Abstract
Context:
Diabetes is associated with a deficit of insulin-producing β-cells. Animal studies show that β-cells become dedifferentiated in diabetes, reverting to a progenitor-like stage, and partly converting to other endocrine cell types.
Objective:
To determine whether similar processes occur in human type 2 diabetes, we surveyed pancreatic islets from 15 diabetic and 15 nondiabetic organ donors.
Design:
We scored dedifferentiation using markers of endocrine lineage, β-cell-specific transcription factors, and a newly identified endocrine progenitor cell marker, aldehyde dehydrogenase 1A3.
Results:
By these criteria, dedifferentiated cells accounted for 31.9% of β-cells in type 2 diabetics vs 8.7% in controls, and for 16.8% vs 6.5% of all endocrine cells (P < .001). The number of aldehyde dehydrogenase 1A3-positive/hormone-negative cells was 3-fold higher in diabetics compared with controls. Moreover, β-cell-specific transcription factors were ectopically found in glucagon- and somatostatin-producing cells of diabetic subjects.
Conclusions:
The data support the view that pancreatic β-cells become dedifferentiated and convert to α- and δ-“like” cells in human type 2 diabetes. The findings should prompt a reassessment of goals in the prevention and treatment of β-cell dysfunction.
Type 2 diabetes is associated with progressive β-cell failure, resulting from combined loss of insulin secretory function and β-cell number (1). Prospective studies of subjects at high risk of developing or newly diagnosed with type 2 diabetes underscore that, whereas insulin resistance remains relatively stable in time, β-cell function undergoes a rapid, steady decline (2–4). However, despite its insidiously progressive course, β-cell failure can be partly and temporarily reversed by dietary or pharmacological interventions (2, 5). Although the progression of β-cell failure could be ascribed to β-cell death, its apparent reversibility suggests that cellular loss is not permanent (5, 6). Interestingly, insulin sensitizers appear to outperform insulin secretagogues in staving off β-cell dysfunction (7, 8). In the light of the sensitizers' role to decrease β-cell “afterload,” these findings can be construed to indicate a mechanistic link between increased demand for insulin secretion and β-cell loss. Cellular pathologies such as apoptosis, autophagy, oxidative stress, and nutrient overload (“toxicity”) can affect either β-cell function or mass (9, 10).
Animal studies demonstrate that pancreatic β-cells of mice become dedifferentiated in response to hyperglycemia, reverting to a progenitor-like state (11–15). In addition, β-cells convert to other endocrine cells, including glucagon (Gcg)-producing “α-like”-cells (11, 16), thus providing a potential explanation for the hyperglucagonemia of diabetes (17, 18).
The cellular plasticity of the endocrine pancreas remains largely untested in the pathophysiology of human diabetes (19), owing to the limitations of assessing cellular pathologies in vivo. Absent the ability to genetically label pancreatic endocrine cells to provide a definitive demonstration of dedifferentiation in humans, animal studies allow us to formulate testable hypotheses on the expected features of dedifferentiated human β-cells (6, 20). To understand whether human β-cells become dedifferentiated, we undertook to survey diabetic and nondiabetic pancreata from organ donors, using the next assumptions derived from experimental models: 1) dedifferentiated β-cells should no longer contain insulin, or other pancreatic hormones (to exclude cells arising from converted β-cells) (11); 2) they should retain endocrine features, as assessed by immunoreactivity with general endocrine markers (21); and 3) they should express progenitor cell markers (11). In addition, we considered the possibility that ectopic expression of transcription factors normally restricted to β-cells might indicate conversion of one endocrine cell type to another (11). Under these assumptions, the prediction that β-cells become dedifferentiated in type 2 diabetes was borne out by the studies described below.
Research Design and Methods
Subjects
We obtained pancreata from thirty organ donors. Thirteen had a history of type 2 diabetes, 1 of drug-induced diabetes, and 1 of diabetes of unclear type. The fifteen controls were organ donors without a history of diabetes, with normal plasma glucose during their stay in the intensive care unit (Supplemental Table 1). The institutional review boards at Columbia University and at the University of Pisa have approved all procedures.
Antibodies
We used the next primary antibodies: synaptophysin (Syn) (LS-C174787; LsBio), NK transcription factor-related 6.1 (NKX6.1) (F55A12; DSHB), chromogranin A (MAB5268; Millipore), Gcg (A056501–2; DAKO) (LS-B4738; LsBio) (M182; TaKaRa) (G2654; Sigma-Aldrich), somatostatin (Ssn) (A0566; DAKO) (sc-7819; Santa Cruz Biotechnology, Inc), pancreatic polypeptide (PP) (A0619; DAKO) (AB939; Millipore) (NB100–1793; Novus Biological), insulin (sc-9168; Santa Cruz Biotechnology, Inc) (A056401–2; DAKO), Musculoaponeurotic fibrosarcoma oncogene protein A (MAFA) (ab26405; Abcam), Forkhead box-containing protein O1 (FOXO1) (LS-B4151; LsBio), aristaless related homeobox (Arx) (MABN102; Millipore), aldehyde dehydrogenase 1A3 (ALDH1A3) (NBP2–15339; Novus Biological), and ghrelin (ab57222; Abcam).
Immunohistochemical and morphometric analyses
We fixed and processed tissue for immunohistochemistry as previously described (22, 23). We focused the survey on the head and neck region of the pancreas (24). We performed histochemical reactions in controls and persons with diabetes at the same time, using the same lot of antibodies at dilutions that we had tested to maximize sensitivity and minimize nonspecific staining. We controlled each reaction by omitting primary or secondary antibodies to determine signal specificity. We obtained frozen sections from samples collected at Columbia/Presbyterian Hospital to perform transcription factors analysis. We applied antigen retrieval at pH 9.0 (Nacalai USA) to facilitate antigen retrieval and nuclear transcription factors detection. We used Alexa-conjugated donkey secondary antibodies (Jackson ImmunoResearch and Molecular Probes) as described (22). We used confocal microscopy and Laser Scanning Microscope Software (Zeiss LSM 510 and 710) to survey colocalization and capture images. We performed the quantification in a blinded fashion using the CytoNuclear FL function of the HALO software to analyze individual cells in whole-slide fluorescent images. This tool scans images on multiple wavelengths corresponding to each fluorophor, locating cells and measuring the intensity of immunofluorescence against a preset standard. Each marker is measured in distinct cellular compartments, ie, nucleus and cytoplasm. The analysis scores numbers of positive cells for each marker and calculates the number of cells showing colocalization of different markers. To perform quantitative analyses, we scored at least 3 random sections per donor and 5 random islets per section. We analyzed 81 ± 8 cells/islet. We scored islets containing at least one dedifferentiated cell (Syn-positive and hormone-negative) as “dedifferentiated.” To quantify the ratios of hormone-producing and hormone-nonproducing cells, we divided the number of insulin-, Gcg-, Ssn-, PP-, or ghrelin-positive cells by the number of Syn-positive cells.
Electron microscopy (EM)
EM was performed as described (25, 26). We fixed islets in 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) for 1 hour at 4°C. After rinsing in cacodylate buffer, tissue was postfixed in 1% cacodylate-buffered osmium tetroxide for 2 hours at room temperature, then dehydrated in a graded series of ethanol, transferred to propylene oxide and embedded in Epon-Araldite. Ultrathin sections (60–80 nm thick) were cut with a diamond knife, placed on formvar-carbon coated copper grids (200 mesh), and stained with uranyl acetate and lead citrate. Morphometric analyses were performed as previously described. Microphotographs were obtained at ×10 000 and analyzed as described (25, 26).
Islet isolation and insulin secretion
Islets were prepared from pancreata of thirteen organ donors by collagenase digestion and density gradient purification (23), followed by culture in M199 medium (containing 5.5 mmol/L glucose), supplemented with 10% adult bovine serum, antibiotics (penicillin, 100 U/mL; streptomycin, 100 μg/mL; gentamicin, 50 μg/mL; and amphotericin B, 0.25 μg/mL) at 37°C in 5% CO2. Insulin release was determined by the batch incubation technique (27, 28). Groups of approximately 30 islets of comparable size were incubated at 37°C for 45 minutes in Krebs-Ringer bicarbonate (pH 7.4), with 0.5% albumin and 3.3 mmol/L glucose. Then, medium was removed, assayed to measure “basal” insulin secretion, and replaced with Krebs-Ringer bicarbonate containing 16.7 mmol/L glucose. After an additional 45-minute incubation, medium was removed, and insulin levels were measured to assess “stimulated” insulin release. Insulin secretion was expressed as stimulation index, ie, ratio of stimulated over basal insulin secretion.
RNA extraction
We extracted total RNA from batches of 100–120 handpicked islets using the PicoPure RNA Isolation kit (Arcturus), adapted to cell pellets. We rinsed islets with 1 mL of PBS, centrifuged them at 3000g for 5 minutes, resuspended them in 0.1 mL of extraction buffer, and incubated them at 42°C for 30 minutes. Thereafter, we centrifuged samples at 3000g for 2 minutes and processed the supernatant for RNA isolation. We removed genomic DNA by incubation with DNA nuclease I (QIAGEN), and eluted the RNA in 30 μL of elution buffer. We assessed RNA quantity and purity by absorbance at 260 and 280 nm, using the NanoDrop 2000C spectrophotometer and by testing samples on Nano LabChip of the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc). The respective mean ± SD of these parameters were: 51.1 ± 21.8 ng/μL for the amount of RNA, 2.1 ± 0.0 for the A260:A280 ratio, and 8.1 ± 0.5 for the RNA integrity number value.
Reverse transcription and quantitative polymerase chain reaction
Quantitative analysis of FOXO1, MAFA, and NKX6.1 transcripts was performed by real-time PCR, as described (29). We synthesized cDNA templates from 200 ng of RNA using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). TaqMan Fast Advanced Master Mix (Applied Biosystems) was used to perform real-time PCR, using 10-ng cDNA and 1 μL of TaqMan Gene Expression Assay (Applied Biosystems) in each well. Assays used were Hs01054576_m1 for FOXO1, Hs01651425_s1 for MAFA and Hs00232355_m1 for NKX6.1. We used importin 8 as a reference transcript, and evaluated expression using the Hs00183533_m1 assay. We performed PCR in the fast mode using the ViiA 7 system (Applied Biosystems). For each sample, we performed triplicate amplifications and used average measurements for data analysis. Fold differences in expression were determined by the 2−ΔCT method.
Statistical methods
We used 2-tailed Student's t test for data analysis and the customary threshold of P < .05 to declare a statistically significant difference. We present quantitative data as mean ± SEM.
Results
Increased β-cell dedifferentiation in type 2 diabetes
We assessed dedifferentiation in pancreata from organ donors with and without diabetes. We arbitrarily defined a dedifferentiated cell as a Syn-positive (ie, endocrine) cell that failed to react with antibodies to the 5 pancreatic hormones: insulin, Gcg, PP, Ssn, and ghrelin. A summary of patient features is reported in Supplemental Table 2. We surveyed the head and neck of the pancreas (24) and scored hormone-positive cells using antibodies to insulin, Gcg, Ssn, or PP, and general endocrine cells using antibodies to Syn (11). In a subset of patients (n = 5 for each group), we also examined the ratio of β- to α-cells. Consistent with previous reports (6, 30), we detected a 32% decrease (from 77% to 53%) of insulin-positive cells in diabetic donors (P < 1 × 10−6), and a 68% increase of Gcg-positive cells (from 22% to 37%) (P = .009), leading to a rise of the α- to β-cell ratio from 33% to 63% (P = .0002).
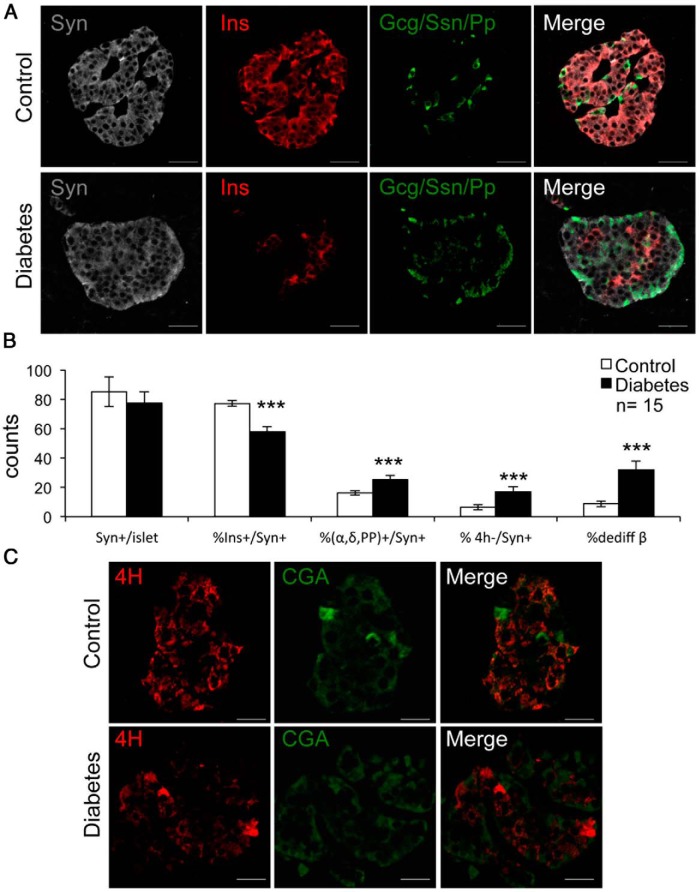
In the complete cohort (n = 15 for each group), we analyzed the number of Syn-positive/hormone-negative cells per islet. There were no differences in the number of Syn-positive cells between the 2 groups, indicating that there is no loss of cells with general endocrine features in type 2 diabetes (Figure 1, A and B). The percentage of Syn-positive/insulin-positive cells in persons with diabetes declined by 26% compared with controls (57% vs 77%) (P < .001) (Figure 1B). In contrast, the percentage of Syn-positive and Gcg/Ssn/Pp-positive cells rose by 36% (16% vs 25%) (P < .001), and the percentage of all surveyed cells testing positive for Syn and negative for the 4 hormones rose by 61% vs normal subjects (6.5% vs 16.8%) (P < .001). When normalized by the number of β-cells, the percentage of insulin-negative/Syn-positive cells increased 350% in persons with diabetes, from 8.7% to 31% (P < .001) (Figure 1, A and B). We obtained similar results when we used chromogranin A as a general endocrine marker (Figure 1C).
Figure 1.
Representative images of dedifferentiated β-cells. A, Immunofluorescent histochemistry on pancreatic section using insulin (Ins) (red), combined Gcg, Ssn, PP (green), and Syn (gray). B, Quantitative analysis of the data in A. C, Immunofluorescent histochemistry with the 4-hormone cocktail (4H) (red) and chromogranin A (CGA) (green). Data in B are mean ± SEM. ***, P < .001 by Student's t test. Scale bars, 20 μm; n = 15 for each group.
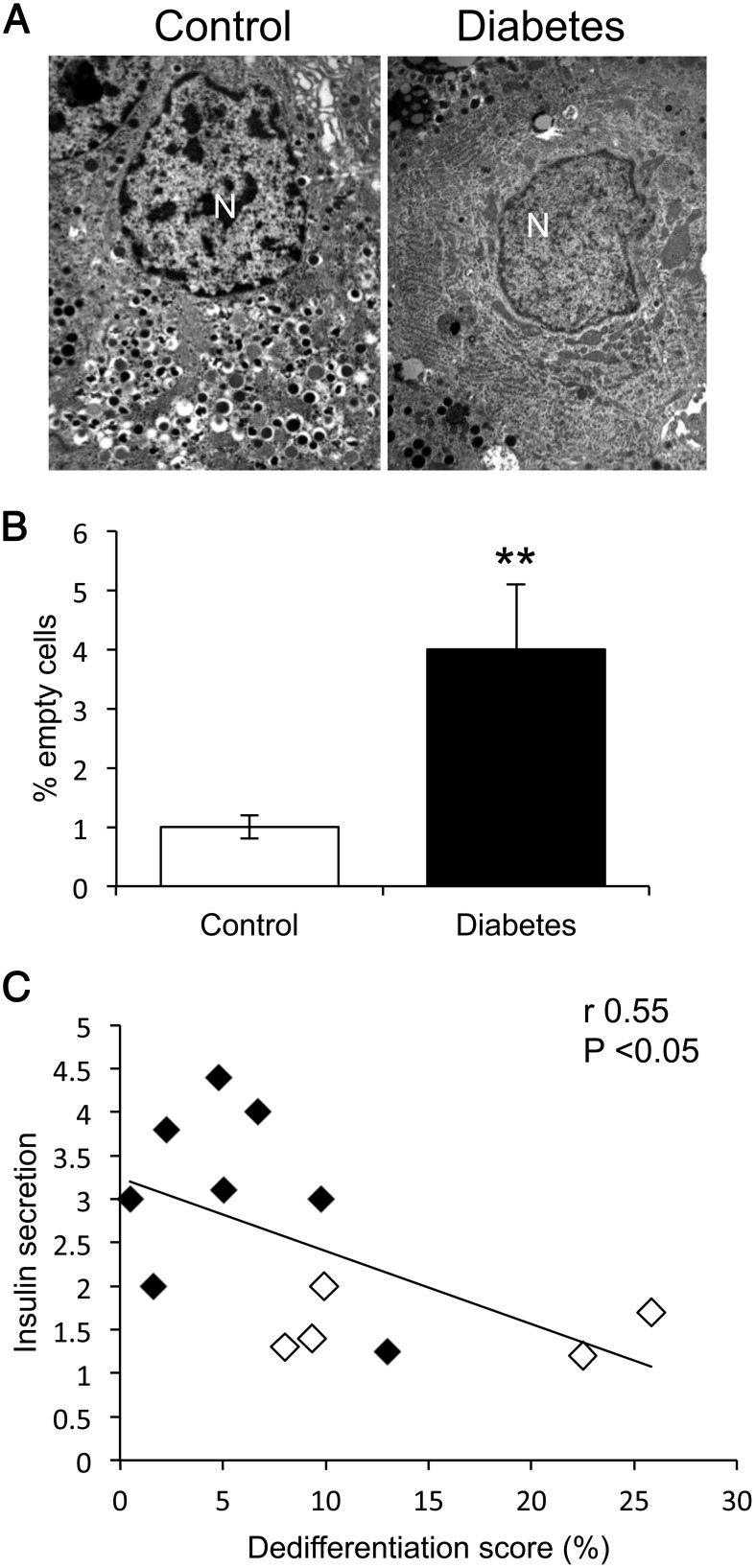
It is possible that the failure to detect insulin by immunohistochemistry reflects a decrease in insulin content but not a complete absence. To address this possibility, we analyzed insulin granule content by EM in a subset of 1290 and 1377 islet cells, respectively, from nondiabetic and type 2 diabetic pancreata. The percentage of cells that did not contain any secretory granules rose 4-fold in persons with diabetes, from 1.0 ± 0.2% to 4.0 ± 1.1% (P < .01) (Figure 2, A and B).
Figure 2.
EM and correlation of insulin secretion with dedifferentiation. A, Representative images of healthy and degranulated cells. B, Quantitative analysis of EM findings. Data are mean ± SEM. **, P < .01 by Student's t test. N, nucleus. C, We plotted linear correlation analyses (Spearman's r) between the dedifferentiation score, calculated as % ratio of SYN+4H−/SYN+ cells, and glucose-induced insulin secretion in isolated islets obtained from 13 donors. Controls are denoted by filled symbols, persons with diabetes by open circles.
We also sought to establish a functional correlation between dedifferentiation and insulin secretion. To this end, we assessed insulin release in response to glucose in a subset of 13 donors and found a substantial decrease in type 2 persons with diabetes (1.5 ± 0.3 vs 3.7 ± 1.0) (P < .01). Interestingly, insulin secretion was inversely correlated with the dedifferentiation score, defined as the ratio of Syn-positive/hormone-negative cells to Syn-positive cells (r = 0.55, P < .05) (Figure 2B). In contrast, we found no statistically significant correlation between dedifferentiation score and donors' age, body mass index, or duration of diabetes. There was a weak trend for an association between dedifferentiation score and age among persons with diabetes (Supplemental Figure 1).
Notably, there were large interislet variations within the same donor, with seemingly healthy islets lacking dedifferentiated cells mingled with islets characterized by extensive loss of hormone-positive cells. Islets with near-complete dedifferentiation, however, were unique to type 2 persons with diabetes (14, 30).
A transcriptional signature of dedifferentiated β-cells
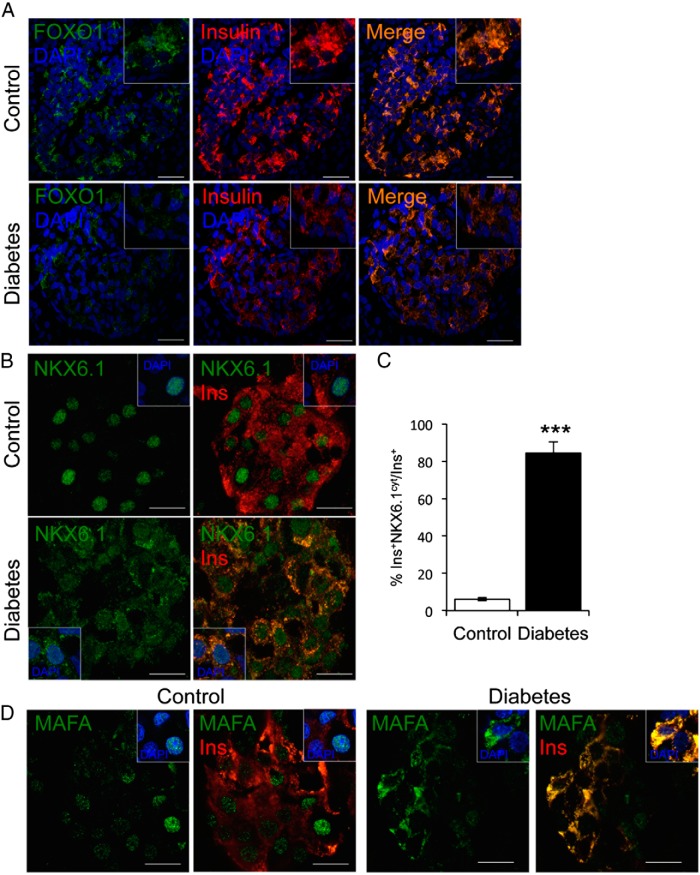
We next assessed expression and localization of transcription factors required for maintenance of β-cells in rodents: FOXO1, NKX6.1, and MAFA (11, 12, 14). As reported, transcripts encoding the 3 proteins were decreased in persons with diabetes (14). We determined that FOXO1 localization is restricted to β-cells (31) and that its levels decline in type 2 persons with diabetes, paralleling the loss of insulin immunoreactivity (Figure 3A). NKX6.1 localized to the nucleus of β-cells in control donors, whereas it localized to both nucleus and cytoplasm in 84% of insulin-positive cells in persons with diabetes (P < 1 × 10−5) (Figure 3, B and C). Similar to NKX6.1, the subcellular localization of MAFA was altered in β-cells of persons with diabetes, with diffuse cytoplasmic immunoreactivity (Figure 3D). However, because MAFA is also found in α-cells (14), we focused further analyses on FOXO1 and NKX6.1.
Figure 3.
Transcription factor analysis in pancreatic islets. A, Immunofluorescence on fresh-frozen pancreatic sections with FOXO1 (green), insulin (red), and DAPI (blue). B, Immunofluorescence with NKX6.1 (green), insulin (red), and DAPI (blue). C, Quantitative analysis of the data, shown as mean ± SEM. D, Immunofluorescence with MAFA (green), insulin (red), and DAPI (blue). Insets show representative cells. ***, P < .001 by Student's t test. Scale bars, 20 μm (A) and 10 μm (B and D); n = 5 for each group.
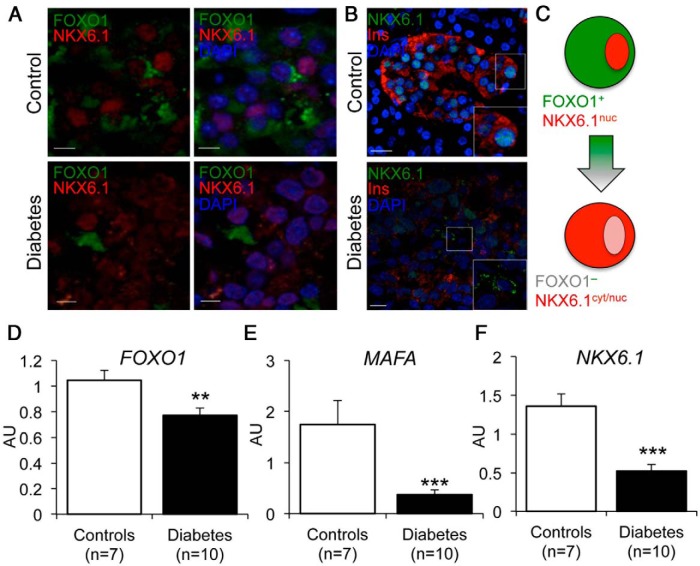
We surveyed pancreata for colocalization of FOXO1 and NKX6.1 and found that they colocalized to the same cells in control donors, with NKX6.1 in the nucleus and FOXO1 in the cytoplasm (Figure 4A). In contrast, pancreata from type 2 persons with diabetes showed cells with cytoplasmic NKX6.1 that lacked FOXO1 immunoreactivity (Figure 4A). Interestingly there were also insulin-negative cells with cytoplasmic NKX6.1 (Figure 4B). These cells may represent dedifferentiating β-cells that have lost FOXO1 and are in the process of losing NKX6.1 (Figure 4C). The findings suggest that cytoplasmic localization of NKX6.1 is a marker of dedifferentiating β-cells. In addition, we also determined mRNA levels of pancreatic and duodenal homeobox 1, NKX6.1, and FOXO1 in control and diabetic islets, and found that all 3 markers were decreased (Figure 4, D–F).
Figure 4.
Altered localization and expression of FOXO1 and NKX6.1 in dedifferentiating β-cells. A, Immunofluorescence of pancreatic islets with FOXO1 (green), NKX6.1 (red), and DAPI (blue). Scale bars, 5 μm. B, Immunofluorescence of pancreatic islets with NKX6.1 (green), insulin (red), and DAPI (blue). Scale bars, 10 μm. C, Proposed model of dedifferentiating β-cells. D–F, qRT-PCR analysis of FOXO1 (D), MAFA (E), and NKX6.1 (F) in isolated human islets. Data are shown as mean ± SEM. **, P < .01; ***, P < .001 by Student's t test (n = 7 for controls, n = 10 diabetes).
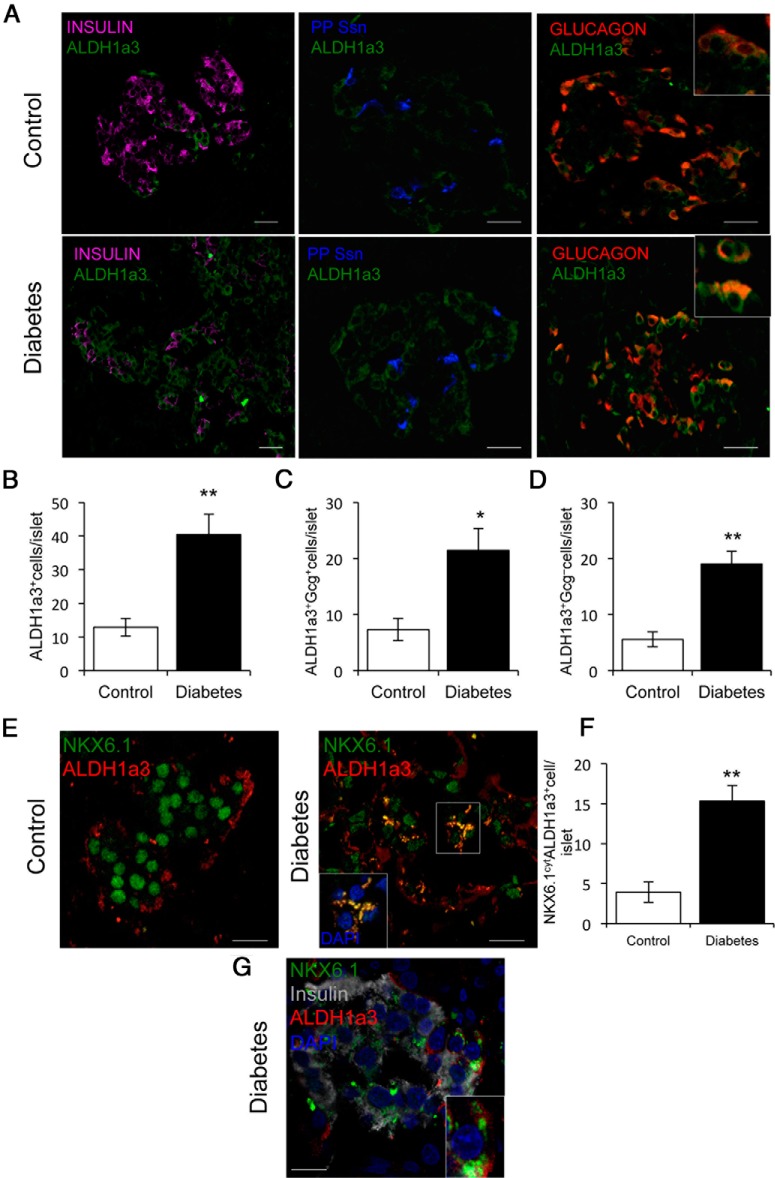
A key feature of β-cell dedifferentiation in animal models is regression to a progenitor-like stage (11, 12, 15). Analyses of gene expression datasets in diabetic mice indicated that progenitor cell marker, ALDH1A3 (32), is enriched in dedifferentiated islet endocrine cells (33). Thus, we used ALDH1A3 immunoreactivity to interrogate human pancreata. Mean counts of ALDH1A3-positive cells per islet increased 3-fold in type 2 persons with diabetes (P = .01) (Figure 5, A and B). Nearly 60% of ALDH1A3-positive cells in controls were immunoreactive with Gcg, indicating that they are α-cells. The number of Gcg-positive/ALHD1A3-positive cells increased 3-fold in persons with diabetes (P = .05) (Figure 5, A and C). Most importantly, 40% of ALDH1A3-positive cells were hormone negative (insulin, PP, and Ssn), consistent with their identity as progenitor-like cells (Figure 5A). This critical population increased over 3-fold in persons with diabetes compared with controls (P = .007) (Figure 5D). Are these cells dedifferentiated β-cells? To address this question we determined colocalization of ALDH1A3 with NKX6.1 or insulin and found a nearly 4-fold increase of ALDH1A3+ cells with cytoplasmic NKX6.1 in persons with diabetes compared with controls (P = .009) (Figure 5, E and F). Moreover, these cells were immunohistochemically insulin-negative (Figure 5G). These data are consistent with the possibility that, as β-cells lose their identity (indicated by loss of insulin and NKX6.1 mislocalization), they acquire ALDH1A3 immunoreactivity.
Figure 5.
ALDH1A3 localization in human islets. A, Immunofluorescence of ALDH1a3 (green) with insulin (magenta), combined Ssn and PP (blue), and Gcg (red). Scale bars, 20 μm. B–D, Quantitative analysis of the data shown as mean ± SEM. *, P = .05; **, P < .01 by Student's t test; n = 5 for each group. E, ALDH1A3 (red) colocalization with cytoplasmic NKX6.1 (green). Scale bars, 10 μm. F, Quantitative analysis of the data expressed as mean ± SEM. **, P < .01 by Student's t test; n = 5 for each group. G, Immunofluorescence of ALDH1a3 (red) with insulin (gray), NKX6.1 (green), and DAPI (blue). Inset shows ALDH1a3 colocalization with NKX6.1 in insulin negative cell. Scale bar, 10 μm.
Evidence of β-cell conversion to other cell types
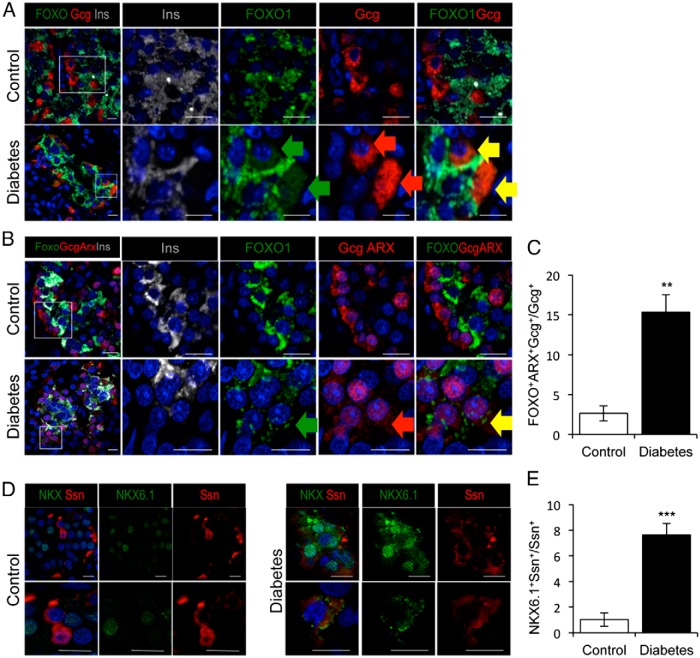
Type 2 diabetes is a state of relative Gcg overproduction (34). It is unclear whether this can be explained by increased pancreatic α-cell mass or function (17, 18, 34). Because the number of Syn+ cells does not change between persons with diabetes and controls, the increase of α-cells in persons with diabetes cannot be accounted for by reduced numbers of β- or other endocrine cells. Thus, we examined α-cell markers in persons with diabetes. We found that up to 12% of Gcg-immunoreactive cells in diabetic pancreata tested weakly positive for cytoplasmic FOXO1 immunoreactivity (P = .05). We did not detect such cells in controls; the variance among our diabetic sample was entirely due to a single outlier with an inordinately high number of these cells (Figure 6A). Thus, in a second experiment, we evaluated a larger sample by immunohistochemistry with FOXO1, Gcg, and the α-cell transcription factor, ARX (35). We found that 15% of Gcg-positive cells scored positive for ARX and cytoplasmic FOXO1 in persons with diabetes, a 7-fold rise compared with controls (P = .005) (Figure 6, B and C). Because cytoplasmic FOXO1 is inactive, the findings are compatible with the explanation that these cells represent former β-cells that, through loss of FOXO1 function, are undergoing conversion to Gcg-producing, α-like-cells. We found no evidence of FOXO1 expression in δ-cells (data not shown).
Figure 6.
Evidence of β-cell conversion to non-β-cells. A, Mislocalization of FOXO1 (green) to Gcg-immunoreactive cells (red). B, Mislocalization of FOXO1 (green) to ARX- and Gcg-immunoreactive cells (red). C, Quantitative analysis of triple positive (FOXO1, ARX, and Gcg) cells, as determined by the assay in C. D, Mislocalization of NKX6.1 (green) to Ssn-immunoreactive cells (red). E, Quantitative analysis of double positive (NKX6.1 and Ssn) cells. Insulin immunofluorescence is shown in gray (A and B). Scale bars, 10 μm (A, B, and D). In all panels, nuclei are counterstained with DAPI (blue). Green, red, and yellow arrows in panel indicate FOXO1+GCG+ cells (A) and FOXO1+GCG+Arx+ (B). C and E, Data as mean ± SEM. *, P = .05; **, P < .01; ***, P < .001 by Student's t test; n = 5 for each group.
Next, we examined the coexpression of NKX6.1 with Gcg and Ssn. We found no evidence of NKX6.1 colocalization with Gcg, but 7.5% of Ssn-positive cells scored positive for cytoplasmic NKX6.1 (P = .001) (Figure 6, D and E). These data are consistent with the possibility that, as β-cells lose NKX6.1, they convert to Ssn-producing cells. We did not find any PP-positive cell that express either FOXO1 or NKX6.1 (data not shown).
Discussion
The pathogenesis of β-cell failure in type 2 diabetes is complex, as it integrates both qualitative (ie, secretory) as well as quantitative (ie, cell number) defects in insulin production, possibly spawning an inappropriate Gcg response (17, 36). In light of recent suggestions that β-cell loss in type 2 diabetes is due to dedifferentiation, we surveyed pancreata from type 2 diabetic and nondiabetic organ donors to examine this question. We found a near 3-fold increase in the number of pancreatic islet cells that no longer produce any of the 4 major pancreatic hormones, yet retain endocrine features. Moreover, we report that transcription factors FOXO1 and NKX6.1, markers of the well-appointed β-cell, are either decreased or mislocalized in β-cells from persons with diabetes. And that FOXO1 and NKX6.1 are ectopically found in Gcg- or Ssn-immunoreactive cells of type 2 persons with diabetes, respectively. These data are consistent with the possibility that insulin-producing β-cells become dedifferentiated and undergo conversion to Gcg- or Ssn-immunoreactive cells during the course of type 2 diabetes (Supplemental Table 3). Evidence of cell conversion is necessarily correlative in human studies. For example, it is possible, although in our view unlikely, that cells with weak cytoplasmic FOXO1 are in fact α-cells undergoing conversion to β-cells. Our findings uphold key findings from experimental models, suggesting that β-cells are not permanently lost in persons with type 2 diabetes (11–15). A limitation of this work is that we cannot rule out that some “hormone-negative” cells still possess low levels of hormone production. Nonetheless, from a clinical standpoint such cells would likely be unhealthy, and should be targeted for prevention and reversal of β-cell dysfunction.
Our data expand and strengthen conclusions from previous human studies in several key aspects (6, 14, 20, 37). First, we determined the number of cells that retained endocrine properties, as assessed by Syn or chromogranin A immunohistochemistry, but lost hormone immunoreactivity. Second, we correlated these findings with EM evidence of β-cell degranulation and reduced glucose-induced insulin secretion. Finally, we performed a transcription factor analysis that included localization of FOXO1 in islets as a marker of both β-cell dedifferentiation and conversion to other cells.
Progenitor cells in the failing islet
In rodents, dedifferentiated β-cells revert to a progenitor-like stage characterized by expression of transcription factor Neurogenin3 (11, 12, 15). We were unable to detect NEUROGENIN3 immunoreactivity in either pancreas or intestine of humans, where it should be abundant (38), indicating that this is a technical problem; thus, we cannot conclude that NEUROGENIN3 is absent in human diabetic pancreata. Nevertheless, using information gleaned from gene expression profiling of animal models of β-cell dedifferentiation (12, 33), we found that progenitor cell marker ALDH1A3 (32) is present in former β-cells, ie, cells with cytoplasmic NKX6.1, supporting the hypothesis that dedifferentiation entails regression to a progenitor-like stage. We do not know whether ALDH1A3 is simply a marker, or an effector of differentiation processes. There are precedents linking ALDH1 activity (but not the A3 isoform) with pancreas differentiation. In mice, Aldh1 is enriched in pancreatic centroacinar cells (39), a potential source of endocrine progenitor cells (40). In human fetal pancreas, ALDH1 is enriched in progenitor cells (41). And functional inhibition of its enzymatic product retinoic acid impairs terminal differentiation of β-cells (41). Thus, expression of ALDH1A3 could be envisioned as a compensatory mechanism to protect against dedifferentiation. It should be noted that ALHD1A3 immunoreactivity is a standard marker of progenitor cells in cancer (32).
Therapeutic implications
The present findings provide correlative evidence for a role of FOXO1 in human β-cell failure. The clinical relevance of this observation is that FOXOs integrate insulin/hormone-dependent pathways with glucose/nutrient-dependent pathways in the pathogenesis of β-cell “stress” (42), thus providing a unifying mechanism that supersedes the debate on whether insulin resistance or hyperglycemia are to blame for precipitating β-cell failure, and offering a potential explanation for the benefits of glucose-lowering agents as well as insulin sensitizers on β-cell function (2). Indeed, the notion that dedifferentiated cells lie quiescent and can be redifferentiated to produce insulin can explain why restoration of β-cell function is possible for years on end after the onset of hyperglycemia (5, 43, 44). However, it should be noted that the rapid onset of β-cell recovery is likely to also entail an amelioration of insulin secretion by residual β-cells (45).
The therapeutic conundrum of type 2 diabetes is that β-cell dysfunction progresses more rapidly than insulin resistance (2, 3), yet insulin sensitizers appear to outperform insulin secretagogues as first-line treatment (7, 8). We suggest that these data, as well as data indicating that early β-cell “rest” is beneficial to preserve β-cell function (46), are consistent with dedifferentiation as a key mechanism of β-cell failure. It is possible that insulin secretagogues accelerate dedifferentiation by depleting β-cells of insulin, whereas decreasing the afterload of insulin resistance, and lessening the demand for insulin, are conducive to β-cell rest. We envision dedifferentiation as a mechanism to protect β-cells from apoptosis by stealth, preserving them for redifferentiation under more favorable metabolic conditions. In this regard, a recent publication demonstrating that, in rodents, β-cell dedifferentiation can be reversed (15), provides impetus to harness appropriate biochemical, cellular, and genetic pathways to modulate this mechanism in humans. An interesting question that should be addressed in future studies is whether this mechanism is also at play in type 1 diabetes, to protect β-cells against immune destruction (47).
Acknowledgments
We thank members of the Accili and Marchetti laboratories for helpful comments on the manuscript.
Author contributions: F.C. and R.B. designed, executed, and analyzed the experiments and wrote the manuscript; J.Y.K.-M. performed and analyzed experiments; M.M., L.M., and M.S. selected patients and obtained pancreata, performed experiments, including EM, and islet isolation, including insulin secretion; P.R.S. and L.E.R. performed surgeries from which donor samples were obtained; D.A. and P.M. designed and reviewed the experiments and wrote the manuscript; and D.A. and P.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by National Institutes of Health Grants DK64819 and DK63608 (Columbia University Diabetes Research Center), by the JPB Foundation, the Brehm Coalition, and the Juvenile Diabetes Research Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALDH1A3
- aldehyde dehydrogenase 1A3
- ARX
- Aristaless related homeobox
- EM
- electron microscopy
- FOXO1
- Forkhead box-containing protein O1
- Gcg
- glucagon
- MAFA
- Musculoaponeurotic fibrosarcoma oncogene protein A
- NKX6.1
- NK transcription factor-related 6.1
- PP
- pancreatic polypeptide
- Ssn
- somatostatin
- Syn
- synaptophysin.
References
- 1. Ferrannini E. The stunned β cell: a brief history. Cell Metab. 2010;11:349–352. [DOI] [PubMed] [Google Scholar]
- 2. Defronzo RA, Tripathy D, Schwenke DC, et al. Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes. 2013;62:3920–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. β-Cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med. 1998;15:290–296. [DOI] [PubMed] [Google Scholar]
- 5. Savage PJ, Bennion LJ, Flock EV, et al. Diet-induced improvement of abnormalities in insulin and glucagon secretion and in insulin receptor binding in diabetes mellitus. J Clin Endocrinol Metab. 1979;48:999–1007. [DOI] [PubMed] [Google Scholar]
- 6. Marselli L, Suleiman M, Masini M, et al. Are we overestimating the loss of β cells in type 2 diabetes? Diabetologia. 2014;57:362–365. [DOI] [PubMed] [Google Scholar]
- 7. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. [DOI] [PubMed] [Google Scholar]
- 8. U.K. Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 9. Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of β cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758–768. [DOI] [PubMed] [Google Scholar]
- 10. Talchai C, Lin HV, Kitamura T, Accili D. Genetic and biochemical pathways of β-cell failure in type 2 diabetes. Diabetes Obes Metab. 2009;11(suppl 4):38–45. [DOI] [PubMed] [Google Scholar]
- 11. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic β cells. Cell Rep. 2013;4:1262–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises β-cell identity and results in diabetes mellitus. Genes Dev. 2013;27:2563–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123:3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brereton MF, Iberl M, Shimomura K, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun. 2014;5:4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dunning BE, Gerich JE. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28:253–283. [DOI] [PubMed] [Google Scholar]
- 18. Yoon KH, Ko SH, Cho JH, et al. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. [DOI] [PubMed] [Google Scholar]
- 19. Dor Y, Glaser B. β-Cell dedifferentiation and type 2 diabetes. N Engl J Med. 2013;368:572–573. [DOI] [PubMed] [Google Scholar]
- 20. White MG, Marshall HL, Rigby R, et al. Expression of mesenchymal and α-cell phenotypic markers in islet β-cells in recently diagnosed diabetes. Diabetes Care. 2013;36:3818–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lukinius A, Stridsberg M, Wilander E. Cellular expression and specific intragranular localization of chromogranin A, chromogranin B, and synaptophysin during ontogeny of pancreatic islet cells: an ultrastructural study. Pancreas. 2003;27:38–46. [DOI] [PubMed] [Google Scholar]
- 22. Kitamura T, Kitamura YI, Kobayashi M, et al. Regulation of pancreatic juxtaductal endocrine cell formation by FoxO1. Mol Cell Biol. 2009;29:4417–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marchetti P, Bugliani M, Lupi R, et al. The endoplasmic reticulum in pancreatic β cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Misawa R, Zielinski MC, et al. Regional differences in islet distribution in the human pancreas–preferential β-cell loss in the head region in patients with type 2 diabetes. PLoS One. 2013;8:e67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masini M, Bugliani M, Lupi R, et al. Autophagy in human type 2 diabetes pancreatic β cells. Diabetologia. 2009;52:1083–1086. [DOI] [PubMed] [Google Scholar]
- 26. Masini M, Marselli L, Bugliani M, et al. Ultrastructural morphometric analysis of insulin secretory granules in human type 2 diabetes. Acta Diabetol. 2012;49(suppl 1):S247–S252. [DOI] [PubMed] [Google Scholar]
- 27. Lupi R, Del Guerra S, Fierabracci V, et al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002;51(suppl 1):S134–S137. [DOI] [PubMed] [Google Scholar]
- 28. Del Guerra S, Lupi R, Marselli L, et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–735. [DOI] [PubMed] [Google Scholar]
- 29. Bugliani M, Liechti R, Cheon H, et al. Microarray analysis of isolated human islet transcriptome in type 2 diabetes and the role of the ubiquitin-proteasome system in pancreatic β cell dysfunction. Mol Cell Endocrinol. 2013;367:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(suppl 4):32–42. [DOI] [PubMed] [Google Scholar]
- 31. Al-Masri M, Krishnamurthy M, Li J, et al. Effect of forkhead box O1 (FOXO1) on β cell development in the human fetal pancreas. Diabetologia. 2010;53:699–711. [DOI] [PubMed] [Google Scholar]
- 32. Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10:1378–1384. [DOI] [PubMed] [Google Scholar]
- 33. Kim-Muller JY, Zhao S, Srivastava S, et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab. 2014;20:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henquin JC, Accili D, Ahren B, Boitard C, Seino S, Cerasi E. Long in the shade, glucagon re-occupies centre court. Diabetes Obes Metab. 2011;13(suppl 1):v–viii. [DOI] [PubMed] [Google Scholar]
- 35. Spijker HS, Ravelli RB, Mommaas-Kienhuis AM, et al. Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes. 2013;62:2471–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Polonsky KS. The past 200 years in diabetes. N Engl J Med. 2012;367:1332–1340. [DOI] [PubMed] [Google Scholar]
- 37. Mezza T, Muscogiuri G, Sorice GP, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, Cortina G, Wu SV, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. [DOI] [PubMed] [Google Scholar]
- 39. Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki T, Kadoya Y, Sato Y, et al. The expression of pancreatic endocrine markers in centroacinar cells of the normal and regenerating rat pancreas: their possible transformation to endocrine cells. Arch Histol Cytol. 2003;66:347–358. [DOI] [PubMed] [Google Scholar]
- 41. Li J, Feng ZC, Yeung FS, et al. Aldehyde dehydrogenase 1 activity in the developing human pancreas modulates retinoic acid signalling in mediating islet differentiation and survival. Diabetologia. 2014;57:754–764. [DOI] [PubMed] [Google Scholar]
- 42. Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. [DOI] [PubMed] [Google Scholar]
- 43. Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;1:444–447. [DOI] [PubMed] [Google Scholar]
- 44. Wajchenberg BL. β-Cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. [DOI] [PubMed] [Google Scholar]
- 45. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. [DOI] [PubMed] [Google Scholar]
- 46. Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. [DOI] [PubMed] [Google Scholar]
- 47. Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes. 2011;60:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]