Abstract
Context:
Biomarkers to predict bone loss in premenopausal women after breast cancer treatment have not been examined.
Objective:
To determine whether baseline FSH predicts subsequent bone loss.
Design:
Secondary data analysis of the Exercise for Bone Health: Young Breast Cancer Survivors study, in which women were randomized to a 12-month exercise program or monthly health newsletter.
Setting:
Community dwelling women.
Participants:
A total of 206 women age less than or equal to 55 years at breast cancer diagnosis who had received adjuvant chemotherapy and were at least 1 year after diagnosis.
Intervention:
Serum collected at baseline (an average of 302 ± 148 d after completing chemotherapy) was analyzed for FSH.
Main Outcome Measure:
Change in bone mineral density.
Results:
In linear regression models, baseline FSH levels predicted bone loss over the ensuing 12 months at the lumbar spine and femoral neck including after adjustment for age, ethnicity, treatment group (exercise vs control), baseline bone density, and high-sensitivity C-reactive protein (P < .001). In multiply adjusted models, the 12-month rate of change in bone density was +0.007% in the lowest tertile of FSH (FSH = 9 ± 7 IU/L, mean ± SD), −0.96% in the middle tertile (mean FSH = 41 ± 11 IU/L), and −2.2% in the highest tertile (mean FSH = 86 ± 19 IU/L), P for trend <.001.
Conclusions:
Among premenopausal women with breast cancer treated with chemotherapy, baseline FSH levels are strongly associated with subsequent bone loss. Further studies are needed to establish the optimal timing of FSH measurement in relation to breast cancer treatment and to investigate potential strategies to prevent bone loss.
Breast cancer is the most common nonskin cancer diagnosed among women in the United States. Breast cancer accounts for 1 in 3 cancer diagnoses and is the second leading cause of cancer death in women. One in 8 women in the United States will develop breast cancer in her lifetime. In 2013, approximately 49 000 new cases of invasive breast cancer were diagnosed, and approximately 4800 breast cancer deaths occurred in women under age 50. Increasing numbers of premenopausal women diagnosed with breast cancer are becoming long-term survivors, due to more successful screening and treatment. From 1990 to 2010, breast cancer death rates dropped by 34% with a larger decline among women under age 50 than among women aged 50 and older (1). This increase in survival has occurred for all racial and ethnic groups. Successful breast cancer therapy may put premenopausal women at risk for long-term health consequences, including chemotherapy-induced ovarian dysfunction. Significant loss of ovarian function results in bone loss in all women, regardless of age. Identifying women who will lose bone density after breast cancer therapy can provide opportunity for intervention as bisphosphonate treatment can be used to prevent reductions in bone mineral density (BMD) in the setting of estrogen deficiency.
In premenopausal women, chemotherapy leads to ovarian dysfunction by depletion of ovarian follicles and damage to steroid-producing granulosa and theca cells, thereby lowering estradiol and inhibin B production (2). This leads to loss of feedback inhibition on pituitary gonadotrophs, increasing FSH levels (3). Disruption of the gonadal axis may be temporary or permanent with age more than 40 years and the type/duration of chemotherapy being the primary determinants of whether ovarian failure will occur after breast cancer therapy. In early ovarian dysfunction, serum estradiol levels may be preserved while gonadotropin levels, including FSH, begin to rise. Similar to natural menopause, chemotherapy induced ovarian failure ultimately results in amenorrhea, hypoestrogenemia, and sustained increases in FSH levels. Chemotherapy-induced ovarian failure has been defined as amenorrhea for more than or equal to 3 months, FSH more than or equal to 30 IU/L and nonpregnant at 1 year (4); however, lower FSH values may signal more subtle degrees of ovarian dysfunction that could have an impact on the skeleton.
FSH as a biomarker to predict bone loss in premenopausal women after breast cancer treatment has not been examined. Previous studies suggest clinical assessments such as questionnaires on menstruation do not reliably classify menopausal status in breast cancer patients (5, 6). Additionally, FSH itself has emerged as an important regulator of bone mass (7). FSH has been shown to increase mRNA expression of genes involved in osteoclastic phenotypes and function, including Rank and Cathepsin K, in a dose-dependent manner (8) and affect osteoclast activity and bone mass independent of estrogen levels in mice (9). Therefore, FSH may contribute directly to accelerated bone loss in the setting of ovarian dysfunction as well as through being a marker for ovarian dysfunction, resulting in an improved ability to predict bone loss compared with either clinical parameters such as menstruation history or estrogen levels. Finding a reliable biomarker that predicts bone loss would have significant implications for preserving bone health in premenopausal women with breast cancer, as an accurate biomarker could be used to predict which women will benefit from prophylactic treatment to prevent bone loss. Given the long skeletal half-life of bisphosphonates and potential for long-term complications such as atypical fractures, a means to identify which premenopausal women will benefit from therapy and which women do not need treatment is critical. We hypothesized that in premenopausal women with breast cancer treated with chemotherapy, baseline FSH levels predict bone loss over the ensuing 12 months.
Materials and Methods
We analyzed hormonal, dietary, and biomarker data from the Exercise for Bone Health: Young Breast Cancer Survivors study, a randomized, controlled trial of 206 premenopausal women age less than or equal to 55 years at diagnosis who had received adjuvant chemotherapy, were at least 1 year after diagnosis, and were cancer free to determine whether baseline parameters, including FSH, could predict ongoing bone loss; complete data was available for 188 women. Women withdrew from the study due to subject preference (n = 11), weight exceeding the bone density table limit (n = 2), moved out of the region (n = 2), pregnancy (n = 1), death (n = 1), and lost to follow-up (n = 1). Reasons for withdrawing were not different between treatment groups. Women were recruited between July 2006 and March 2010 and each followed for 1 year with the final study visit in March 2011. Women were recruited from the California Cancer Registry, had been diagnosed with local or regional stage breast cancer at age 50 or younger, and were residing in San Francisco, San Mateo, Marin, and Solano Counties in Northern California. All women provided written informed consent. Women were randomized to the exercise intervention, a 12-month exercise program with a combination of resistance training and aerobic exercise administered through the YMCA or the control group, which received a monthly health newsletter. Biologic samples were processed and stored at −80°C until completion of the study. Batched assays were performed at the Maine Medical Center Research Institute. FSH was measured at baseline by ELISA (Alpco). Areal BMD at the lumbar spine, femoral neck, and total hip was assessed by DXA (Hologic Delphi) at baseline and at 12 months. Daily phantom scanning and quality assurance testing were performed.
Rate of change in BMD was examined in terms of absolute change in bone density calculated as the month 12 value minus the baseline value in g/cm2 as well as percent change from baseline by dividing the change in bone density by the baseline value and multiplying by 100. The relationship between change in bone density and FSH was examined using linear regression with outcomes of absolute change in bone density and percent change in bone density over the 12-month study period. Models were adjusted for age, baseline bone density, race, and treatment group (exercise vs control) initially. Further adjustments were made for variables that remained significantly related to bone loss or had a scientific rationale for possible effects on bone density such as tamoxifen. Final models included age, baseline bone density, race, treatment group (exercise vs control), high-sensitivity C-reactive (hsCRP) protein, tamoxifen use, menstruating at baseline (yes/no), serum estradiol, days between completing chemotherapy and study baseline visit, and serum C-telopeptide. In addition, subject characteristics were examined among tertiles of FSH using ANOVA for overall comparisons and post-ANOVA comparisons were done using the Sidak test to identify differences between tertiles.
Results
Study participants were 45.9 ± 5.5 years of age with an average body mass index (BMI) of 26.1 ± 6.4 kg/m2. Most the participants were White and most had received tamoxifen as part of their treatment regimen. Less than 20% of participants were continuing to menstruate (Table 1) at the time of study enrollment which occurred an average of 302 ± 148 days after completing chemotherapy. All women were premenopausal at the time of breast cancer diagnosis before chemotherapy per the National Comprehensive Cancer Network guidelines.
Table 1.
Baseline Characteristics for the 188 Women With Premenopausal Breast Cancer Treated With Adjuvant Chemotherapy
| Age (y) | 45.9 ± 5.5 |
| BMI (kg/m2) | 26.1 ± 6.4 |
| Race (% White) | 64% |
| Lumbar spine BMD (g/cm2) | 1.002 ± 0.11 |
| Femoral neck BMD (g/cm2) | 0.797 ± 0.10 |
| 25 hydroxyvitamin D (ng/mL) | 31.2 ± 10.1 |
| FSH (IU/L) | 45.8 ± 34.2 |
| Currently menstruating (% yes) | 16% |
| Time from end of chemotherapy to baseline visit (d) | 302 ± 148 |
| Treatment includes tamoxifen (% yes) | 57% |
| Chemotherapy received (%) | |
| Adriamycin | 57% |
| Cyclophosphamide | 72% |
| Fluorouracil | <1% |
| Methotrexate | <1% |
| Phenylalanine mustard | <1% |
| Paclitaxel | 50% |
| Docetaxel | 30% |
| Other | 17% |
| Do not know | 4% |
Values are mean ± SD or %. BMD, bone mineral density.
In linear regression models in the whole cohort, baseline FSH levels predicted bone loss over the ensuing 12 months at the lumbar spine and femoral neck including after adjustment for age, ethnicity, treatment group (exercise vs control), and baseline bone density (P < .001). Further adjustment for tamoxifen use, days since chemotherapy, continuing to menstruate (yes/no), and serum estradiol did not alter the relationship; none of these additional variables were significantly related to bone loss. In univariate or minimally adjusted models containing FSH, age, ethnicity, treatment group, and baseline bone density, bone turnover markers were not related to bone loss. In multivariate models adjusted for all covariates, serum C-telopeptide was related to bone loss at the lumbar spine with P = .04 (Table 2). Other baseline variables including calcitropic hormones and dietary intakes were not significantly related to bone loss and were not significant in the linear regression models.
Table 2.
Regression Coefficients and Standardized (β) Regression Coefficients From the Fully Adjusted Linear Regression Model Examining the Effects of Baseline FSH on Change in BMD at the Lumbar Spine Over the Ensuing 12 Months in 188 Women Diagnosed With Premenopausal Breast Cancer
| Coefficient | β-Coefficient | SE | P Value | |
|---|---|---|---|---|
| FSH (IU/L) | −0.023 | −0.219 | 0.008 | .007 |
| Age (y) | −0.141 | −0.222 | 0.048 | .004 |
| Baseline LS BMD (g/cm2) | −4.258 | −0.139 | 2.185 | .05 |
| Treatment group (exercise vs control) | 0.867 | 0.126 | 0.490 | .08 |
| Race/ethnicity (White, Black, Asian, Hispanic) | −0.533 | −0.160 | 0.229 | .02 |
| hsCRP (ng/mL) | −0.0001 | −0.174 | 0.0001 | .01 |
| Tamoxifen use (yes/no) | 0.083 | 0.048 | 0.129 | .52 |
| Menstruating (yes/no) | 0.151 | 0.017 | 0.714 | .83 |
| Time from chemotherapy (d) | −0.002 | −0.090 | 0.002 | .20 |
| Estradiol (pg/mL) | −0.004 | −0.101 | 0.003 | .14 |
| lnCTX (ng/mL) | −1.034 | −0.162 | 0.508 | .04 |
BMD, bone mineral density; hsCRP, high sensitivity C reactive protein; lnCTX, serum C-terminal telopeptide (log transformed); LS, lumbar spine.
In similarly constructed models with bone density at the femoral neck as the outcome, FSH also was significantly related to bone loss (P = .02), including after adjustment for all covariates (Table 3). In contrast to the lumbar spine bone loss models, in either the minimally or multiply adjusted femoral neck bone loss models, serum C-telopeptide was not significantly related to bone loss at the femoral neck.
Table 3.
Regression Coefficients and Standardized (β) Regression Coefficients From the Fully Adjusted Linear Regression Model Examining the Effects of Baseline FSH on Change in BMD at the Femoral Neck Over the Ensuing 12 Months in 188 Women Diagnosed With Premenopausal Breast Cancer
| Coefficient | β-Coefficient | SE | P Value | |
|---|---|---|---|---|
| FSH (IU/L) | −0.0002 | −0.207 | 0.00007 | .02 |
| Age (y) | −0.0003 | −0.056 | 0.0004 | .50 |
| Baseline femoral neck BMD (g/cm2) | −0.0532 | −0.199 | 0.0220 | .02 |
| Treatment group (exercise vs control) | 0.0008 | 0.0145 | 0.0041 | .85 |
| Race/ethnicity (White, Black, Asian, Hispanic) | −0.0005 | −0.021 | 0.0019 | .79 |
| hsCRP (ng/mL) | −0.0000003 | −0.050 | 0.0000005 | .51 |
| Tamoxifen use (yes/no) | 0.0035 | 0.093 | 0.0029 | .23 |
| Menstruating (yes/no) | −0.0016 | −0.022 | 0.0062 | .80 |
| Time from chemotherapy (d) | −0.000007 | −0.039 | 0.00001 | .60 |
| Estradiol (pg/mL) | 0.00001 | 0.031 | 0.00003 | .68 |
| lnCTX (ng/mL) | −0.0029 | −0.058 | 0.0044 | .50 |
BMD, bone mineral density; hsCRP, high sensitivity C reactive protein; lnCTX, serum C-terminal telopeptide (log transformed); LS, lumbar spine.
The relationship between baseline FSH and subject characteristics, bone density, and metabolic parameters was further evaluated by examining the data by tertiles of FSH. There were no significant differences across FSH tertiles in terms of height, BMI, or race (White vs non-White); however, many other parameters were significantly different across tertiles of FSH (Table 4). Similar to the linear regression models using FSH as a continuous variable described above, multiply adjusted models examining bone loss by FSH tertiles that included all covariates demonstrated a significant difference in bone loss across the tertiles of FSH. The change in BMD at 12 months was +0.0008 g/cm2 in the lowest tertile of FSH (mean FSH = 9 ± 7 IU/L, mean ± SD), −0.012 g/cm2 in the middle tertile (mean FSH = 41 ± 11 IU/L), and −0.021 g/cm2 in the highest tertile (mean FSH = 86 ± 19 IU/L), P for trend < .001. In terms of percent change, the change in lumbar spine bone density was 0.18 ± 1.4% (mean ± SD) in the lowest tertile, −1.12 ± 1.3% in the middle tertile, and −2.1 ± 1.1% in the highest tertile of FSH over the 12-month study interval, P for trend <.001 (Figure 1).
Table 4.
Baseline Subject Characteristics by FSH Tertiles in 188 Premenopausal Women Diagnosed With Breast Cancer and Treated With Adjuvant Chemotherapy
| FSH Range in Each Tertile |
P Value | |||
|---|---|---|---|---|
| 0–21.1 IU/L (n = 68) | 21.2–61.6 IU/L (n = 67) | 61.7–124.6 IU/L (n = 67) | ||
| Age (y) | 42.6 ± 6.2 | 46.8 ± 5.4 | 47.6 ± 4.5 | <.001 |
| Weight (kg) | 71.0 ± 18.5 | 70.1.0 ± 18.2 | 71.0 ± 18.5 | .08 |
| Height (cm) | 163.3 ± 7.0 | 163.2 ± 7.4 | 161.2 ± 10.6 | NS |
| BMI (kg/m2) | 26.6 ± 6.4 | 26.3 ± 6.9 | 25.2 ± 5.4 | NS |
| LS BMD (g/cm2) | 1.023 ± 0.130 | 1.001 ± 0.098 | 0.979 ± 0.100 | .06 |
| FN BMD (g/cm2) | 0.836 ± 0.010 | 0.793 ± 0.100 | 0.760 ± 0.093 | <.001 |
| Hip BMD (g/cm2) | 0.962 ± 0.115 | 0.939 ± 0.112 | 0.885 ± 0.098 | .002 |
| Menstruating (% yes) | 43% | 6% | 6% | <.001 |
| Tamoxifen (% yes) | 56% | 85% | 39% | <.001 |
| Race | NS | |||
| White | 62% | 72% | 70% | |
| Non-White | 38% | 28% | 30% | |
| Estradiol (pg/mL) | 100.3 ± 78.4 | 89.5 ± 94.1 | 68.5 ± 49.4 | .05 |
| 25-OH D (ng/mL) | 28.8 ± 9.9 | 32.8 ± 13.8 | 37.2 ± 13.6 | <.001 |
| Alkaline phosphatase (U/L) | 66.8 ± 22.8 | 65.7 ± 18.0 | 76.7 ± 28.5 | NS |
| Urinary NTX (nmBCE/mmol Cr) | 53.6 ± 22.1 | 62.4 ± 25.0 | 84.4 ± 45.9 | NS |
| CTX (ng/mL) | 0.49 (0.34–0.67) | 0.56 (0.42–0.75) | 0.75 (0.54–1.11) | <.001 |
| P1NP (ng/mL) | 64.6 ± 29.9 | 59.2 ± 25.2 | 88.6 ± 48.4 | <.001 |
| Osteocalcin (ng/mL) | 21.4 ± 8.0 | 21.5 ± 10.5 | 31.1 ± 13.0 | <.001 |
| iPTH (pg/mL) | 44.3 ± 21.6 | 55.3 ± 44.9 | 38.6 ± 16.4 | .006 |
| 1,25-(OH)2 vitamin D (pg/mL) | 32.7 ± 12.1 | 31.6 ± 10.7 | 30.7 ± 7.9 | NS |
Values are mean ± SD or %; CTX values are median and interquartile range. LS, lumbar spine; CTX, C-terminal telopeptide; NTX, N-terminal telopeptide; P1NP, total procollagen type 1 amino-terminal propeptide; iPTH, intact PTH; BMD, bone mineral density; 1,25-(OH)2 vitamin D, 1,25 dihydroxyvitamin D; NS, not significant. P values are for overall comparisons among tertiles by ANOVA.
Figure 1.
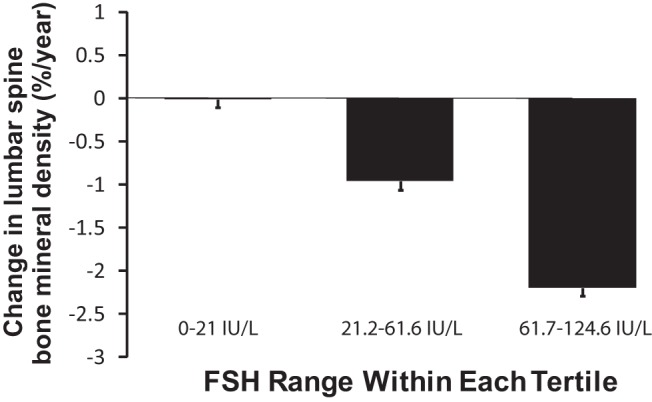
One-year percent change in BMD by tertile of FSH in 188 women diagnosed with premenopausal breast cancer, adjusted for age, baseline bone density, race, treatment group (exercise vs control), hsCRP, tamoxifen use, menstruation at baseline, serum estradiol, days between completing chemotherapy and study baseline visit, and serum C-telopeptide. P value for trend <.001 across tertiles.
Discussion
Between 50% and 70% of premenopausal women with breast cancer treated with adjuvant chemotherapy will develop chemotherapy induced ovarian failure, with significant psychological and physical implications (10, 11). In young breast cancer survivors (≤50 y of age), concerns about menopausal symptoms and infertility are common and are related to level of distress after treatment (12, 13). Similar to natural menopause, chemotherapy-induced chemotherapy induced ovarian failure causes bone loss via reduction in estradiol levels (14). A previous study in premenopausal women with breast cancer demonstrated that those who resumed menstruating after chemotherapy showed little change in BMD over the 5 years after initial treatment. By contrast, the women in this study who developed amenorrhea experienced large decreases in BMD at the lumbar spine and femoral neck (15). Another study of premenopausal breast cancer patients treated with adjuvant chemotherapy showed that during 10 years of follow-up, nearly 90% of women developed menstrual irregularities or amenorrhea. Over the 10-year period after chemotherapy, total bone loss at the lumbar spine was −5% in women with preserved menstruation, −15% in those with irregular menses and −13% in amenorrheic women (16). The changes in lumbar spine BMD correlated significantly with menstrual function. One-third of women with ovarian dysfunction (irregular menses or amenorrhea) developed osteoporosis of the lumbar spine during the follow-up period. Other studies have confirmed that premenopausal women with stage I/II breast cancer treated with adjuvant chemotherapy who experienced chemotherapy induced ovarian failure have rapid and significant bone loss at the lumbar spine (17).
Recognizing chemotherapy induced ovarian dysfunction in premenopausal women after breast cancer treatment is important for several reasons, including the need to prevent early bone loss in this population. Treatment with the iv bisphosphonate, zoledronic acid, every 3 months has been shown to prevent bone loss in premenopausal women who develop chemotherapy-induced chemotherapy induced ovarian failure, defined as amenorrhea for more than or equal to 3 months, FSH more than or equal to 30 IU/L and nonpregnant at 1 year (4). However, bisphosphonate therapy in the premenopausal population carries several significant risks, including osteonecrosis of the jaw, the rare complication of atypical fractures, and potential effects on fetal development (18–21). Bisphosphonates may remain in the skeleton for months to years after cessation of use, complicating long-term reproductive decisions for premenopausal women. A reliable method of targeting which women will benefit from prophylactic bisphosphonate therapy could target this intervention to those women who will derive benefit without having to wait for bone loss to occur to identify which women need therapy.
Clinical parameters do not reliably identify breast cancer patients with chemotherapy induced ovarian dysfunction, as questionnaires on menstruation are subjective and have been shown to lead to inaccurate classification of menopausal status (5, 6). In our study, women in the middle and highest tertiles of FSH had, on average, estradiol levels above the postmenopausal range (<55 ng/mL) despite over 95% of these women reporting that they were not menstruating at baseline. Less than 60% of women in the lowest tertile of FSH reported menstruating at baseline and at 12 months, but they maintained bone mass. Among women with low FSH levels, disruption of their menstrual cycles does not appear to correlate to bone loss. Conversely, although the current National Comprehensive Cancer Network definition of menopause includes FSH level more than or equal to 40 IU/L and low estradiol level, our results show that women with FSH levels as low as 22.5 IU/L (middle tertile) and normal estradiol levels experienced bone loss. This further supports FSH as a sensitive and early marker of bone loss in premenopausal women with breast cancer who undergo treatment with adjuvant chemotherapy.
Based upon our data, baseline measurement of FSH level after completion of breast cancer treatment can predict which women will benefit from prophylactic osteoporosis treatment to prevent bone loss. FSH predicted bone loss better than any other parameter in our models, including self-reported menstrual status and estradiol levels.
As expected, women in the highest tertile of FSH had the highest levels of bone turnover markers (C-telopeptide, osteocalcin, and total procollagen type 1 amino-terminal propeptide). Women in the highest tertile of FSH were older and also had higher levels of 25-OH vitamin D and lower intact PTH levels; this may reflect higher usage of vitamin D supplements in these women.
There are limitations to our study. Participants were not stratified by tamoxifen use. In the Breast Cancer Prevention Trial, tamoxifen use was associated with a 45% reduction in hip fracture (relative risk, 0.55; 95% confidence interval, 0.25–1.15) (22). Tamoxifen treatment has been shown induce ovarian suppression in premenopausal breast cancer patients (low estradiol and low FSH, LH levels) (23). It is possible that tamoxifen treatment accounted for reduced bone loss and low FSH levels in a subset of participants. However, in our study the women in the highest 2 tertiles of FSH were losing bone density despite a higher prevalence of tamoxifen use than the lowest tertile of FSH.
Further studies are needed to establish the population of premenopausal breast cancer patients in which FSH measurement could be useful. It will be important to determine the optimal timing of FSH measurement in relation to breast cancer treatment. Appropriate use of FSH, as a marker of chemotherapy induced ovarian dysfunction and predictor of bone loss after breast cancer treatment, may allow for the timely implementation of preventive measures and treatments to reduce fractures while avoiding treatment in women who will maintain bone mass.
Acknowledgments
This work was supported by National Institutes of Health Grants R01CA112273 and R01CA112273-03S1.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- hsCRP
- high-sensitivity C-reactive.
References
- 1. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. [DOI] [PubMed] [Google Scholar]
- 2. Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110(10):2222–2229. [DOI] [PubMed] [Google Scholar]
- 3. Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–1729. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro CL, Halabi S, Hars V, et al. Zoledronic acid preserves bone mineral density in premenopausal women who develop ovarian failure due to adjuvant chemotherapy: final results from CALGB trial 79809. Eur J Cancer. 2011;47(5):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson BD, Merz CN, Braunstein GD, et al. Determination of menopausal status in women: the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt). 2004;13(8):872–887. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell ES, Woods NF, Mariella A. Three stages of the menopausal transition from the Seattle Midlife Women's Health Study: toward a more precise definition. Menopause. 2000;7(5):334–349. [DOI] [PubMed] [Google Scholar]
- 7. Colaianni G, Cuscito C, Colucci S. FSH and TSH in the regulation of bone mass: the pituitary/immune/bone axis. Clin Dev Immunol. 2013;2013:382698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Zhang W, Yu C, et al. Follicle-stimulating hormone increases the risk of postmenopausal osteoporosis by stimulating osteoclast differentiation. PLoS One. 2015;10(8):e0134986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. [DOI] [PubMed] [Google Scholar]
- 10. Knobf MT. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. Oncologist. 2006;11(2):96–110. [DOI] [PubMed] [Google Scholar]
- 11. Molina JR, Barton DL, Loprinzi CL. Chemotherapy-induced ovarian failure: manifestations and management. Drug Saf. 2005;28(5):401–416. [DOI] [PubMed] [Google Scholar]
- 12. Anderson DJ, Yates P, McCarthy A, et al. Younger and older women's concerns about menopause after breast cancer. Eur J Cancer Care (Engl). 2011;20(6):785–794. [DOI] [PubMed] [Google Scholar]
- 13. Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. [DOI] [PubMed] [Google Scholar]
- 14. Coleman RE, Rathbone E, Brown JE. Management of cancer treatment-induced bone loss. Nat Rev Rheumatol. 2013;9(6):365–374. [DOI] [PubMed] [Google Scholar]
- 15. Vehmanen L, Saarto T, Elomaa I, Mäkelä P, Välimäki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37(18):2373–2378. [DOI] [PubMed] [Google Scholar]
- 16. Vehmanen LK, Elomaa I, Blomqvist CP, Saarto T. The effect of ovarian dysfunction on bone mineral density in breast cancer patients 10 years after adjuvant chemotherapy. Acta Oncol. 2014;53(1):75–79. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19(14):3306–3311. [DOI] [PubMed] [Google Scholar]
- 18. Rathbone EJ, Brown JE, Marshall HC, et al. Osteonecrosis of the jaw and oral health-related quality of life after adjuvant zoledronic acid: an adjuvant zoledronic acid to reduce recurrence trial subprotocol (BIG01/04). J Clin Oncol. 2013;31(21):2685–2691. [DOI] [PubMed] [Google Scholar]
- 19. Rugani P, Luschin G, Jakse N, Kirnbauer B, Lang U, Acham S. Prevalence of bisphosphonate-associated osteonecrosis of the jaw after intravenous zoledronate infusions in patients with early breast cancer. Clin Oral Investig. 2014;18(2):401–407. [DOI] [PubMed] [Google Scholar]
- 20. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–1565. [DOI] [PubMed] [Google Scholar]
- 21. McNicholl DM, Heaney LG. The safety of bisphosphonate use in pre-menopausal women on corticosteroids. Curr Drug Saf. 2010;5(2):182–187. [DOI] [PubMed] [Google Scholar]
- 22. Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326(13):852–856. [DOI] [PubMed] [Google Scholar]
- 23. Berliere M, Duhoux FP, Dalenc F, et al. Tamoxifen and ovarian function. PLoS One. 2013;8(6):e66616. [DOI] [PMC free article] [PubMed] [Google Scholar]


