Abstract
Context:
NNC0195-0092 is a reversible, albumin-binding GH derivative, developed for once-weekly administration.
Objectives:
The objective of the study was to evaluate safety, local tolerability, pharmacodynamics, and pharmacokinetics of multiple, once-weekly doses of NNC0195-0092, compared with daily GH.
Design and Setting:
This was a phase 1, randomized, open-label, active-controlled, multiple-dose, dose-escalation trial.
Patients:
Thirty-four GH-treated adult subjects (male, n = 25) with GH deficiency participated in the study.
Interventions and Main Outcome Measures:
Subjects were sequentially assigned into four cohorts of eight subjects, randomized within each cohort (3:1) to once-weekly NNC0195-0092 (n = 6) for 4 weeks (0.02, 0.04, 0.08, and 0.12 mg/kg) or daily injections of Norditropin NordiFlex (n = 2) for 4 weeks with a dose replicating the pretrial dose of somatropin. A safety assessment was performed prior to initiating treatment at the next dose level of NNC0195-0092. Daily GH treatment was discontinued 14 days before the trial start. Blood samples were drawn for assessment of safety, pharmacokinetics, pharmacodynamics (IGF-1 and IGF-binding protein-3) profiles, and immunogenicity studies.
Results:
Numbers of adverse events were similar at the dose levels of 0.02, 0.04, and 0.08 mg/kg NNC0195-0092 vs daily injections of Norditropin NordiFlex, whereas the number of adverse events was greater at the highest dose level of NNC0195-0092 (0.12 mg/kg). NNC0195-0092 (area under the curve[0–168h]) and peak plasma concentration) increased in a dose-dependent manner, and a dose-dependent increase in IGF-1 levels was observed. IGF-1 profiles were elevated for at least 1 week, and for the 0.02-mg/kg and 0.04-mg/kg NNC0195-0092 doses, the observed IGF-1 levels were similar to the levels for the active control group.
Conclusion:
Four once-weekly doses of NNC0195-0092 (dose range 0.02–0.12 mg/kg) administered to adult patients with GH deficiency were well tolerated, and IGF-1 profiles were consistent with a once-weekly treatment profile. No clinically significant safety and tolerability signals causally related to NNC0195-0092 were identified, nor were any immunogenicity concerns revealed.
GH replacement therapy has proven to be both efficacious and safe in adults with GH deficiency (AGHD) (1–3). GH is currently administered as daily sc injections; however, a long-acting GH formulation that decreases injection frequency may improve treatment adherence and reduce the inconvenience associated with daily injections. In AGHD similar efficacy and safety have been reported with daily administration and long-acting or sustained-release GH preparations (4–6), indicating that a long-acting GH may be as safe and efficacious as once-daily injections of GH.
NNC0195-0092 is a novel reversible, albumin-binding human GH (hGH) derivative, intended for once-weekly sc administration with the aim of improving convenience for patients by reducing injection frequency from 365 to 52 injections per year and potentially improving treatment adherence. The plasma half-life of therapeutic peptides, such as GH, can be extended through binding to serum albumin. Serum albumin has a high affinity and binding capacity for fatty acids, and acylation of fatty acids to therapeutic proteins has been used to facilitate binding of these molecules to circulating albumin. Indeed, acylation in insulin detemir, a long-acting insulin analog (7), and liraglutide, a long-acting glucagon-like peptide-1 derivative (8), has been successfully used to enable albumin binding, which contributes to a protracted action of the molecules (7, 8). In NNC0195-0092, fatty acids with noncovalent, albumin-binding properties have been attached by acylation. Based on unpublished nonclinical experiments, the albumin-binding properties are expected to prolong the absorption phase and reduce the clearance. In humans, noncovalent binding of the molecule to albumin in the blood significantly prolongs the in vivo half-life in accordance with a reduced clearance (9).
The safety, pharmacokinetics (PK), and IGF-1 profiles generated in a recent single-dose and multiple-dose clinical trial of NNC0195-0092 involving healthy adult male subjects indicated the feasibility of a once-weekly dosing regimen for NNC0195-0092 (9). We now report the first data obtained from a multiple-dose trial of NNC0195-0092 in AGHD.
Subjects and Methods
Subjects
Adults of both genders (aged 20–70 y, body mass index 18–35 kg/m2) diagnosed with GH deficiency (GHD) according to either of two recent consensus guidelines criteria (10, 11) and receiving stable GH replacement therapy for 3 months or longer were eligible for inclusion. An inclusion criterion, IGF-1 level within −2.0 to +2.0 SD score (SDS) of the age and sex in the normal range, was recommended to be deleted during the trial because IGF-1 SDS was +2.0 SDS in some subjects, despite stable GH replacement for 3 months or longer according to local IGF-1 values. The deletion of this inclusion criterion was a per-protocol amendment and was reviewed and approved by the relevant regulatory agencies prior to implementation. The amendment was applied after the first dosing cohort.
Exclusion criteria were as follows: malignant disease, proliferative retinopathy, heart insufficiency (New York Heart Association class >2), poorly controlled diabetes mellitus (hemoglobin A1c [HbA1c] >8.0 mmol/L [or 8.0%]), or insulin therapy. In addition, patients were excluded if they had stable pituitary replacement therapy for less than 3 months or if they had any history of illnesses, disease, or medication that, in the investigator's opinion, may pose a risk or confound the results after the administration of the trial product. All subjects ceased GH therapy 2 weeks prior to the first dose of NNC0195-0092 or Norditropin NordiFlex (Novo Nordisk, Denmark). Before initiating any trial-related activity, written informed consent was obtained from all participants.
Trial design and procedures
The trial (number NCT01706783) was conducted at four hospitals (Denmark: Aarhus University Hospital, Odense University Hospital, Copenhagen University Hospital; Sweden: Karolinska University Hospital) between October 2012 and November 2013, in accordance with the Declaration of Helsinki (12) and the International Conference on Harmonisation and good clinical practice guidelines (13), and relevant approvals according to local guidelines were obtained from Danish and Swedish health authorities and independent ethics committee institutional review boards (Denmark: De Videnskabsetiske Komitéer for Region Midtjylland, Viborg; Sweden: Regionala Etikprövningsnämnden i Stockholm, FE 289 Karolinska Institutet, Stockholm).
This was a phase 1, prospective, randomized, open-label, active-controlled, multiple-dose, dose-escalating, sequential dose-group trial. The primary objective was to evaluate the safety and tolerability of once-weekly NNC0195-0092 in adults with GHD vs once-daily Norditropin NordiFlex. Secondary objectives were to evaluate the PK and pharmacodynamics (PD) of multiple, once-weekly dosing of NNC0195-0092 as well as local tolerability (injection site reactions) vs daily injections of Norditropin NordiFlex. PD was assessed using serum concentrations of IGF-1 and IGF-binding protein-3 (IGFBP-3).
Subjects were sequentially assigned into four dose cohorts of eight subjects (0.02, 0.04, 0.08, and 0.12 mg/kg) and within each cohort were randomized (3:1) to either once-weekly NNC0195-0092 (n = 6) for 4 weeks or daily injections of Norditropin NordiFlex (n = 2) for 28 days. Progress to the higher-dose level in a new cohort of subjects took place after evaluation (by an internal safety assessment group at Novo Nordisk A/S) of interim data on safety, PK, and IGF-1 levels from the previous dose level. In accordance with the trial protocol, the internal nonindependent safety group comprised the vice president of clinical pharmacology, the responsible international trial manager, the responsible international medical director, a representative from global safety, and a representative from the drug metabolism and pharmacokinetics, cell, and antibody analysis department. The safety group mandate was to approve progress to the next planed dose level if one or fewer nonacceptable adverse events (AEs) had been reported and/or detected by laboratory measurements and no concerns were raised based on the PK and PD properties. The safety data from a minimum of seven subjects in a cohort were evaluated by the safety group prior to dose escalation.
Doses of NNC0195-0092 were administered once weekly for 4 weeks in the morning after overnight fasting by sc injections, using a syringe and needle (gauge 31; length 6 mm). NNC0195-0092 was supplied as a freeze-dried powder in single-use vials (6.7 mg/vial) for reconstitution in 1.1 mL of sterile water. Norditropin NordiFlex (10 mg per 1.5 mL) was administered once daily by sc injection for 28 days, with NordiFlex pen (gauge 31; length 6 mm). All injections were made at alternating injection sites in the left or right thigh, and sequential doses for doses above 2 mL were made immediately after each other, 3 cm apart. The viscosity of NNC0195-0092 is similar to Norditropin. Trial drug administrations (Norditropin NordiFlex and NNC0195-0092) were performed at the trial site under the supervision of the investigator, except for the daily dose of Norditropin between days 15 and 22 (visits 7–9). Compliance was assessed by examination of returned trial product.
Subjects were closely monitored at the trial site for 3 days after dosing and then at daily clinic visits for an additional 4 days after the first and fourth doses of NNC0195-0092 and correspondingly after the Norditropin NordiFlex dose administration on days 1 and 22. Blood samples were taken for safety assessment (biochemistry and hematology) at baseline and 7 days after the first and fourth NNC0195-0092 dose (24, 48, 72, and 168 h); fasting samples for blood glucose and insulin were taken at baseline and after the dose (72 and 168 h). PK and PD (IGF-1 and IGFBP-3) sampling time points are provided below. After the second and third doses of NNC0195-0092 and correspondingly after the Norditropin NordiFlex dose administration on days 8 and 15, subjects were followed up at two daily clinic visits (dosing day and 168 h). The pretrial GH replacement therapy could be resumed at day 29, 7 days after the last dose of trial medication.
Trial procedures and assay methods
After the first and fourth doses of NNC0195-0092 and after dose administration on days 1 and 22 for Norditropin NordiFlex, PK assessments were collected at baseline, 30 minutes before dosing, and 7 days after the first and fourth NNC0195-0092 dose (0, 0.25, 1, 2, 4, 6, 8, 12, 16, 20, 24, 26, 28, 30, 36, 42, 48, 50, 56, 64, 72, 96, 120, 144, and 168 h), with the same sampling time points after Norditropin NordiFlex dose administration on days 1 and 22. After the first and fourth doses after NNC0195-0092 and after the dose administration on days 1 and 22 for Norditropin NordiFlex, PD assessments were collected at baseline, 30 minutes before dosing, and 7 days after the first and fourth NNC0195-0092 dose (0, 12, 24, 48, 72, 96, 120, 144, and 168 h), with the same sampling time points after the Norditropin NordiFlex dose administration on days 1 and 22.
Serum concentrations of NNC0195-0092 were analyzed using a NNC0195-0092-specific luminescent oxygen channeling immunoassay with no cross-activity of hGH, validated in accordance with Food and Drug Administration guidelines (14). The sensitivity of the NNC0195-0092 immunoassay is 0.50 ng/mL. The intra- and interassay precision was determined at 1.00, 1.50, 30.0, and 520 ng/mL. The intraassay coefficient of variation (CV) was 9.8% or less and the interassay CV was 8.8% or less at these concentrations.
The concentration of GH in serum from subjects treated with Norditropin NordiFlex was assessed at the central laboratory using a commercially available kit (Siemens IMMULITE 2000 [Siemens Medical Solutions Diagnostics GmbH]). Analysis of serum IGF-1 and IGFBP-3 concentrations was performed using commercially available assay kits (Immuno Diagnostic Systems immunoassay ISYS assay) at the analytical central laboratory Laboriatorium für Klinische Forschung GmbH (Schwentinental, Germany). GH, IGF-1, and IGFBP-3 assay performance was in accordance with the assay information provided by the manufacturer. IGF-1 SDSs were calculated according to Bidlingmaier et al (15).
Serum samples for determination of antibodies against NNC0195-0092 or hGH were collected at baseline (prior to treatment start), the end of treatment (168 h after the last dose), and the follow-up visit after 4 weeks of drug washout. For subjects treated with NNC0195-0092, antibodies against NNC0195-0092 were evaluated using a bridging ELISA. For subjects treated with hGH, a similar validated assay was applied to detect antibodies against hGH. Both assays were developed and validated by Novo Nordisk A/S. In both assays, samples were acid pretreated, and upon neutralization, they were incubated overnight in the presence of horseradish-peroxidase-labeled and biotin-labeled NNC0195-00992 (or hGH). Samples were then transferred to streptavidin-coated microtiter plates, and, upon incubation and plate washing, plates were developed using 3,3′,5,5′-tetramethylbenzidine substrate and read spectrofotometrically at 450/620 nm dual wavelength. A tiered approach for analysis was applied for both assays. Samples above screening cut point (tier 1 described above) were analyzed in a confirmation assay (tier 2), which included a preincubation step with excess unlabeled compound to verify the specificity of the assay signal. Only samples positive in the confirmation assay (tier 2) were considered positive for antibodies against NNC0195-0092 or hGH.
Laboratory safety assessments of hematological (white blood cell count, red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, platelets), biochemistry (sodium, potassium, chloride, calcium [total], inorganic phosphate, creatinine, urea, uric acid, total protein, albumin, bilirubin [total], creatinine kinase, alkaline phosphatase, γ-glutamyltransferase, aspartate aminotransferase, aminotransferase, C-reactive protein), urinalysis (protein, glucose, erythrocytes, leukocytes, bilirubin), HbA1c, glucose, and insulin were all analyzed at the central laboratory (Laboratorium fur Klinische Forschung).
Statistical analysis
A significance level of 5% was used without adjustment for multiple testing. All tests were two-sided superiority tests. Dose proportionality was investigated by statistical analyses. Other endpoints were evaluated using descriptive statistics. Sample size was not based on formal calculations but was determined to expose the lowest possible number of subjects to NNC0195-0092 while enabling adequate assessment of safety, PK, and PD data to continue to the next dose level. The number of subjects enrolled in this trial was based on prior experience with similar trials (9) and interaction with regulatory agencies. All subjects who received at least one dose of the trial drug were included in the analyses. To ensure seven completers within each cohort, treatment + active control, subjects who were withdrawn or who dropped out prior to day 29 were replaced, unless this was due to AEs related to the trial drug. The replacement subject was assigned to the same treatment as the subject being replaced.
The molecular mass of Norditropin is 22 125 g/mol, so 1 g is equivalent to 45.2 nmol. The molecular mass of NNC0195-0092 is 23 305 g/mol, so 1 g is equivalent to 42.9 nmol. Thus, for the molar equivalent conversion of Norditropin in nanograms per milliliter, multiply by 0.0452. For NNC0195-0092, the equivalent multiplication factor is 0.0429.
Pharmacokinetics
The following PK parameters were determined using standard noncompartmental methods: the NNC0195-0092 peak plasma concentration (Cmax), the time to Cmax (tmax), the area under the curve (AUC) from 0 to 168 hours after dosing (AUC[0–168h]) (NNC0195-0092 only), and terminal half-life. AUC for 0–24 hours was determined for Norditropin NordiFlex only. All evaluations for NNC0195-0092 were based on evaluation of data collected for up to 7 days (168 h) after the first and fourth dosing, and at the end of the trial. All PK parameters were analyzed using descriptive statistics. Dose proportionality was investigated using a linear regression model with the log-transformed end point (AUC[0–168h] or Cmax for the fourth dosing of NNC0195-0092) as the dependent variable and the log-transformed dose as covariate. The accumulation index (RAcc) was calculated as AUC(0–168h) for the fourth dosing divided by AUC(0–168h) for the first dosing. In descriptive statistics and all formal statistical testing, all subjects receiving Norditropin NordiFlex were pooled into one dose group, irrespective of the allocated dose cohort.
Pharmacodynamics
The following PD end points were calculated for IGF-1 and IGFBP-3: AUC(0–168h), Cmax, and tmax derived from serum concentration vs time profiles. AUC end points were approximated by a linear trapezoidal technique. Change in ΔIGF-1 and ΔIGFBP-3 statistical comparison between dose levels of NNC0195-0092, and between NNC0195-0092 doses and Norditropin NordiFlex, were performed using an analysis of covariance model with treatment as a factor and the predose value as covariate. AUC(0–168h) and Cmax values were log transformed before analysis. Mean ratios/differences for NNC0195-0092 doses and mean ratios/differences for NNC0195-0092 vs Norditropin NordiFlex were estimated with corresponding 95% confidence intervals and P values. IGF-1 SDSs were calculated according to Bidlingmaier et al (15), and IGFBP-3 SDSs were calculated using reference ranges supplied by the kit manufacturer (Immuno Diagnostic Systems) and presented using descriptive statistics. In accordance with the trial protocol, IGF-1 and IGFBP-3 were considered as PD parameters only.
Safety and tolerability
The safety of NNC0195-0092 and Norditropin NordiFlex was assessed from the incidence of AEs from the first dose of NNC0195-0092 or Norditropin NordiFlex on day 1 and until day 49 after first dosing (1, 2, 3, 4, 6, 7, 8, 15, 22, 23, 24, 25, 26, 27, 28, 29, and 49 d) as well as the following safety assessments: safety laboratory assessments, physical examinations, vital signs, and 12-lead electrocardiogram (ECG).
Injection site tolerability was assessed by manual and visual inspection of injection sites by the investigator. Pain/tenderness, itching, rash, redness, induration, and skin dimpling at the injection site were assessed by the investigator and graded on a scale from 0 (none) to 3 (severe). Redness, induration, and dimpling at the injection site were measured in millimeters.
The primary end point of the trial, safety, was assessed as the incidence of AEs from the first dose of trial product until day 49 after dosing. AEs reported during this period were summarized by dose, Medical Dictionary for Regulatory Activities system organ class, and preferred term. No AEs were subject to adjudication. Evaluation of severity and causality was based on the investigator's judgment, taking into account the study population, study indication, concomitant illnesses, other AEs, the overall clinical picture, and clinical significance of the AE. Other safety end points, including physical examination, changes from baseline in vital signs, ECG and laboratory safety parameters (hematology, biochemistry, urinalysis), and hGH antibodies, were considered secondary safety end points and were evaluated using descriptive statistics. Comparison of local tolerability between multiple dosing of NNC0195-0092 and once-daily dosing of Norditropin NordiFlex, assessed from the number of injection site reactions from the first dose of each trial drug until day 49, was evaluated by descriptive statistics.
Safety analysis was based on the safety analysis set: 26 subjects receiving NNC0195-0092 and eight subjects receiving Norditropin NordiFlex.
Results
Subjects
Forty adults with GHD were screened. Thirty-four subjects were planned to be randomized; however, two subjects replaced two other subjects withdrawn for reasons other than AEs, and one subject replaced another withdrawn due to an AE that was considered unlikely to be related to the trial drug. Thus, a total of 35 subjects (males/females: 25/10) with AGHD were randomized into the trial, with one subject randomized to Norditropin NordiFlex but never exposed, leaving 34 exposed subjects (males/females: 25/9) exposed to treatment (NNC0195-0092, 0.02 mg/kg, n = 7; 0.04 mg/kg, n = 6; 0.08 mg/kg, n = 6; 0.12 mg/kg, n = 7; Norditropin NordiFlex, n = 8) and 30 subjects (males/females: 21/9) who completed the trial (Table 1). The five subjects withdrawn from the trial were equally split across the five cohorts: two were withdrawn due to AEs and not replaced (NNC0195-0092, 0.02 mg/kg, n = 1, gastroenteritis, serious AE, unlikely causality; NNC0195-0092, 0.08 mg/kg, weight increase and edema, mild severity AEs, probable causality). The three other subjects withdrawn were not due to AEs and were replaced, one (NNC0195-0092, 0.12 mg/kg, n = 1) was withdrawn at 6.4 weeks due to alcohol consumption within 24 hours of blood sampling after the dosing (protocol defined withdrawal criterion), and two were withdrawn for other reasons (NNC0195-0092, 0.04 mg/kg, n = 1, withdrew consent; Norditropin NordiFlex, n = 1, difficult phlebotomy). All the results and conclusions are based on data from all 34 exposed subjects.
Table 1.
Summary of Baseline Characteristics
| Norditropin NordiFlex (n = 8) | NNC0195-0092 (mg/kg) |
||||
|---|---|---|---|---|---|
| 0.02 (n = 7) | 0.04 (n = 6) | 0.08 (n = 6) | 0.12 (n = 7) | ||
| Age, y | 57.0 (14.9) | 51.6 (12.2) | 56.8 (13.1) | 55.3 (12.8) | 43.3 (16.9)a |
| Sex, M/F | 6/2 | 7/0 | 3/3 | 3/3 | 6/1 |
| Body weight, kg | 81.4 (17.0) | 84.1 (15.3) | 88.3 (16.6) | 77.8 (23.2) | 80.1 (15.3) |
| BMI, kg/m2 | 26.8 (3.7) | 27.3 (2.7) | 30.0 (4.1) | 25.5 (4.7) | 25.9 (4.6) |
| GHD onset | |||||
| Childhood, idiopathic | 0 (0) | 2 (28.6) | 0 (0) | 0 (0) | 1 (14.3) |
| Childhood, acquired | 1 (12.5) | 1 (14.3) | 1 (16.7) | 2 (33.3) | 2 (28.6) |
| Adult | 7 (87.5) | 4 (57.1) | 5 (83.3) | 4 (66.7) | 4 (57.1) |
Abbreviation: BMI, body mass index. Data are presented as mean (SD).
Mean age of subjects in the 0.12-mg/kg group was below the mean age of trial subjects (52.7 [14.3] y).
Baseline characteristics were similar across dose groups and are summarized in Table 1. Mean (SD) age in the NNC0195-0092 0.12 mg/kg group (43.3 [16.9] y) was lower vs the other groups (Table 1). During the trial, the mean dose for subjects randomized to Norditropin NordiFlex was 0.004 mg/kg · d (range 0.001–0.008 mg/kg), corresponding to a mean total daily dose of 0.3 mg (range 0.15–0.5 mg). The Norditropin NordiFlex dose during the trial was replicated according to subjects' individual GH dose during the last 3 months of stable GH replacement therapy prior to the trial start. The four NNC0195-0092 doses administered during the dose-escalation trial were determined based on IGF-1 responses during multiple weekly doses of NNC0195-0092 (0.02–0.24 mg/kg per dose) in healthy adult male subjects (9).
Pharmacokinetics
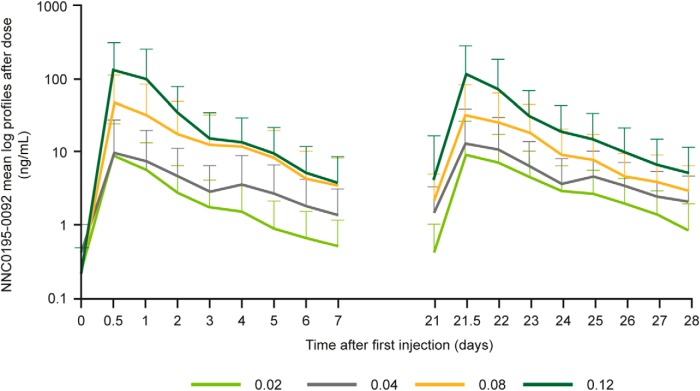
The mean serum concentration of NNC0195-0092 increased in a dose-dependent manner after multiple-dose exposure (Figure 1). Mean NNC0195-0092 AUC from 0 hours to next dosing (AUC0-τ) and Cmax increased with dose (Table 2) and were consistent with dose proportionality across the range of doses tested. A limited degree of accumulation was observed for the 0.02–0.08 mg/kg, but not the 0.12 mg/kg, cohorts with the RAcc ranging from 1.0 to 2.0 across cohorts (Table 2). For Norditropin NordiFlex, Cmax appeared stable and no accumulation took place (RAcc = 0.9).
Figure 1.
PK mean profiles (log scale) after the first administration and after fourth administration of NNC0195-0092.
Table 2.
Summary of PK End Points for NNC0195-0092 and Norditropin NordiFlex
| Dose, mg/kg | n | AUC0-τ, ngh/mL | Cmax, ng/mL | tmax, h | RAcc |
|---|---|---|---|---|---|
| NNC0195-0092 | |||||
| After first dose | |||||
| 0.02 | 7 | 475 (94.5) | 14.4 (119.5) | 11.1 (7.8) | |
| 0.04 | 6 | 777 (71.3) | 19.8 (115.0) | 25.6 (35.8) | |
| 0.08 | 6 | 2753 (173.6) | 64.2 (193.1) | 16.6 (11.0) | |
| 0.12 | 7 | 6382 (71.6) | 142.5 (129.1) | 22.5 (11.0) | |
| After fourth dose | |||||
| 0.02 | 7 | 666 (70.9) | 14.4 (193.2) | 9.0 (10.6) | 2.0 (2.0) |
| 0.04 | 6 | 986 (93.3) | 20.6 (201.2) | 5.4 (4.6) | 1.3 (0.3) |
| 0.08 | 6 | 2085 (102.2) | 45.4 (127.6) | 15.2 (15.8) | 1.3 (0.6) |
| 0.12 | 7 | 5431 (61.7) | 114.8 (113.3) | 17.2 (12.0) | 1.0 (0.2) |
| Norditropin NordiFlex | |||||
| Norditropin NordiFlex, wk 1/d 1) | 8 | 21 (202.1) | 1.6 (195.7) | 3.8 (5.0) | |
| Norditropin NordiFlex, wk 4/d 22) | 8 | 17 (153.4) | 1.5 (185.1) | 5.3 (6.3) | 0.9 (0.3) |
Abbreviation: AUC0-τ, AUC from 0 hour to next dosing Data are presented as mean (SD). Area under the serum concentration-time curve from time zero to time (AUCτ) and Cmax are presented as geometric mean (CV percentage). The RAcc was calculated as mean (SD) = AUC(0–168 h, dose 4)/AUC(0–168 h, dose 1). For Norditropin NordiFlex, the RAcc was calculated for AUC0-τ, week 4, day 22 per AUC0-τ, week 1, day 1.
Pharmacodynamics
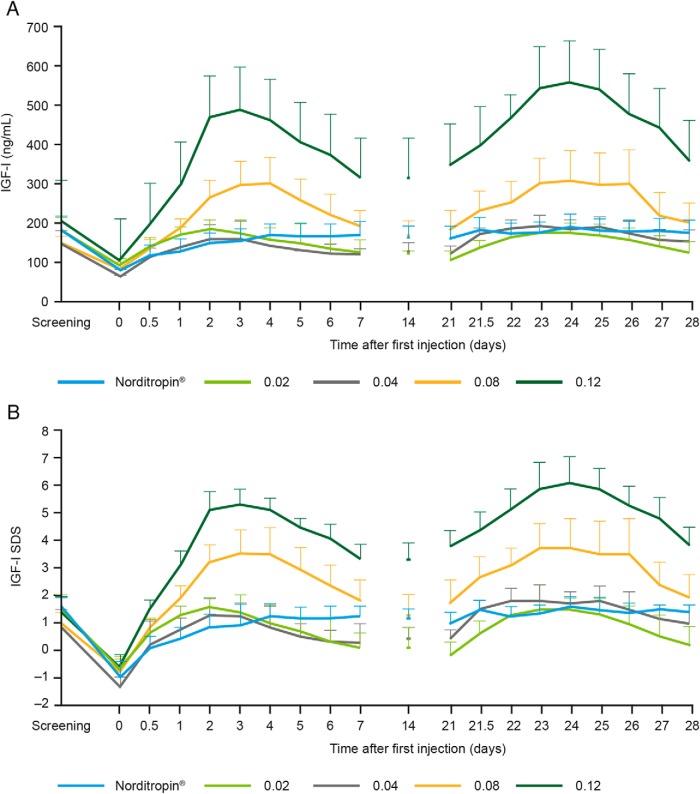
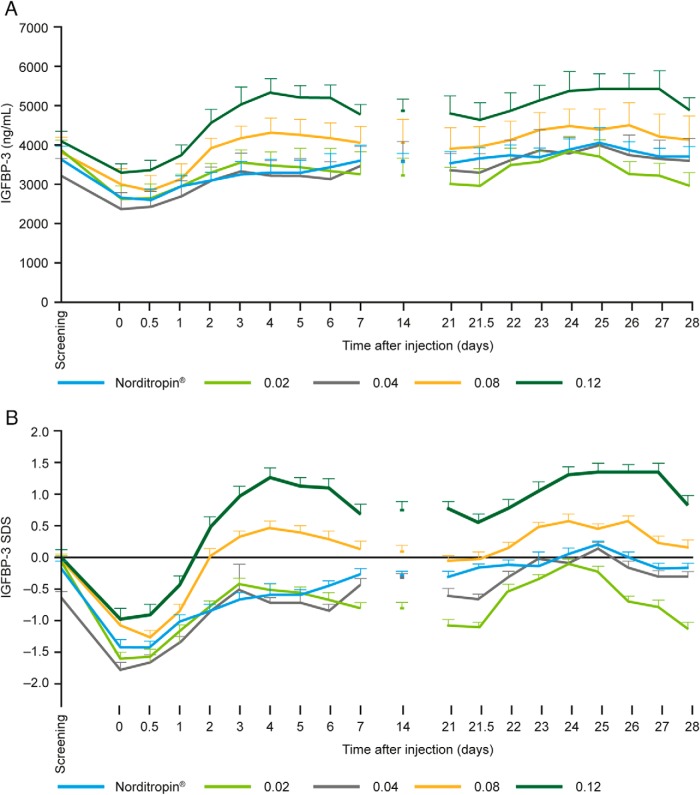
Mean pretrial IGF-1 levels for all NNC0195-0092 groups as well as for the Norditropin NordiFlex group were ranging across groups from +0.9 SDS to +1.5 SDS (Figure 2B). After the GH washout period and administration of NNC0195-0092, a dose-dependent IGF-1 response to NNC0195-0092 was observed, with an increase in IGF-1 levels at all dose levels tested and, in the active control arm, an increase to a level similar to the pretrial IGF-1 level (Figure 2). For 0.02 mg/kg and 0.04 mg/kg NNC0195-0092, the observed IGF-1 levels were similar to IGF-1 levels obtained during standard daily hGH treatment (Table 3). The IGF-1 SDS response showed a dose-dependent increase after the washout period with the IGF-1 SDS maintained for 7 days after dosing and showed no significant accumulation (Figure 2). The dose response in IGF-1 levels was reflected in the IGF-1 AUC0–168h and Cmax. At 0.02 mg/kg and 0.04 mg/kg NNC0195-0092, the estimated IGF-1 AUC0–168h was similar to that with Norditropin NordiFlex, and IGF-1 SDS remained within normal levels (>−2 SDS; <+2 SDS) (Figure 2). At 0.08 mg/kg, peak values of IGF-1 SDS exceeded +2, and at 0.12 mg/kg IGF-1 SDS was greater than +2 at all time points assessed (Figure 2). Mean IGFBP-3 also showed a dose-dependent increase after the administration of NNC0195-0092 (Figure 3). The IGFBP-3 response to 0.02, 0.04, and 0.08 mg/kg NNC0195-0092, as assessed by AUC0–168h and Cmax, was similar to that with Norditropin NordiFlex (Table 3).
Figure 2.
Mean levels (±SEM) of IGF-1 nanograms per milliliter (A) and IGF-1 SDS (B) vs time pretrial, baseline (washout), after the first administration (d 0), and the fourth administration (d 21) of NNC0195-0092 or Norditropin NordiFlex to adults with GHD.
Table 3.
Statistical Analysis of PD End Points (IGF-1 and IGFBP-3) After Multiple Doses of NNC0195-0092 Compared With Daily Norditropin NordiFlex
| Dose Level, mg/kga | Estimated Mean Ratio AUC0–168h | 95% CI | P Value | Estimated Mean Ratio Cmax | 95% CI | P Value |
|---|---|---|---|---|---|---|
| IGF-1 | ||||||
| 0.02 mg/kg/Norditropin NordiFlex | 0.87 | [0.66, 1.13] | .2713 | 0.95 | [0.72, 1.24] | .6896 |
| 0.04 mg/kg/Norditropin NordiFlex | 1.08 | [0.82, 1.43] | .5719 | 1.13 | [0.85, 1.50] | .3940 |
| 0.08 mg/kg/Norditropin NordiFlex | 1.37 | [1.04, 1.82] | .0275 | 1.48 | [1.12, 1.97] | .0086 |
| 0.12 mg/kg/Norditropin NordiFlex | 2.32 | [1.77, 3.04] | <.0001 | 2.52 | [1.92, 3.32] | <.0001 |
| IGFBP-3 | ||||||
| 0.02 mg/kg/Norditropin NordiFlex | 0.92 | [0.84, 1.01] | .0929 | 0.98 | [0.88, 1.09] | .6646 |
| 0.04 mg/kg/Norditropin NordiFlex | 1.01 | [0.91, 1.12] | .8380 | 1.03 | [0.92, 1.15] | .5697 |
| 0.08 mg/kg/Norditropin NordiFlex | 1.02 | [0.93, 1.13] | .6460 | 1.04 | [0.93, 1.16] | .5043 |
| 0.12 mg/kg/Norditropin NordiFlex | 1.19 | [1.08, 1.31] | .0010 | 1.19 | [1.06, 1.32] | .0031 |
Abbreviation: CI, confidence interval.
Assessments at dose 4 for NNC0195-0092 and at week 4/day 22 for Norditropin NordiFlex.
Figure 3.
Mean levels (±SEM) of IGFBP-3 nanograms per milliliter (A) and IGFBP-3 SDS (B) vs time after the first administration (d 0) and the fourth administration (d 21) of NNC0195-0092 or Norditropin NordiFlex to adults with GHD.
Safety
Four once-weekly doses of NNC0195-0092 within the dose range 0.02–0.12 mg/kg were administered to adult patients with GHD. No significant safety and tolerability signals causally related to NNC0195-0092 were identified.
A total of 87 AEs were reported (NNC0195-0092: 79 events in 18 subjects [69%]; Norditropin NordiFlex: eight events in five subjects [62%]). A dose-dependent frequency in incidence and severity of AEs was observed as the number of events was greater at the highest dose level of NNC0195-0092 (NNC0195-0092, 0.02 mg/kg: 11 events in four subjects [57%]; NNC0195-0092, 0.04 mg/kg: 15 events in four subjects [67%]; NNC0195-0092, 0.08 mg/kg: 13 events in four subjects [67%]; NNC0195-0092, 0.12 mg/kg: 40 events in six subjects [86%]). Events seen with NNC0195-0092 reported in more than two subjects included the following: edema (three events in three subjects), gastroenteritis (three events in three subjects); increases in fasting plasma glucose (FPG; four events in four subjects), C-reactive protein (three events in three subjects), and increased weight (six events in five subjects).
Twenty-four of the 79 events were rated as possibly or probably related to NNC0195-0092 and 23 (96%) were reported at the highest NNC0195-0092 dose levels; all but four of these events (increased weight [three events in three subjects]; increased FPG [one event in one subject]) were resolved by the end of the trial. The subject with increased FPG had an elevated FPG at screening, which did not normalize during the trial.
Five AEs (6% of the 79) in four subjects receiving NNC0195-0092 were graded severe (0.02 mg/kg, gastroenteritis [n = 1]; 0.04 mg/kg, sepsis and urticaria [n = 1]; 0.12 mg/kg, renal cancer [n = 1]; 0.12 mg/kg, and transient increase in creatinine kinase) [n = 1]); all were rated as unlikely to be related to trial drug except for the transient increase in creatinine kinase.
In total, five serious adverse events (SAEs; three of these were also judged severe) were reported in four subjects receiving NNC0195-0092 (0.02 mg/kg, gastroenteritis [severe] [n = 1, male, aged 34 y]; 0.04 mg/kg [causality unlikely], decreased international normalized ratio [nonsevere] [causality possible] [n = 1, male, 66 y]; 0.04 mg/kg, sepsis [severe] [causality unlikely], and atrial fibrillation (AF) [nonsevere] [causality unlikely] [n = 1, male, 68 y]; 0.12 mg/kg, renal cell carcinoma [severe] [causality unlikely] [n = 1, female, 64 y]). All patients recovered from their SAEs during continuation of NNC1095-0092 at the assigned dose level, except the subject in the NNC0195-0092 0.04 mg/kg cohort with gastroenteritis who voluntarily withdrew from the trial and the subject with renal cell carcinoma who was reported recovered by investigators in the follow-up period after the 4-week trial treatment period. Only the decreased international normalized ratio was assessed as possibly related to trial drug, although this was considered as potentially an incidental finding due to insufficient anticoagulation therapy for the subject's underlying conditions (AF and deep vein thrombosis). The subject diagnosed with sepsis and AF went to the emergency department due to fever and confusion five days after inclusion in the trial. During hospitalization the subject's ECG showed AF and the patient had septicemia. The subject was treated and the AF was evaluated recovered after 4 days and the subject recovered from septicemia after 12 days and was discharged from the hospital.
In the subject diagnosed with renal cell carcinoma, hematuria measured by urine dipsticks was present on the first day after dosing; no hematuria was present 2 days after this occurrence, and the subject was without any specific symptoms during the trial treatment period. The subject did not have any medical history of urine tract diseases prior to inclusion in the trial. The subject stopped taking the trial drug after the 4-week trial period as per protocol. Significant hematuria reoccurred 3 days after stopping the trial drug, and the subject was referred for further investigations of the urinary tract. A computed tomography urography identified a well-defined tumor process in the right kidney without signs of metastasis. The investigator judged the causality relating to the trial drug to be unlikely. A nephrectomy was performed 1 month later, and the subject was reported as recovered by investigators in accordance with per-protocol described SAE follow-up procedure, ie, SAE must be followed up until the outcome of the event is recovered, recovered with sequelae, or fatal.
The impact of NNC0195-0092 on insulin resistance or glucose tolerance was not investigated in this trial. Two subjects (Norditropin NordiFlex, n = 1; NNC0195-0092, n = 1) had type 2 diabetes at baseline. Increased FPG levels defined as 6.1 mmol/L or greater were observed in nine patients (four reported as AEs) (Norditropin NordiFlex, n = 4; [individual values were 6.6 [type 2 diabetes >6 y at baseline]; 7.9; 6.8; and 6.1] mmol/L, respectively]); NNC0195-0092, 0.08 mg/kg, n = 1 [6.4 mmol/L]; 0.12 mg/kg, n = 4 [individual values were 8.1; 7.1; 6.7; 6.8 mmol/L, respectively]). No significant change in HbA1c levels from baseline was observed in either the NNC0195-0092 or the Norditropin NordiFlex groups (decrease of 0.3% in all groups).
Local tolerability
Two transient injection-site reactions were reported after the NNC0195-0092 injections, both of mild severity (0.04 mg/kg: blue discoloration [not reported whether the discoloration was due to bleeding or bruising]; 0.12 mg/kg: redness). No injection-site reactions were reported after the Norditropin NordiFlex injections. Two sequential injections were required only if the trial dose exceeded 2 mL and was relevant only for the highest-dose group in subjects weighing more than 100 kg. Two sequential injections were required for four subjects. No complaints of pain or discomfort were reported in these subjects. No additional local tolerability issues, assessed as pain, tenderness, itching, rash, redness, induration, and other reactions at the injection site, were identified or reported.
Antibodies
No anti-NNC0195-0092 antibodies or anti-hGH antibodies were detected during the trial in subjects treated with NNC0195-0092.
Discussion
NNC0195-0092 is a novel reversible albumin-binding GH derivative intended for once-weekly treatment of GHD with the aim of reducing the frequency of injections from 365 to 52 injections per year. NNC0195-0092 comprises a single-point mutation in the hGH backbone to which a noncovalent, albumin-binding moiety is attached. This may prolong the absorption phase after a sc injection and, more importantly, the ability to bind reversibly to albumin in the blood significantly reduces plasma clearance and prolongs the in vivo half-life (9). A similar mechanism, successfully used to extend the half-life of insulin and glucagon-like peptide-1 (7, 8), is not associated with any significant tolerability issues (8, 16).
The safety and tolerability of daily GH injections are well established (17, 18); however, an increased frequency of injection-site reactions has been observed in studies of sustained-release formulations of GH (19, 20) as well as long-acting GH preparations (21, 22) in AGHD. Over the past 15 years, several attempts to develop a GH preparation to be administered less frequently than daily injections have been undertaken. For one candidate, lipoatrophy at the injection site was reported with once-weekly sc injections of a pegylated GH formulation in adults with AGHD (21). The increased time that long-acting GH preparations remain in the sc tissue warrant careful evaluation of local tolerability reactions. Thus, in the development of long-acting GH preparations, convenience in relation to the injection procedure and local tolerability is of utmost importance.
Over the NNC0195-0092 dosing range tested in this trial (0.02–0.12 mg/kg), drug exposure parameters (Cmax and AUC) were proportional to dose. Variation was observed in tmax between dose levels and between subjects. Except for the first dose in the 0.04 mg/kg dose group in which the data for two outliers confounded results, there was a trend for increasing tmax with increasing dose. This trend is related to the slightly more extended plateau around tmax for the concentration-time profiles at the higher dose levels, which in turn is related to the nonlinear PK observed at the higher doses.
The PD responses were proportional to all doses of NNC0195-0092 tested, and at the 0.02 mg/kg and 0.04 mg/kg NNC0195-0092 doses, the estimated IGF-1 AUC0–168h was similar to that observed with Norditropin NordiFlex. The IGF-1 SDS remained within normal levels, supporting once-weekly dosing of NNC0195-0092 for treatment of AGHD at a starting dose of 0.02–0.04 mg/kg · wk for individual dose titration. A gender difference in IGF-1 response to GH treatment, with women requiring higher doses than men to achieve similar levels of IGF-1, has previously been described (23). The small sample sizes in the current trial, however, did not permit analysis by gender, and the unequal gender distribution between treatment groups could have affected the IGF-1 results and is a potential limitation in the trial. In addition, inclusion of subjects with prestudy IGF-1 levels above and below usual therapeutic guidelines was allowed and could have biased the comparisons between the treatment and active control groups; however, it was observed that the active control group achieved IGF-1 levels consistent with usual therapeutic guidelines during the current trial. The mean age of subjects in the 0.12-mg/kg group was lower compared with the other groups; however, given the overlap between groups in age ranges, the observed lower mean age in the highest-dose group is not expected to have affected the PK/PD results significantly.
Although NNC0195-0092 may potentially affect other medications that rely on binding to albumin, it is considered very unlikely that the albumin-binding capability of NNC0195-002 will interfere with other albumin-binding medications because albumin is present at 35–50 mg/mL (24), whereas the Cmax of NNC0195-0092 after the first or fourth dose in this trial was between 14 and 142 ng/mL. The molecular mass of human serum albumin is approximately 66 000 g/mol; 35–50 mg/mL is equivalent to 530 000–758 000 nmol/L. The albumin to NNC0195-0092 M ratio at the highest NNC0195-0092 concentration and lowest normal albumin concentration is approximately 88 000, suggesting albumin occupancy of approximately 0.001%. Thus, any displacement of albumin-binding moieties, including other drugs, is not likely to be of clinical relevance.
In conclusion, four once-weekly doses of NNC0195-0092 within the dose range of 0.02–0.12 mg/kg were administered to adult patients with GHD. No clinically significant safety and tolerability signals causally related to NNC0195-0092 were identified, nor were any immunogenicity concerns revealed. The IGF-1 profiles were consistent with a once-weekly treatment profile of NNC0195-0092 at a starting dose of 0.02–0.04 mg/kg · wk.
Acknowledgments
Medical writing services were provided by Penny Butcher at Watermeadow Medical, UK, funded by Novo Nordisk A/S, who worked with the authors to draft, revise, and finalize the manuscript. We thank Geraldine Majken Gul Rasmussen for her excellent technical and practical skills. We also acknowledge Professor Jens Sandahl Christiansen for his large scientific knowledge, enthusiasm, and always-present support and kindness for which he will be remembered and missed.
All the authors have had a part in the writing and final editing of the report, all have been given a copy of the manuscript, all have approved the final version of the manuscript, and all are prepared to take public responsibility for the work, sharing responsibility and accountability for the results.
The clinical trial registration number for this study (clinicaltrials.gov) was NCT01706783.
This work was supported by Novo Nordisk A/S.
Disclosure Summary: M.H.R. is an employee of Novo Nordisk. J.J. is a researcher for Novo Nordisk. L.F.N. is an employee of Novo Nordisk. C.H. is a coinvestigator and member of the NordiNet IOS International Study Committee, Novo Nordisk; is a member of Pfizer's Study Advisory Board for the Swedish KIMS database; is a member of the Sandoz Study Advisory Board for the PATRO database; is a member of the European Hyponatraemia Network; and has received lecture fees from Pfizer and Otsuka. M.A. is a coinvestigator for Novo Nordisk. U.F.-R. is a principal investigator for Novo Nordisk and Novartis Pharmaceuticals; is a speaker for Pfizer, Inc, Novo Nordisk, Ipsen, and Novartis Pharmaceuticals; and is an advisory group member for Pfizer, Inc and Novartis Pharmaceuticals. J.S.C. has received research funding from Novo Nordisk; is an advisory board member of Novo Nordisk and Merck Serono; has received lecture fees from Novo Nordisk, Pfizer, Inc and fees from Eli Lilly & Co. M.K., D.M., and M.T. have nothing to disclose.
Footnotes
- AE
- adverse event
- AF
- atrial fibrillation
- AGHD
- adults with GHD
- AUC
- area under the curve
- AUC(0–168h)
- AUC from 0 to 168 hours after dosing
- Cmax
- peak plasma concentration
- CV
- coefficient of variation
- ECG
- electrocardiogram
- FPG
- fasting plasma glucose
- GHD
- GH deficiency
- hGH
- human GH
- IGFBP-3
- IGF-binding protein-3
- PD
- pharmacodynamics
- PK
- pharmacokinetics
- SAE
- serious AE
- SDS
- SD score
- HbA1c
- hemoglobin A1c
- RAcc
- accumulation index
- tmax
- time to Cmax.
References
- 1. Salomon F, Cuneo RC, Hesp R, Sonksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med. 1989;321:1797–1803. [DOI] [PubMed] [Google Scholar]
- 2. Cummings DE, Merriam GR. Growth hormone therapy in adults. Annu Rev Med. 2003;54:513–533. [DOI] [PubMed] [Google Scholar]
- 3. Hazem A, Elamin MB, Bancos I, et al. Body composition and quality of life in adults treated with GH therapy: a systematic review and meta-analysis. Eur J Endocrinol. 2011;166:13–20. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman AR, Biller BM, Cook D, et al. Efficacy of a long-acting growth hormone (GH) preparation in patients with adult GH deficiency. J Clin Endocrinol Metab. 2005;90:6431–6440. [DOI] [PubMed] [Google Scholar]
- 5. Biller BM, Ji HJ, Ahn H, et al. 12-month effects of once-weekly sustained-release growth hormone treatment in adults with GH deficiency. Pituitary. 2013;16:311–318. [DOI] [PubMed] [Google Scholar]
- 6. Kim Y, Hong JW, Chung YS, et al. Efficacy and safety of sustained-release recombinant human growth hormone in Korean adults with growth hormone deficiency. Yonsei Med J. 2014;55:1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurtzhals P, Havelund S, Jonassen I, Kiehr B, Ribel U, Markussen J. Albumin binding and time action of acylated insulins in various species. J Pharm Sci. 1996;85:304–308. [DOI] [PubMed] [Google Scholar]
- 8. Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen MH, Braendholt Olsen MW, Alifrangis L, Klim S, Suntum M. A reversible albumin-binding growth hormone derivative is well-tolerated and possesses a potential once-weekly treatment profile. J Clin Endocrinol Metab. 2014;99:E1819–E1829. [DOI] [PubMed] [Google Scholar]
- 10. Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700. [DOI] [PubMed] [Google Scholar]
- 11. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609. [DOI] [PubMed] [Google Scholar]
- 12. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Last amended by the 59th World Medical Association General Assembly (Seoul). 2008. [Google Scholar]
- 13. ICH harmonised tripartite guideline for good clinical practice. International Conference on Harmonisation; Geneva, Switzerland: May 1, 1996. http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.html. [Google Scholar]
- 14. US Department of Health and Human Services. US Department of Health and Human Services, Food and Drug administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), May 2001, BP: guidance for industry. Bioanalytical method validation.
- 15. Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin-like growth factor-1 (IGF-1) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-1 immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99:1712–1721. [DOI] [PubMed] [Google Scholar]
- 16. Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569–1581. [DOI] [PubMed] [Google Scholar]
- 17. Appelman-Dijkstra NM, Claessen KM, Roelfsema F, Pereira AM, Biermasz NR. Long-term effects of recombinant human GH replacement in adults with GH deficiency: a systematic review. Eur J Endocrinol. 2013;169:R1–R14. [DOI] [PubMed] [Google Scholar]
- 18. Höybye C, Christiansen JS. Growth hormone replacement in adults—current standards and new perspectives. Best Pract Res Clin Endocrinol Metab. 2015;29:115–123. [DOI] [PubMed] [Google Scholar]
- 19. Silverman BL, Blethen SL, Reiter EO, Attie KM, Neuwirth RB, Ford KM. A long-acting human growth hormone (Nutropin Depot): efficacy and safety following two years of treatment in children with growth hormone deficiency. J Pediatr Endocrinol Metab. 2002;15(suppl 2):715–722. [DOI] [PubMed] [Google Scholar]
- 20. Biller BMK, Ji H-J, Ahn H, et al. Effects of once-weekly sustained-release growth hormone: double-blind, placebo-controlled study in adult growth hormone deficiency. J Clin Endocrinol Metab. 2011;96:1718–1726. [DOI] [PubMed] [Google Scholar]
- 21. Touraine P, D'Souza GA, Kourides I, et al. Lipoatrophy in GH deficient patients treated with a long-acting pegylated GH. Eur J Endocrinol. 2009;161:533–540. [DOI] [PubMed] [Google Scholar]
- 22. Yuen KC, Conway GS, Popovic V, et al. A long-acting human growth hormone with delayed clearance (VRS-317): results of a double-blind, placebo-controlled, single ascending dose study in growth hormone-deficient adults. J Clin Endocrinol Metab. 2013;98:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Span JP, Pieters GF, Sweep CG, Hermus AR, Smals AG. Gender difference in insulin-like growth factor I response to growth hormone (GH) treatment in GH-deficient adults: role of sex hormone replacement. J Clin Endocrinol Metab. 2000;85:1121–1125. [DOI] [PubMed] [Google Scholar]
- 24. Peters T. All about albumin: biochemistry, genetics, and medical applications. San Diego: Academic Press; 1996. [Google Scholar]