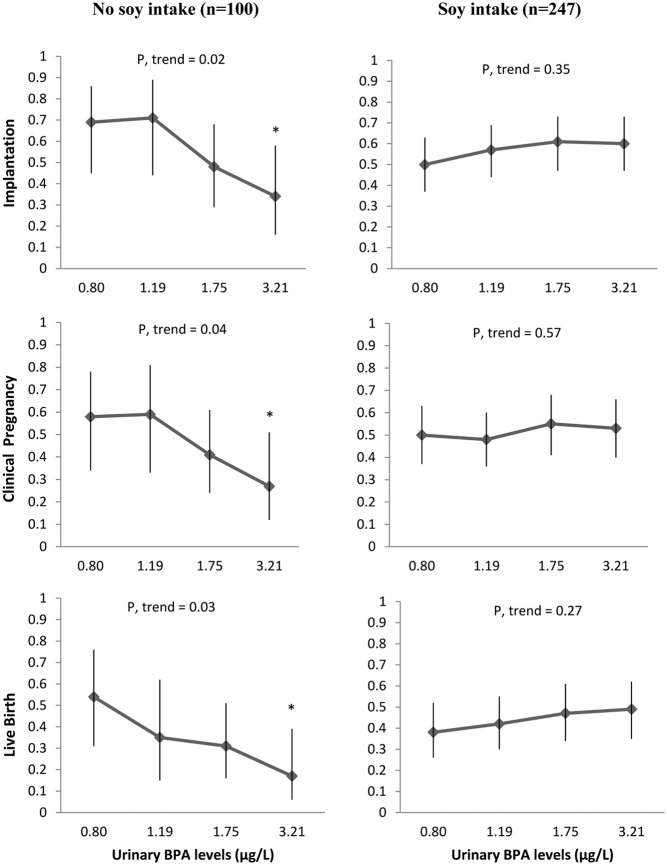
Soy food consumption modified the association of urinary BPA concentration with live birth rates (P for interaction = .01). Among women who did not consume soy foods, the adjusted live birth rates per initiated cycle in increasing quartiles of cycle-specific urinary BPA concentrations were 54%, 35%, 31%, and 17% (P for trend = .03). The corresponding live birth rates among women reporting pretreatment consumption of soy foods were 38%, 42%, 47%, and 49% (P for trend = 0.35). A similar pattern was found for implantation (P for interaction = .02) and clinical pregnancy rates (P for interaction = .03) per initiated cycle, where urinary BPA was inversely related to these outcomes among women not consuming soy foods but unrelated to them among soy consumers.
Conclusion:
Soy food intake may protect against the adverse reproductive effects of BPA. As these findings represent the first report suggesting a potential interaction between soy and BPA in humans, they should be further evaluated in other populations.
Bisphenol A (BPA) is a high production volume chemical widely used in the manufacture of a variety of consumer products (1). Exposure to BPA in the general population is widespread with detectable urinary concentrations in more than 90% of individuals in a representative sample of US residents (2). Aglycone (unconjugated) BPA has weak estrogenic activity through binding with multiple estrogen receptors (3–8) and is also a ligand of the androgen receptor, the peroxisome proliferator–activated receptor γ, and the thyroid hormone receptor (9). Furthermore, the endocrine activity of BPA has been shown to adversely affect fertility in animal models. Specifically, experimental data in rodents have shown that BPA hampers ovarian follicle development and uterine receptivity (10–17).
Experimental data also suggest that the adverse reproductive effects of BPA can be modulated by specific dietary factors. Although data in this area are still emerging, 2 independent groups have reported that the reproductive effects of BPA in rodents are modified by the phytoestrogen content of diets. In mice fed a phytoestrogen-free diet, there was a clear dose-response relationship between BPA and congression failure (defined as the chromosomes' failure to align on an otherwise normal meiotic spindle), with abnormalities present in 2.0%, 3.5%, 3.6%, and 6.4% of the oocytes at increasing doses of BPA, whereas BPA was unrelated to oocyte abnormalities in the animals fed a soy-based diet (18). In the Agouti mouse model, maternal supplementation with phytoestrogens prevented BPA from shifting the coat color distribution of their offspring (19). We have recently reported little evidence of associations between urinary BPA concentrations and adverse reproductive and pregnancy outcomes among women undergoing infertility treatment with assisted reproductive technologies (20). However, we also found that soy intake was positively related to the probability of having a live birth during infertility treatment with assisted reproductive technologies (21). Therefore, the objective of this article was to examine the hypothesis that soy intake modifies the association between BPA and fertility in women undergoing assisted reproduction.
Subjects and Methods
Study population
Study participants were women enrolled in the EARTH Study, an ongoing prospective cohort established in 2004 to evaluate the role of environmental and nutritional determinants of fertility (22). Women 18 to 45 years old were eligible to participate, and approximately 60% of those contacted by the research nurses enrolled. The current analysis includes 239 women who completed at least 1 IVF cycle between 2007 and 2012 at the Massachusetts General Hospital (MGH) Fertility Center, had provided at least 1 urine sample for the measurement of BPA per IVF cycle, and had data on pretreatment soy food intake. The study was approved by the Human Studies Institutional Review Boards of the MGH, Harvard T. H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC). Participants signed an informed consent form after the study procedures were explained by a research nurse, and all questions were answered.
Clinical management and assessment of outcomes
Clinical information was abstracted from the patient's electronic medical record by research nurses. Subsequent to an infertility evaluation, each patient was given an infertility diagnosis by a physician at the MGH Fertility Center according to the Society for Assisted Reproductive Technology definitions as described previously (23). The participant's date of birth was collected at entry, and weight and height were measured by the nurse. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared.
Women underwent 1 of 3 controlled ovarian stimulation IVF treatment protocols on day 3 of induced menses after completing a cycle of oral contraceptives: (1) luteal phase GnRH-agonist protocol, (2) follicular phase GnRH-agonist/flare protocol, or (3) GnRH-antagonist protocol. The leuprolide dose was reduced at, or shortly after, the start of ovarian stimulation with FSH/human menopausal gonadotropin in the luteal phase GnRH-agonist protocol. The FSH/human menopausal gonadotropin and GnRH-agonist or GnRH-antagonist protocols were continued to the day of trigger with human chorionic gonadotropin (hCG). Throughout the monitoring phase of the subject's IVF treatment cycle, estradiol levels were obtained (Elecsys estradiol II reagent kit; Roche Diagnostics). Oocyte retrieval was completed when at least 3 follicles reached a diameter of 16 to 18 mm on transvaginal ultrasound, and the estradiol level reached at least 800 pg/mL. Patients were monitored during gonadotropin stimulation for serum estradiol, follicle size measurements and counts, and endometrial thickness through 2 days before egg retrieval. hCG was administered approximately 36 hours before the scheduled egg retrieval procedure to induce ovulation.
Embryologists determined the total number of oocytes retrieved per cycle and classified them as germinal vesicle, metaphase I, metaphase II (mature), or degenerated. Oocytes underwent either conventional IVF or intracytoplasmic sperm injection as clinically indicated. Embryologists determined the fertilization rate 17 to 20 hours after insemination as the number of oocytes with 2 pronuclei divided by the number of mature oocytes inseminated. The resulting embryos were assessed for cell count and assigned a quality score, ranging from 1 (best) to 5 (worst) based on morphological characteristics. We classified embryos as best quality if they had 4 cells on day 2, 8 cells on day 3, and a morphologic quality score of 1 or 2 on days 2 and 3. In women who underwent an embryo transfer, clinical outcomes were assessed. Implantation was defined as a serum β-hCG level of >6 mIU/mL, typically measured 17 days (range 15–20 days) after egg retrieval. An elevation in β-hCG with the confirmation of an intrauterine pregnancy on an ultrasound at 6 weeks was considered a clinical pregnancy. A live birth was defined as the birth of a neonate on or after 24 weeks of gestation.
Urine sample collection and BPA measurements
Women provided up to 2 spot urine samples per IVF cycle, with the first one collected between day 3 and day 9 of the gonadotrophin phase and the second one collected on the day of oocyte retrieval, before the procedure or administration of intravenous fluids. Urine was collected in a sterile, clean polypropylene specimen cup. Specific gravity (SG), which was used to correct BPA concentrations for urine dilution, was measured at room temperature using a handheld refractometer (National Instrument Company, Inc) calibrated with deionized water before each measurement. Eighty percent of the cycles (278 of 347 cycles) used 2 urine samples to characterize cycle-specific BPA levels. The urine was divided into aliquots, frozen, and stored at −80°C. Samples were shipped on dry ice overnight to the CDC where they were stored at or below −40°C until analysis. The urinary concentrations of the sum of free and conjugated BPA species (total BPA) were measured using online solid-phase extraction coupled with isotope dilution–HPLC-tandem mass spectrometry, as described before (24). First, 100 μL of urine was treated with β-glucuronidase/sulfatase (Helix pomatia, H1; Sigma-Aldrich) to hydrolyze the BPA-conjugated species. BPA was then retained and concentrated on a C18 reversed-phase size-exclusion solid-phase extraction column (Merck KGaA), separated from other urine matrix components using a pair of monolithic HPLC columns (Merck KGaA), and detected by negative ion–atmospheric pressure chemical ionization–tandem mass spectrometry. The limit of detection (LOD) for BPA was 0.4 μg/L. In addition to study samples, each analytical run included low-concentration and high-concentration quality control materials, prepared with spiked pooled human urine and reagent blanks to assure the accuracy and reliability of the data (24). BPA concentrations were corrected for urine dilution by SG using the formula: Pc = P[(1.015 − 1)/SG − 1], where Pc is the SG-corrected BPA concentration (micrograms per liter), P is the measured BPA concentration (micrograms per liter), and 1.015 is the mean SG in the study population (25). The geometric mean of the SG-adjusted BPA concentrations from 2 spot urine samples collected during each IVF cycle was used as a measure of cycle-specific urinary BPA concentration. For cycles with only 1 urine sample (∼20%), the BPA concentration for that single urine sample was used as the cycle-specific urinary BPA concentration. Samples with a BPA concentration below the LOD were assigned a value equal to the LOD divided by the square root of 2 before adjustment for urine dilution by SG as described previously (26).
Dietary assessment
At enrollment, a brief, nurse-administered questionnaire was used to collect data on demographics, medical history, and lifestyle. Participants also completed a detailed take-home questionnaire with additional questions on lifestyle factors, reproductive health, and medical history. Completion of this questionnaire took, on average, 30 minutes. The questionnaire included a section querying the frequency of consumption of 15 soy-based foods, a method previously found to produce valid estimates of intake (27–30). Women were asked to report how often, on average, they consumed each of these 15 foods during the preceding 3 months and to describe the usual serving size for each food in relation to a specified “medium” serving size. The 15 food items included in this questionnaire were tofu, tempeh, soy sausages, soy burgers, soy packages, miso soup, soy milk, soy yogurt, tofu cream, soy beans, soy nuts, soy drinks, soy protein, and soy bars. There were 9 possible frequencies of intake ranging from never or less than once per month to twice or more per day, and 3 possible usual serving sizes: medium (the specified serving size), small (less than specified), and large (more than specified). A more comprehensive dietary assessment was later added to the study with the introduction of a previously validated food frequency questionnaire (31), which asked participants to report how often, on average, they consumed specified amounts of 131 food items during the previous year. From this questionnaire we estimated dietary pattern adherence scores (32).
Statistical analysis
Demographic and baseline reproductive characteristics of the women are presented as medians ± interquartile ranges (IQRs) or percentages. Women were divided into quartiles of urinary BPA concentrations (based on their cycle-specific geometric mean of the SG-adjusted BPA as described above) with the lowest quartile considered the reference group. Associations between urinary BPA concentrations and demographics and baseline reproductive characteristics among women who consumed soy and women who did not were evaluated using Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables. Multivariable generalized linear mixed models were used to evaluate the association between urinary BPA concentrations and IVF outcomes among women who consumed soy and women who did not, with a random intercept to account for multiple IVF cycles in the same woman. A Poisson distribution and log link function were specified for oocyte counts, a normal distribution and identity link function were specified for endometrial wall thickness and estradiol (E2) trigger levels, and a binomial distribution and logit link function were specified for embryo quality, fertilization rates, and clinical outcomes (implantation, clinical pregnancy, and live birth). To test whether the relations between urinary BPA concentrations and IVF outcomes were modified by diet, a product of quartiles of BPA and a dichotomized soy variable (women who consumed soy vs who did not) was entered into the model. Tests for linear trends (33) were conducted using the median values of each quartile of urinary BPA concentration as a continuous variable. To allow for better interpretation of the results, population marginal means (34) were presented accounting for all the covariates in the model.
Confounding was assessed using prior knowledge on biological relevance and descriptive statistics from our study population. Specifically, we considered as potential confounders factors related to BPA and IVF outcomes that were not potential intermediates of this relation. These included factors previously related to IVF outcomes in this and other studies (35–39), as well as factors associated with BPA exposure and IVF outcomes in this study, regardless of whether they had been previously described as predictors of IVF outcomes. Final models were adjusted for age (continuous), BMI (continuous), race (white vs nonwhite), and infertility diagnosis (male, female, and unexplained). Sensitivity analyses were conducted to evaluate the possibility of residual confounding. In these analyses, covariates considered of clinical relevance were added to the multivariable model even if they did not have the statistical properties of a confounder (day 3 FSH, peak E2, and number of embryos transferred). We also restricted analyses to subpopulations of clinical relevance (cycles with an embryo transfer) and to subpopulations with additional data on potential confounders not available in the entire study population (women with complete diet assessment for adjustment for intake of folate, vitamin B12, and dietary patterns). All tests were two-tailed, and the level of statistical significance was set at .05. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc).
Results
The study population comprised 239 women with a median age of 35.0 years (IQR, 32–38 years) and a median BMI of 23.0 kg/m2 (IQR, 21.0–25.8 years), most of whom (71%) had never smoked. Of the women, 176 (74%) consumed soy foods. The mean isoflavone intake among soy food consumers was 3.4 mg/d. The average number of cycles per woman was 1.5 (range, 1–6 cycles); 168 (70%) women underwent 1 treatment cycle, 44 (18%) underwent 2 cycles, and 27 (11%) underwent ≥3 cycles. Urinary BPA concentrations ranged from <0.4 μg/L (LOD) to 16.6 μg/L, with a median (IQR) of 1.3 μg/L (0.9–1.9 μg/L). There was some indication of racial differences (P = .08) with higher BPA concentrations in Caucasian than in Asian women, among women who consumed soy foods (Table 1). No other baseline characteristics were significantly related to urinary BPA concentrations among women who consumed soy and who did not, respectively.
Table 1.
Demographic and Reproductive Characteristics of 239 Women in the EARTH Study According to Quartiles of Urinary BPA Concentrations by Soy Food Intake
| 63 Women Who Did Not Consume Soy |
176 Women Who Consumed Soy |
|||||
|---|---|---|---|---|---|---|
| Q1 (n = 16) | Q4 (n = 15) | P for Trenda | Q1 (n = 43) | Q4 (n = 45) | P for Trenda | |
| Soy food intake, servings/d | 0 (0) | 0 (0) | .99 | 0.2 (0.1–0.5) | 0.2 (0.1–0.4) | .39 |
| Urinary BPA, μg/L | 0.7 (0.6–0.9) | 2.4 (2.0–3.0) | <.0001 | 0.7 (0.6–0.8) | 2.7 (2.2–4.2) | <.0001 |
| Personal characteristics | ||||||
| Age, y | 37.0 (32.0–39.0) | 35.0 (33.0–39.0) | .27 | 35.0 (32.5–37.0) | 35.0 (32.0–38.0) | .97 |
| Race/ethnicity, n (%) | .65 | .08 | ||||
| White | 15 (93.8) | 13 (86.7) | 32 (74.4) | 40 (88.9) | ||
| Black | 0 (0) | 0 (0) | 0 (0) | 2 (4.4) | ||
| Asian | 0 (0) | 0 (0) | 7 (16.3) | 2 (4.4) | ||
| Other | 1 (6.2) | 2 (13.3) | 4 (9.3) | 1 (2.2) | ||
| BMI, kg/m2 | 22.8 (20.8–23.4) | 23.2 (20.1–26.2) | .34 | 23.0 (21.1–25.4) | 22.2 (21.2–24.5) | .76 |
| Current smoker, n (%) | .75 | .86 | ||||
| Never smoker | 12 (75.0) | 10 (66.7) | 32 (74.4) | 31 (68.9) | ||
| Ever smoker | 4 (25.0) | 5 (33.3) | 11 (25.6) | 14 (31.1) | ||
| Baseline reproductive characteristics | ||||||
| Initial infertility diagnosis | .72 | .23 | ||||
| Male factor | 5 (31.3) | 7 (46.7) | 18 (41.8) | 15 (33.3) | ||
| Female | 4 (25.0) | 6 (40.0) | 10 (23.3) | 15 (33.3) | ||
| Unexplained | 7 (43.7) | 2 (13.3) | 15 (34.9) | 15 (33.3) | ||
| Initial treatment protocol | .28 | .25 | ||||
| Antagonist | 1 (6.3) | 2 (13.3) | 3 (7.0) | 7 (15.6) | ||
| Flareb | 3 (18.7) | 1 (6.7) | 8 (18.6) | 9 (20.0) | ||
| Luteal phase agonistc | 12 (75.0) | 12 (80) | 32 (74.4) | 29 (64.4) | ||
| E2 trigger levels, pmol/Ld | 2192.5 (1682.0–2497.5) | 2242.0 (1577.0–2693.0) | .32 | 2017.5 (1453.0–2354.0) | 1937.0 (1381.0–2661.0) | .59 |
| Day 3 FSH levels, IU/Ld | 6.9 (6.0–7.5) | 6.8 (5.6–7.8) | .84 | 6.8 (5.5–8.7) | 7.3 (6.4–8.6) | .19 |
| Embryo transfer dayd | .86 | .23 | ||||
| Day 2 | 1 (6.7) | 1 (7.1) | 1 (2.4) | 1 (2.3) | ||
| Day 3 | 9 (60.0) | 8 (57.1) | 29 (70.7) | 29 (64.4) | ||
| Day 5 | 5 (33.3) | 5 (35.7) | 11 (26.8) | 15 (34.1) | ||
| No. of embryos transferred | .01 | .41 | ||||
| No embryos | 1 (6.3) | 1 (7.1) | 2 (4.7) | 1 (2.2) | ||
| 1 embryo | 3 (18.8) | 0 (0) | 5 (11.6) | 8 (17.8) | ||
| 2 embryos | 7 (43.7) | 12 (78.6) | 27 (62.8) | 28 (62.2) | ||
| 3+ embryos | 5 (31.2) | 2 (14.3) | 9 (20.9) | 8 (17.8) | ||
Abbreviation: Q, quartile. Data are medians (IQR) or n (%).
From Kruskal-Wallis test for continuous variables and χ2 tests for categorical variables.
Luteal phase GnRH-agonist protocol.
Follicular phase GnRH-agonist/flare protocol.
Indicates that those variables have some missing data.
Soy food consumption modified the association between urinary BPA concentrations and clinical outcomes of women undergoing infertility treatment with assisted reproductive technologies (Figure 1). Women who did not consume soy foods had lower rates of implantation, clinical pregnancy, and live birth across increasing quartiles of urinary BPA concentrations. On the other hand, urinary BPA concentrations were unrelated to these outcomes among women consuming soy foods (Figure 1). This differences in dose-response associations were statistically significant for implantation (P for interaction = .02), clinical pregnancy (P for interaction = .03), and live birth rates (P for interaction = .01) per initiated treatment cycle. Urinary BPA, however, was unrelated to intermediate outcomes (including measures of oocyte yield, oocyte quality, fertilization, and embryo quality) among both sets of women (Table 2).
Figure 1.
Effect of the modification by soy intake of the relation between implantation (P for interaction = .02), clinical pregnancy (P for interaction = .03), and live birth (P for interaction = .01) and urinary BPA concentrations (micrograms per liter) (n = 347 fresh cycles). Implantation (95% confidence interval) per initiated cycle across quartiles of urinary BPA concentrations (represented by the medians for each quartile) by soy intake is presented. Models are adjusted for age (continuous), BMI (continuous), race (white, black, Asian, and others), and infertility diagnosis (male, female, and unexplained). Tests for trend were performed using the median level of urinary BPA level in each quartile as a continuous variable in the model. Implantation was defined as a serum β-hCG level of >6 mIU/mL typically measured 17 days (range, 15–20 days) after egg retrieval, clinical pregnancy as the presence of an intrauterine pregnancy confirmed by ultrasound, and live birth as the birth of a neonate on or after 24 weeks of gestation. *, P < .05 comparing that quartile vs first quartile.
Table 2.
Adjusted Rates in Preclinical Outcomes per Initiated Cycle According to Urinary BPA Concentrations by Soy Intake Among 239 Women (Contributing to 347 Fresh Cycles) From the EARTH Study
| MII Oocyte Yield |
Total Oocyte Yield |
Proportion With >1 Best Embryo Qualitya |
Fertilization Rate, All Cycles |
|||||
|---|---|---|---|---|---|---|---|---|
| No Soy Intake | Soy Intake | No Soy Intake | Soy Intake | No Soy Intake | Soy Intake | No Soy Intake | Soy Intake | |
| Urinary BPA, μg/L | ||||||||
| <LOD 0.96 | 9.58 (7.74–11.85) | 8.90 (7.90–10.01) | 11.46 (9.26–14.19) | 10.33 (9.21–11.61) | 0.33 (0.14–0.60) | 0.41 (0.29–0.55) | 0.74 (0.64, 0.83) | 0.72 (0.67, 0.77) |
| 0.97–1.41 | 8.06 (6.29–10.33) | 9.0 (8.03–10.07) | 10.42 (8.19–13.27) | 10.77 (9.64–12.03) | 0.46 (0.20–0.74) | 0.47 (0.35–0.60) | 0.72 (0.59, 0.82) | 0.71 (0.66, 0.76) |
| 1.42–2.25 | 8.70 (7.21–10.48) | 10.41 (9.24–11.73) | 10.02 (8.30–12.10) | 11.69 (10.38–13.15) | 0.41 (0.22–0.63) | 0.44 (0.31–0.58) | 0.60 (0.50, 0.69) | 0.76 (0.71, 0.81) |
| 2.28–16.55 | 9.18 (7.37–11.42) | 7.97 (7.03–9.03) | 11.16 (8.96–13.92) | 9.49 (8.39–10.72) | 0.37 (0.17–0.63) | 0.46 (0.33–0.60) | 0.72 (0.61, 0.81) | 0.74 (0.68, 0.79) |
| P for trendb | 0.77 | 0.12 | 0.91 | 0.40 | 0.55 | 0.61 | 0.92 | 0.54 |
| P for interaction | 0.36 | 0.58 | 0.85 | 0.10 | ||||
Data are presented as rates (95% CI) adjusted for age (continuous), BMI (continuous), races (white, black, Asian, and others), and infertility diagnosis (male, female, and unexplained). A total of 100 fresh cycles were carried out among women who did not consume soy and 247 fresh cycles among women who consumed soy.
We classified embryos as best quality if they had 4 cells on day 2, 8 cells on day 3, and a morphologic quality score of 1 or 2 on days 2 and 3.
Tests for trend were performed using the median level of urinary BPA level in each group as a continuous variable in the model.
We also conducted a series of sensitivity analyses to address the possibility of residual confounding. Results remained unchanged after adjustment for day 3 FSH levels, peak E2 levels, and number of embryos transferred and when analyses were restricted to cycles with an embryo transfer (data not shown). The same pattern was also present in the subgroup of women for whom a complete pretreatment dietary assessment was conducted with a validated food frequency questionnaire (n = 192 women, 272 cycles). After additional adjustment for dietary patterns or intakes of folic acid and vitamin B12, the magnitudes of dose-response associations between BPA and implantation remained similar, although relations were not statistically significant (Supplemental Table 1).
Discussion
We evaluated whether the relation between urinary concentrations of BPA with IVF outcomes differed according to soy food intake in a prospective cohort study of 239 women who underwent 347 IVF cycles. We found suggestive evidence that BPA might relate to lower implantation, clinical pregnancy, and live birth rates only among women who did not consume soy foods but was unrelated to these outcomes among women who consumed soy foods. This pattern persisted in sensitivity analyses aimed to address potential sources of unmeasured confounding including intake of nutrients previously related to live birth rates in this cohort (35). We did not find any evidence, however, for an interaction between BPA and markers of ovarian response, oocyte quality, embryo quality, or fertilization rate.
The findings in the current analysis suggesting that soy intake modifies the relation between BPA and infertility treatment outcomes probably explain why we failed to find an association between urinary BPA concentrations and outcomes of women undergoing infertility treatment in our recent publication (20). Specifically, we observed no overall effect of BPA on a wide range of intermediate and clinical treatment endpoints, ranging from markers of oocyte and embryo quality to live birth rates. However, as our current analysis shows, there were significant associations between urinary BPA concentrations and pregnancy outcomes among women who do not consume soy products. It is also possible that the lack of association between BPA and couple fecundity observed in the Longitudinal Investigation of Fertility and the Environment (LIFE) Study (40) could also be the result of a strong underlying interaction with soy intake as was the case in our study. Our results have potential implications beyond BPA and soy. That is, when we explore the effects of environmental chemicals, it is important to consider modifiers such as diet, stress, or other factors.
Our findings are in agreement with experimental data in rodents showing a protective effect of soy on the adverse reproductive effects of BPA (18, 19). Muhlhauser et al (18) demonstrated that the effects of BPA on the growing oocyte are modulated by phytoestrogens in the diet. They performed an experiment in which female rodents treated with increasing doses of BPA were fed either a casein-based diet or a soy-based diet. Among mice fed the casein-based diet, there was a linear dose-response relationship between BPA and the frequency of spindle/chromosome alignment abnormalities. Among mice fed the soy-based diet, however, there was not a clear relation between BPA and congression failure. In a separate experimental model, Dolinoy et al (19) found that maternal supplementation with genistein, a phytoestrogen primarily found in soy, restored the coat color distribution in BPA-exposed offspring. Specifically, Agouti females exposed to BPA were assigned to receive either a phytoestrogen-free or a genistein-supplemented diet. Of the offspring of mice not supplemented with genistein, 21% had a yellow coat, whereas the frequency of yellow coat offspring among genistein-supplemented mice was 10%.
To our knowledge, this is the first time that an interaction between BPA and soy has been suggested in humans. Although the consistency of our findings with those of previous experiments in rodents suggests that they may represent a true biological interaction, it is not possible to discern the underlying biological mechanism for interaction from our data. Work in rodents suggests that the effects on DNA methylation may be one of the mechanisms underlying this interaction. In the Agouti mouse model, maternal exposure to BPA results in decreased methylation at the CabpIAP metastable epiallele in BPA-exposed offspring, indicating that BPA-induced DNA hypomethylation is not locus dependent (19). In contrast, dietary exposure to genistein induces gene hypermethylation (41) and reverses BPA-induced DNA hypomethylation (19). Genistein inhibits tyrosine kinase (42), scavenges free radicals (43), and, like BPA, exhibits mixed estrogenic and antiestrogenic properties (44) depending on timing, dose, and tissue. Whether these or other, yet to be described, biological mechanisms underlie the observed interaction is not known, highlighting the need for additional study of potential interactions between diet and BPA.
The present study has some limitations. First, because all study participants were women undergoing infertility treatment with assisted reproduction, it is not possible to know whether our findings are generalizable to women trying to become pregnant without medical interventions. However, women in this study are comparable to women attending fertility clinics in the United States (45), suggesting that these findings may be applicable to other women seeking infertility treatment. Second, as is the case for all studies based on diet questionnaires, measurement error and misclassification of intake are of concern. Nevertheless, similar questionnaires have been found to correlate well with biological markers of intake (27–30). Moreover, because soy food intake was assessed before the initiation of infertility treatment, it is unlikely that the error was related to treatment outcome. Therefore, the expected effect of this type of error is that the association between soy intake and treatment outcomes is actually stronger than that observed in this study. Third, although we included 347 urine samples in this analysis, some of the BPA quartiles, particularly among the 61 women who did not consume soy, have relatively small size. Misclassification of BPA exposure based on a spot urine sample is possible because BPA is a short-lived chemical, and exposures are probably episodic in nature, which might attenuate associations. Last, some legumes as well as bakery products made with soy protein–enriched flour contain small amounts of phytoestrogens, specifically of isoflavones (46). Nevertheless, soy and soy products are the primary sources of exposure to isoflavones in Western populations (47–50). In fact, among contemporary women in the United States, soy foods account for >90% of total isoflavone intake and among women in the Boston area, where the study was conducted, they account for nearly 97% of intake (49). As a result, the effect of failing to account for nonsoy sources of isoflavones is probably negligible. The strengths of our study include its prospective design and complete follow-up of participants, which minimizes the risk of reverse causation, and the comprehensive adjustment of possible confounding variables is made possible by standardized assessment of a wide range of participant characteristics. In addition, the similarity in the distribution of urinary BPA concentrations (51) and of isoflavone intake (27) between women in this study and women in the general population suggests that the findings may be generalizable.
In summary, we found that soy food intake may be protective against the adverse reproductive effects of BPA. These findings are in agreement with data from 2 independent experimental models in rodents. However, they represent the first report of a possible interaction between soy and BPA in humans, and as such it is important that these findings be further evaluated in other populations. Moreover, these results highlight the need to study how diet and other lifestyle factors amenable to change may modify the potentially deleterious health effects of BPA in particular and of environmental chemicals in general.
Acknowledgments
We gratefully acknowledge Xiaoyun Ye, Xiaoliu Zhou, Josh Kramer, and Tao Jia (Centers for Disease Control and Prevention, Atlanta, Georgia) for measuring the urinary concentrations of BPA. We also acknowledge all members of the EARTH Study Team, specifically the Harvard T. H. Chan School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd and Patricia Morey, and the physicians and staff at the Massachusetts General Hospital fertility center and give a special thanks to all the study participants.
Author contributions: J.E.C. and R.H. were involved in study concept and design and critical revision for important intellectual content of the manuscript. J.E.C. drafted the manuscript and had a primary responsibility for final content. L.M.-A. analyzed data. P.L.W. contributed to method modification and provided statistical expertise. A.J.G. reviewed the statistical analysis. J.E.C., L.M.-A., Y.-H.C., A.J.G., P.L.W., and R.H. interpreted the data. I.S. and A.M.C. were involved in acquisition of the data. All authors were involved in the critical revision of the manuscript and approved the final manuscript.
This work was supported by the National Institutes of Health (grants R01ES022955, R01ES009718, R01ES000002, T32DK007703-16, and P30DK46200). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the US Department of Health and Human Services or the CDC.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- BPA
- bisphenol A
- CDC
- Centers for Disease Control and Prevention
- E2
- estradiol
- hCG
- human chorionic gonadotropin
- IQR
- interquartile range
- IVF
- in vitro fertilization
- LOD
- limit of detection
- MGH
- Massachusetts General Hospital
- SG
- specific gravity.
References
- 1. Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA. 2014;311:859–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gould JC, Leonard LS, Maness SC, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142:203–214. [DOI] [PubMed] [Google Scholar]
- 4. Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. [DOI] [PubMed] [Google Scholar]
- 5. Dong S, Terasaka S, Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut. 2011;159:212–218. [DOI] [PubMed] [Google Scholar]
- 6. Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-α-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsushima A, Kakuta Y, Teramoto T, et al. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERRγ. J Biochem. 2007;142:517–524. [DOI] [PubMed] [Google Scholar]
- 8. Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-γ. Environ Health Perspect. 2008;116:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richter CA, Birnbaum LS, Farabollini F, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brieño-Enríquez MA, Robles P, Camats-Tarruella N, et al. Human meiotic progression and recombination are affected by bisphenol A exposure during in vitro human oocyte development. Hum Reprod. 2011;26:2807–2818. [DOI] [PubMed] [Google Scholar]
- 11. Rivera OE, Varayoud J, Rodríguez HA, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011;32:304–312. [DOI] [PubMed] [Google Scholar]
- 12. Xi W, Lee CK, Yeung WS, et al. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol. 2011;31:409–417. [DOI] [PubMed] [Google Scholar]
- 13. Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol. 2010;30:393–400. [DOI] [PubMed] [Google Scholar]
- 15. Berger RG, Hancock T, deCatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol. 2007;23:138–144. [DOI] [PubMed] [Google Scholar]
- 16. Berger RG, Shaw J, deCatanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17β-estradiol. Reprod Toxicol. 2008;26:94–99. [DOI] [PubMed] [Google Scholar]
- 17. Crawford BR, Decatanzaro D. Disruption of blastocyst implantation by triclosan in mice: impacts of repeated and acute doses and combination with bisphenol-A. Reprod Toxicol. 2012;34:607–613. [DOI] [PubMed] [Google Scholar]
- 18. Muhlhauser A, Susiarjo M, Rubio C, et al. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol Reprod. 2009;80:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mínguez-Alarcón L, Gaskins AJ, Chiu YH, et al. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum Reprod. 2015;30:2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanegas JC, Afeiche MC, Gaskins AJ, et al. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil Steril. 2015;103:749–755.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. [DOI] [PubMed] [Google Scholar]
- 23. Mok-Lin E, Ehrlich S, Williams PL, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. [DOI] [PubMed] [Google Scholar]
- 25. Smith KW, Braun JM, Williams PL, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120:1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meeker JD, Ehrlich S, Toth TL, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J Am Diet Assoc. 2009;109:245–254. [DOI] [PubMed] [Google Scholar]
- 28. Kirk P, Patterson RE, Lampe J. Development of a soy food frequency questionnaire to estimate isoflavone consumption in US adults. J Am Diet Assoc. 1999;99:558–563. [DOI] [PubMed] [Google Scholar]
- 29. Frankenfeld CL, Patterson RE, Kalhorn TF, Skor HE, Howald WN, Lampe JW. Validation of a soy food frequency questionnaire with plasma concentrations of isoflavones in US adults. J Am Diet Assoc. 2002;102:1407–1413. [DOI] [PubMed] [Google Scholar]
- 30. Frankenfeld CL, Patterson RE, Horner NK, et al. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. Am J Clin Nutr. 2003;77:674–680. [DOI] [PubMed] [Google Scholar]
- 31. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 32. Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosner B. Fundamentals of Biostatistics. 5th ed Pacific Grove, CA: Duxbury Press; 1982. [Google Scholar]
- 34. Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34:216–221. [Google Scholar]
- 35. Gaskins AJ, Afeiche MC, Wright DL, et al. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol. 2014;124:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaskins AJ, Chiu YH, Williams PL, et al. Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. Am J Clin Nutr. 2015;102:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chavarro JE, Ehrlich S, Colaci DS, et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril. 2012;98:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhillon RK, McLernon DJ, Smith PP, et al. Predicting the chance of live birth for women undergoing IVF: a novel pretreatment counselling tool. Hum Reprod. 2016;31:84–92. [DOI] [PubMed] [Google Scholar]
- 39. van Loendersloot LL, van Wely M, Repping S, Bossuyt PM, van der Veen F. Individualized decision-making in IVF: calculating the chances of pregnancy. Hum Reprod. 2013;28:2972–2980. [DOI] [PubMed] [Google Scholar]
- 40. Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. 2014;101:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 43. Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993;20:1–12. [DOI] [PubMed] [Google Scholar]
- 44. Price KR, Fenwick GR. Naturally occurring oestrogens in foods–a review. Food Addit Contam. 1985;2:73–106. [DOI] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2013 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinic Reports. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 46. US Department of Agriculture. USDA database for the isoflavone content of selected foods. Release 2.0. http://www.ars.usda.gov/nutrientdata.
- 47. Horn-Ross PL, Lee M, John EM, Koo J. Sources of phytoestrogen exposure among non-Asian women in California, USA. Cancer Causes Control. 2000;11:299–302. [DOI] [PubMed] [Google Scholar]
- 48. Ritchie MR, Cummings JH, Morton MS, Steel CM, Bolton-Smith C. A newly constructed and validated isoflavone database for the assessment of total genistein and daidzein intake. Br J Nutr. 2006;95:204–213. [DOI] [PubMed] [Google Scholar]
- 49. Huang MH, Norris J, Han W, et al. Development of an updated phytoestrogen database for use with the SWAN food frequency questionnaire: intakes and food sources in a community-based, multiethnic cohort study. Nutr Cancer. 2012;64:228–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54:184–201. [DOI] [PubMed] [Google Scholar]
- 51. Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables (February, 2015). Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf. [Google Scholar]