Abstract
Background
Manganese (Mn) inhalation has been associated with neuropsychological and neurological sequelae in exposed workers. Few environmental epidemiologic studies have examined the potentially neurotoxic effects of Mn exposure in ambient air on motor function and hand tremor in adult community residents. Mn exposed residents were recruited in two Ohio towns: Marietta, a town near a ferro-manganese smelter, and East Liverpool, a town adjacent to a facility processing, crushing, screening, and packaging Mn products.
Methods
Chronic (≥10 years) exposure to ambient air Mn in adult residents and effects on neuropsychological and neurological outcomes were investigated. Participants from Marietta (n = 100) and East Liverpool (n = 86) were combined for analyses. AERMOD dispersion modeling of fixed-site outdoor air monitoring data estimated Mn inhalation over a ten year period. Adult Mn-exposed residents’ psychomotor ability was assessed using Finger Tapping, Hand Dynamometer, Grooved Pegboard, and the Computerized Adaptive Testing System (CATSYS) Tremor system. Bayesian structural equation modeling was used to assess associations between air-Mn and motor function and tremor.
Results
Air-Mn exposure was significantly correlated in bivariate analyses with the tremor test (CATSYS) for intensity, center frequency and harmonic index. The Bayesian path analysis model showed associations of air-Mn with the CATSYS non-dominant center frequency and harmonic index; while the Bayesian structural equation model revealed associations between air-Mn and lower Finger Tapping scores. Household income was significantly associated with motor dysfunction but not with tremor.
Conclusion
Tremor and motor function were associated with higher exposure to airborne Mn.
Keywords: Manganese, Neurotoxicity, Psychomotor, Tremor, Environment, Adult residents
Graphical Abstract
1. Introduction
Manganese (Mn) is a naturally occurring essential element for living organisms, including humans. Industrial processes using Mn can result in high occupational and environmental Mn exposures from dust particles (Bowler et al., 2007a,b; Mergler et al., 1999; Myers et al., 2003; Solís-Vivanco et al., 2009). Exposure to Mn in the environment may also result from excess Mn in water, soil, and foods (ATSDR, 2012).
Exposure to excessive amounts of Mn may lead to adverse health outcomes (ATSDR, 2012). Neurological and neuropsychological sequelae from Mn overexposure include problems with motor efficiency and speed, tremor, postural sway and rigidity (similar to Parkinson's disease), mood disturbances and cognitive impairment (Bowler et al., 1999; Mergler et al., 1999). Most health studies on Mn overexposure report on occupational exposures to Mn (Meyer-Baron et al., 2013). Few studies on adults have examined adverse effects of environmental exposures to Mn, which tend to be much lower compared to occupational exposures (Bowler et al., 2011; Haynes et al., 2010; Kim et al., 2011; Mergler et al., 1999; Rodriquez-Agudelo et al., 2006; Solís-Vivanco et al., 2009; Baldwin et al., 1999; Menezes-Filho et al., 2011).
The published literature on environmental Mn studies examining motor and tremor function is sparse (Solís-Vivanco et al., 2009; Rodriquez-Agudelo et al., 2006; Baldwin et al., 1999; Kim et al., 2011; Bowler et al., 2012). Solís-Vivanco et al. (2009) investigated eight communities in a Mexican mining district and found an association between attention and air-Mn in 288 adults. In the same sample, Rodriquez-Agudelo et al. (2006) report elevated odds ratios for reduced coordination and declines in motor function with exposure to air-Mn concentrations ranging from 0.003 to 5.86 μg/m3.
In Canada, low Mn concentrations in the range of 0.009–0.035 μg/m3 (based on TSP air sampling near a ferro-alloy and silico-alloy plant) were associated with nervous system dysfunction (Baldwin et al., 1999)). We reported previously on motor function and tremor in adults by comparing two communities: one with elevated air-Mn due to ferro-alloy smelting operations at a large metals refinery, and a demographically similar town lacking such industry (Kim et al., 2011; ATSDR, 2007, 2009). We found an increased risk in the exposed town for abnormal Unified Parkinson's Disease Rating Scale (UPDRS) motor findings for bradykinesia and the Computerized Adaptive Testing System (CATSYS) postural sway performance in comparison to the unexposed residents. We concluded that the UPDRS and CATSYS findings may indicate subclinical effects of Mn exposure. Using the same exposed study population, we used modeled air-Mn (M = 0.18 μg/m3; SD = 0.13), blood Mn (M = 9.65 μg/L; SD = 3.21), distance from the smelter (M = 4.75 miles; SD = 1.64) and length of residence (M = 36.1 years; SD = 15.8), and found significant clinically-elevated generalized anxiety (Bowler et al., 2012). Higher scores on anxiety were related to poorer performances on UPDRS motor function and bradykinesia.
Building on the previous study conducted in Marietta, OH, this present report presents additional data collected in East Liverpool, OH, a town with previously documented elevated air concentrations of Mn (ATSDR, 2010). Air-Mn concentrations for 24-h samples had a range as high as 0.02 to 25.0 μg/m3 and average of 1.50 (AM) and 0.56 μg/m3 (GM). Both East Liverpool and Marietta were identified in U.S. EPA's School Air Toxics (SAT) initiative as having increased potential for non-cancer health effects from exposure to Mn (U.S. EPA, 2010a, b).
Given the need for further clarification of the effects of environmental Mn exposure, this study evaluated chronic (≥10 years) air-Mn exposure and associations between tremor and motor function. Motor and neurological functions were assessed with established and sensitive clinical tests. The two objectives of this study were to examine the relationship between: 1) motor function as measured by psychomotor speed, strength and tactile manipulative function and Mn-air exposure and 2) measurable tremor and Mn-air exposure.
2. Methods
2.1. Study design and participants
A cross-sectional design was used. Data were collected from residents in two towns, East Liverpool and Marietta, OH. Participant selection for the Marietta sample was described elsewhere (Bowler et al., 2012; Kim et al., 2011).
The same methods of selection/recruitment, inclusion and exclusion criteria were followed for East Liverpool residents and they were administered the same neurological and neuropsychological evaluations using identical procedures.
The Marietta study took place in 2009 and residents were randomly selected based on residential parcel records in a defined exposure zone (within 12 air miles from the smelter Mn source). In 2011, East Liverpool residents, similarly to Marietta, were sent invitation letters whose addresses were located within two air miles downwind of the Mn source (vendor address lists were cross-checked with East Liverpool parcel maps and the Columbiana County 9-1-1 Emergency Response mapping system database). The difference in using recruitment zones for Marietta (within 12 air miles) and East Liverpool (2 air miles) was supported by different particle size fractions of Mn in the two towns; East Liverpool had larger particle sizes, and Mn was dispersed over a shorter distance. Single family units, multifamily units, and trailer addresses were included and resulted in a total of 3041 IRB-approved recruitment letters mailed (1732 in Marietta and 1309 in East Liverpool). In both towns, up to two participants per household could take part in the study. Residents who returned post cards and/or were reached by telephone (269 in Marietta and 436 in East Liverpool) were screened for eligibility (122 were interested and eligible in Marietta and 123 in East Liverpool) and scheduled for testing appointments. Subsequently, the number of eligible residents tested in Marietta was 100 (in 76 households) and 86 in East Liverpool (72 households) (14 “no shows” in East Liverpool due to personal or work scheduling conflicts).
Study inclusion criteria comprised the following: a minimum of 10 years residency and ages between 30 and 75 years. Exclusions included major physical or psychiatric illness, a degenerative disorder, significant prior chemical exposure(s) requiring medical treatment, and/or having worked at either the Mn smelter in Marietta or the Mn plant in East Liverpool. Each participant's self-reported medical history was reviewed for meeting the inclusion/exclusion criteria in clinical interviews conducted by the P.I. (RMB) and was additionally reviewed by the collaborating physician (YK).
After all other study procedures were completed, questionnaires and test folders were reviewed for completeness and a gift card for $50.00 from a local store was given to each participant.
IRB approval was obtained for both towns from San Francisco State University, the Ohio Department of Health (ODH), and for the U.S. EPA, the University of North Carolina at Chapel Hill.
2.2. Data collection, testing and administration
In each town data collection took place at locations convenient for participants. Testing rooms met the privacy needs for sensitive evaluations. A general health questionnaire, individual interview with the principal investigator (PI), and neurological, neuropsychological, and mood tests were administered. The health questionnaire developed for this study contained sections on residency, symptoms, medical history, medications, work history and behaviors, diet, and personal demographic information. The questionnaire also asked about specific symptoms of neurological and neuropsychological function. Due to the large number of neuropsychological and neurological tests, which included cognitive and mood tests, separate manuscripts report the results of mood (Bowler et al., 2012) and cognitive tests (Bowler et al., 2015).
All motor and neurological functions were measured bilaterally and administered by trained psychometricians who followed standardized instructions. Motor speed, grip strength, and tactile manipulative ability were measured using the Finger Tapping Test, the Hand Dynamometer, and the Grooved Pegboard, respectively. The Finger Tapping test (Lezak et al., 2012; Bowler and Lezak, 2015) is a measure of psychomotor speed requiring examinees to tap a lever with their index finger as fast as possible within five 10-second trials alternating between the dominant and non-dominant hand. The score is the average number of taps for each hand, recorded by a counter connected with the lever attached to a board. Tactile manipulative ability was assessed with the Grooved Pegboard test (Kløve, 1963) which requires speeded rotation and insertion of pegs with ridges on them in a 5 × 5 matrix of holes. The score for each hand is based on the time required to complete the task. Grip strength was assessed in kilograms with the use of a Dynamometer (Strauss et al., 2006) which participants were asked to squeeze alternating between hands, twice for each hand, and the scores for each hand were averaged. The raw scores of these motor tests were standardized in T-scores (mean = 50, SD = 10; a higher T-score indicates better motor function) using commonly accepted normative data stratified by age, sex, and education (Heaton et al., 2004). The updated Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery permits demographic adjustments using the Neuropsychological Norms computed for ages of 20–88 years, of 1000 neurologically normal adults, native English language speakers, and educated in the United States.
Testing of bilateral hand tremor was measured with the CATSYS (Danish Product Development, 1996; Després et al., 2000). The trained graduate student who administered the CATSYS tremor tests in both towns was supervised by the senior medical coauthor (YK). The Tremor tests administered bilaterally, included measures of: 1) Tremor Intensity — the mean root square of the accelerations in the 0.9–15 Hz band; 2) Center Frequency — the median frequency of acceleration in the 0.9–15 Hz band with 50% above or below the center frequency; and 3) Harmonic Index — compares tremor frequency patterns with a single harmonic oscillation, which decreases when there are many tremor oscillations (Després et al., 2000). The scoring system developed by Edwards and Beuter (1999) was used. The calculation methods for tremor measures for the normative group included sex and 5 groups of ‘normal’ individuals between the ages of 20 and 70 years (Després et al., 2000). Age was re-examined as a covariate but was not further associated with the already adjusted tremor scores in the two towns.
Although the motor tests in Marietta and East Liverpool were administered by different examiners, all psychometricians demonstrated advanced level neuropsychological testing proficiency, and were extensively trained by the P.I., who observed numerous motor test administrations by each of the testers. Additionally, all examiners followed verbatim the standardized test administration instructions. All completed motor tests were scored twice using standardized instructions — any scoring discrepancies were resolved by the P.I. Test data were double entered into SPSS using identification numbers only.
2.3. Description of the long-term exposure analysis
Dispersion modeling calibrated with measured data was used to estimate chronic ambient air-Mn exposures for each study participant in the two towns, as published by Colledge et al. (2015). Briefly, both communities had over 12 years of environmental total suspended particulate matter (TSP) sampling data generated between 1999 and 2013 that were analyzed for a number of toxic metals, including Mn. As noted in Colledge et al. (2015), sample filters were analyzed for metals using inductively coupled plasma mass spectrometry (ICP-MS) according to the EPA Compendium Method IO 3.5, and includes analysis for aluminum, antimony, arsenic, barium, beryllium, cadmium, chromium, cobalt, copper, lead, manganese, molybdenum, nickel, selenium, silver, thallium, thorium, uranium, vanadium, and zinc. Of these metals, ATSDR (2010) determined that manganese presented the primary health risk in the Marietta and East Liverpool communities.
Only years with data completeness of ≥75% in both towns (1999–2013) were used for statistical analyses. For over 10 years, stationary monitors at various sites in both towns (five in Marietta; three in East Liverpool) collected TSP from the air using high volume (“Hi-Vol”) stationary monitors, and 341 glass fiber HiVol 24-hour. These samples were analyzed to determine metal content, particle size distribution and morphology, and elemental abundance by using LAICP-MS (laser ablation-inductively coupled plasma-mass spectrometry), SEM (scanning electron microscopy), and XRD (X-ray diffractometry) techniques. Both 24-hour and monthly averaged samples were collected and evaluated using Federal Reference Methods (U.S. EPA, 2009).
Receptor points were identified for Mn sources and study participants. AERMOD, a U.S. EPA steady state plume modeling program, was used to predict long-term air-Mn concentrations near each modeled receptor (5 year averages based on 5 years of meteorological data). This model includes a meteorological preprocessor (AERMET), and a topography preprocessor (AERMAP). No emissions data were available for the Mn source facility in East Liverpool, so a site surface air emissions model, scalable to measured data for both towns, was used to quantify the relationship of concentrations to a reference monitoring site. A unit emission rate of 1 g/s was used over the surface areas of both sites, generating relative (but not site-specific) estimated concentrations for all receptors and each area air monitoring location. Ratios of modeled concentrations were calculated as compared to the reference monitoring location closest to the facility. These ratios were multiplied by the actual ambient air-Mn measured at the reference monitoring location to yield receptor-specific long-term (>10 year) Mn concentration averages. This receptor-specific Mn concentration average was used as a surrogate for the corresponding resident's chronic Mn inhalation exposure.
Statistical distributions of community exposure were as follows: Marietta [PM10: 0.18 μg/m3 (AM), range 0.03 to 1.33 μg/m3; PM2.5: 0.05 μg/m3 (AM), range 0.007 to 0.34 μg/m3]; East Liverpool [PM10: 0.31 μg/m3 (AM), range 0.005 to 2.21 μg/m3; PM2.5: 0.03 μg/m3 (AM), range 0.001 to 0.23 μg/m3]. Although the air-Mn levels in East Liverpool were generally higher than in Marietta, different particle sizes in the 2 towns suggest that the Marietta residents may have a slightly higher exposure to respirable (dae ≤ 10 μm) Mn particulate matter. Smelters create finer particulate than grinding operations. The fingerprinting analysis of Mn in both towns, conducted by U.S. EPA at the National Enforcement Investigations Center (“NEIC”), determined that Eramet (Marietta) had an impact radius of at least 10 miles. Particulate connected to Eramet (identified by a lead isotope analysis) was found on filters from Vienna, West Virginia to downtown Marietta, Ohio, thus we fully expected residents to have exposures from this facility at a greater distance than 2 miles. These particles (83%) were smaller than 10 μm, with over half having a dae ranging from 3.4 to 4.6 μm. In East Liverpool, the same NEIC analysis determined that the particles of Mn were much larger (35% were <dae of 10 μm) and would be expected to have more local deposition.
The inhalational fraction was determined through scaling the TSP-Mn. The scaling factor was determined from more limited (≤1 year of collocated sampling) PM10 or PM2.5 sampling. The relative fractions of Mn in total dust were determined through forensic laboratory techniques (dust fingerprinting via SEM and laser ablation) and ambient air sampling. These analyses indicated that East Liverpool residents were exposed to more Mn in total dust, but when comparing the respirable fraction, Marietta residents experienced slightly higher exposures to respirable dust. Since the calculation of respirable particles over the 10-year period was assumed to be a constant percentage of the TSP over time, the statistical analyses using TSP data were appropriate. Despite the fact that fine particles (dae ≤ 2.5 μm) travel further and Marietta had more fine particles, a higher percentage of residents live close to and were exposed to a greater amount of Mn in total dust in East Liverpool.
2.4. Statistical analysis
Because we used the same methods to estimate exposure within the two towns, we were able to pool the two towns together for analyses (n = 186).
The Finger Tapping, Dynamometer, and Grooved Pegboard scores used the revised T-scores of the Heaton norms (Heaton et al., 2004). None of the tremor test scores were normally distributed nor could they be transformed to T-scores. Therefore, the Wilcoxon two-sample test was used for the non-normally distributed tremor and motor function scores when comparing the towns, except where indicated. Spearman's rho was used for calculating correlations between air-Mn concentrations (combined towns) and the motor and tremor function scores.
Demographic differences by town were compared using chi-square tests for categorical variables (sex, employment status, household income). Student t-tests were used when characteristics were continuous and normally distributed and a non-parametric Wilcoxon two-sample test was used for variables that were not normally distributed. Household income was associated with the motor function tests and with exposure to air-Mn; however, none of the tremor variables were associated with household income. Extensive analyses revealed no other potential confounding factors.
We used Bayesian estimation to assess the number of factors in an exploratory factor analysis of the six tremor tests as part of refining the measurement model prior to building Structural Equation Models (SEMs). Maximum likelihood exploratory factor analyses of the six motor function tests were conducted separately from the tremor tests. We hypothesized that the tremor tests would form a latent factor representing subtle changes in fine motor control and that the Finger Tapping, Dynamometer, and Grooved Pegboard tests could also be measured as a latent variable representing generalized motor control. We also hypothesized that combining these tests into factors to represent two levels of neurological impairment would provide us with greater power to detect subtle changes in neurological function. In addition, using a latent modeling approach reduces the number of statistical comparisons. Model fit in the exploratory factor analysis (EFA) was examined using the Comparative Fit Index (CFI > 0.95), the Tucker Lewis Index (TLI > 0.95), and the Root Mean Square Error of Approximation (RMSEA < 0.05). Confirmatory factor analysis (CFA) was used to confirm the factor structure using the Bayesian estimator for the tremor variables due to non-normality. Robust maximum likelihood was used for a CFA of the motor function variables because the motor function scores were z-scores calculated from T-scores.
A Bayesian model was used to assess the associations between individual tremor tests and air-Mn concentrations. In a separate Bayesian model, which included only the motor function tests and air-Mn, we assessed the associations between motor function and air-Mn concentrations for the combined towns. Similarly, separate models were used for the tremor tests based on EFA and CFA models and the best fitting model was identified using SEMs. Bayesian estimation with a non-informative prior was used due to the small sample size, correlated exposure data (MacLehose et al., 2007) and the non-linearity, non-normality and skewed distribution in the air concentration variable. We modeled the median in a two chain, Markov Chain Monte Carlo (MCMC) with Gibbs sampling. Convergence was set at 0.01 and satisfied when the potential scale reduction (PSR) was close to 1.0 (Gelman et al., 2004). The posterior predictive value (PPV) and chi-square test were used to assess model fit. Ideally, the PPV should be about 0.5 and should be centered at 0 with a narrow and symmetric 95% confidence interval, indicating an excellent fitting model (Muthén and Asparouhov, 2012). The chi-square test was used to determine how well the original values could be replicated by random sampling of the MCMC chains. The replicated values produced from the MCMC chains using the Gibbs Sampler compares the replicated chi-square statistic to the observed chi-square value. This is similar to a likelihood ratio test for model fit. One-sided p-values are reported for effect sizes because the distributions are not assumed to be symmetrical.
Parameter estimates produced using a Bayesian estimator can be interpreted as slope coefficients similar to models using a least squares or a maximum likelihood estimator. The estimates (effect sizes) have a standard error, a confidence interval and a p-value associated with them. Effects were considered significant at the 0.05 level of confidence. Descriptive and univariate analyses were conducted in SPSS, version 22 (IBM Corp., 2012). Bayesian analyses were conducted in MPlus, Version 6.12 (Muthen and Muthen, 1998-2010).
3. Results
Table 1 provides demographic and exposure characteristics of the two towns separately and combined. Marietta residents had, on average, more years of education beyond high school, resided fewer years in their respective town, and had significantly higher household income compared to East Liverpool, but no other demographic differences were noted. Income and education were strongly correlated in Marietta (r = 0.58, p < 0.0001), but was less so for EL (r = 0.23, p = 0.01). A significant correlation between household income and age was observed for the combined towns (r = 0.46, p < 0.0001). Partial correlations were used to evaluate the effect of years of residence on test performance. Length of residency was significantly associated with CATSYS center frequency (dominant hand r = 0.313, p < 0.001; non-dominant hand r = 0.305, p < 0.001) and harmonic index (dominant hand r = −0.293, p < 0.001; non-dominant hand r = −0.275, p < 0.001). Analyses of Mn in diet from a diet questionnaire indicated no significant differences between the residents of the two towns in the amount of Mn consumed in foods, or the amount of Mn consumed in foods by sex.
Table 1.
Demographic and exposure characteristics for Marietta and East Liverpool residents and the combined towns.
| Marietta (n = 100) |
East Liverpool (n = 86) |
Differences by town |
Combined towns |
|
|---|---|---|---|---|
| Residentcharacteristic | N (%) | N (%) | p-Value | N (%) |
| Sex | ||||
| Male | 45 (45.0) | 31 (36.1) | 76 (40.9%) | |
| Female | 55 (55.0) | 55 (63.9) | 0.22 | 110(59.1%) |
| Employment status | ||||
| Unemployed | 39 (39.0) | 38 (44.7) | 77 (41.6) | |
| Employed/student | 61 (61.0) | 47 (55.3) | 0.43 | 108(58.4) |
| Annual household incomea | ||||
| $0 to $19,999 | 11 (11.9) | 22 (28.2) | 33 (19.4) | |
| $20,000 to $39,999 | 26 (28.3) | 23 (29.5) | 49 (28.8) | |
| $40,000 to $69,999 | 25 (27.2) | 13 (16.7) | 38 (22.4) | |
| $ 70,000 and above | 30 (32.6) | 20 (25.6) | 0.04 | 50 (29.4) |
| Mean ± SD | Range | Mean ± SD | Range | p-Value | Mean ± SD | Range | |
|---|---|---|---|---|---|---|---|
| Age | 54.4 ± 9.9 | 30.0-74.0 | 56.0 ± 11.9 | 30.0-75.0 | 0.28 | 55.15 ± 10.85 | 30.0-75.0 |
| Years of education | 14.6 ± 2.7 | 8.0-22.0 | 12.9 ± 2.2 | 6.0-18.0 | <0.0001 | 13.76 ± 2.59 | 6.0-22.0 |
| Years of residence in town | 36.1 ± 15.8 | 10.0-65.0 | 47.0 ± 16.4 | 10.0-75.0 | <0.0001 | 41.1 ± 16.92 | 10.0-75.0 |
| Dietary manganese (mg/week)b | 14.8 ± 15.1 | 0.06-74.43 | 11.3 ± 10.3 | 0.06-65.79 | 0.35 | 13.2 ± 13.2 | 0.06-74.43 |
| Male | 15.6 ± 18.0 | 0.06-74.43 | 11.3 ± 12.1 | 0.22-65.79 | 0.715 | 13.9 ± 15.9 | 0.06-74.43 |
| Female | 14.0 ± 12.3 | 0.45-48.16 | 11.4 ± 9.2 | 0.06-41.49 | 0.340 | 12.7 ± 10.9 | 0.06-48.16 |
| Air manganese (TSP),μg/m3 | 0.21 ± 0.22 | 0.03-1.61 | 0.88 ± 1.23 | 0.01-6.32 | <0.001 | 0.52 ± 0.91 | 0.01-6.32 |
Missing for 16 residents.
Mann-Whitney U test: no difference between males and females (p = 0.559).
Bilateral CATSYS tremor intensity, center frequency, and harmonic index scores differed significantly between the towns and were associated with air-Mn for the combined towns (Table 2).
Table 2.
Bivariate associations between tremor test z-scores, motor function test T-scores and air-Mn.
| Tremor or motor test by handedness | Marietta Mean ± SD | EL Mean ± SD | Town differences p-Value | Combined towns Mean ± SD | Air-Mn Spearman correlation (p-Value) |
|---|---|---|---|---|---|
| Intensity, dom. | 0.12±0.08 | 0.13±0.05 | 0.04a | 0.13±0.07 | 0.17 (0.02) |
| Center frequency, dom. | 4.29±2.00 | 7.96±2.28 | <0.0001a | 5.99±2.81 | 0.34 (<0.0001) |
| Harmonic index, dom. | 0.95±0.02 | 0.83±0.06 | 0.0007a | 0.89±0.08 | –0.33 (<0.0001) |
| Intensity, non-dom. | 0.13±0.10 | 0.13±0.05 | 0.02a | 0.13±0.08 | 0.17 (0.02) |
| Center frequency, non-dom. | 4.16±2.12 | 7.96±2.37 | <0.0001a | 5.92±2.93 | 0.33 (<0.0001) |
| Harmonic index, non-dom. | 0.95±0.02 | 0.83±0.06 | <0.0001a | 0.90±0.08 | –.36 (<0.0001) |
| Finger tapping, dom. | 47.89±10.97 | 40.89±8.50 | <0.0001b | 44.68±10.49 | –0.26 (<0.001) |
| Finger tapping, non-dom. | 46.41±9.36 | 41.41±9.64 | 0.0005b | 44.11±9.79 | –0.16 (0.03) |
| Dynamometer, dom. | 36.57±8.63 | 30.55±8.41 | <0.0001b | 33.82±9.02 | –0.06 (0.40) |
| Dynamometer, non-dom. | 37.93±8.93 | 33.43±7.48 | 0.003b | 35.88±8.57 | –0.05 (0.47) |
| Grooved pegboard, dom. | 49.86±9.79 | 45.99±11.23 | 0.013b | 48.08±10.62 | –0.10 (0.17) |
| Grooved pegboard, non-dom. | 48.75±10.26 | 46.98±11.07 | 0.26a | 47.94±10.65 | –0.15 (0.04) |
Dom. = dominant hand; non-dom. = non-dominant hand.
Wilcoxon 2-sample test.
t-Test.
Significant differences between towns were seen for all motor tests with the exception of Grooved Pegboard, non-dominant hand (Table 2). Among the three motor tests, Finger Tapping T–scores were significantly negatively correlated bilaterally with air-Mn for the combined towns, as were the Grooved Pegboard non-dominant hand T–scores (Table 2).
With the exception of the Hand Dynamometer score for the non-dominant hand, which was significantly correlated only with the Dynamometer dominant hand (ρ = 0.65, p < 0.0001), the motor function tests were significantly correlated with one another (ρ = 0.16 to 0.36, all p-values < 0.05).
Using factor analysis, the tremor variables, in whole or in part, did not comprise a latent factor. In contrast, the six motor tests loaded onto three factors with a perfect fit (χ2 = 471, p < 0.0001; CFI = 1.00; TLI = 1.00; RMSEA = 0.00; eigenvalues were 2.50, 1.55 and 1.17). Finger Tapping, dominant and non-dominant hand, formed a strong single factor, as did the Dynamometer dominant and non-dominant hands and Grooved Pegboard dominant and non-dominant hands. Factor loadings ranged from 0.79 to 1.0 with no cross-loadings. The Finger Tapping construct was significantly correlated with the Dynamometer construct (p < 0.0001); remaining pairwise correlations were significant (p < 0.05).
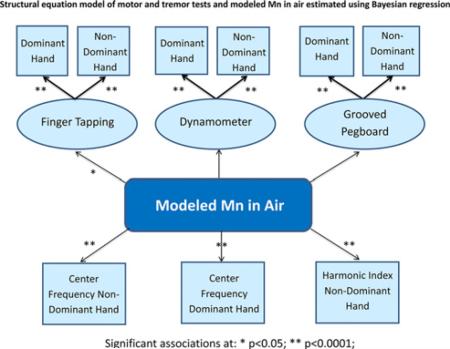
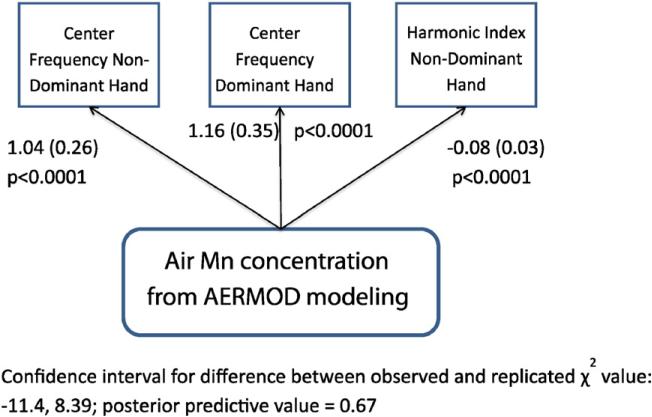
Of the six tremor tests, Center Frequency showed the strongest associations with exposure to air-Mn with beta (slope) coefficients of magnitude greater than one (p-values < 0.0001) (Table 3 and Fig. 1). The Harmonic Index for the non-dominant hand was also significant, but with a smaller effect size. The Bayesian path analysis model showed an excellent fit to the data as evidenced by the large PPV value and narrow 95% confidence interval for the replicated chi-square values (Table 3). Sampling from the MCMC reproduces the true model parameters with a convincing degree of certainty.
Table 3.
Unadjusted Bayesian path analysis model for the associations between CATSYS tremor tests and manganese air concentration (air-Mn) from AERMOD surface air modeling (combined Marietta, East Liverpool, Ohio) averaged over 2003-2013.
| Associations and model fit statistics | Effect estimate β ± SE | One-sided p-value |
|---|---|---|
| Air-Mn and center frequency, non-dominant hand | 1.04 (0.26) | <0.0001 |
| Air-Mn and center frequency, dominant hand | 1.16 (0.35) | <0.0001 |
| Air-Mn and harmonic index, non-dominant hand | –0.08 (0.03) | <0.0001 |
| Model fit statistics | ||
| Posterior predictive value (PPV) | 0.667 | |
| 95% CI for difference between observed and replicated χ2 values | (–11.4, 8.39) | |
| Potential scale reduction (PSR) | 1.004 |
Fig. 1.
Mn Air concentration from AERMOD modeling for CATSYS center frequency and harmonic index.
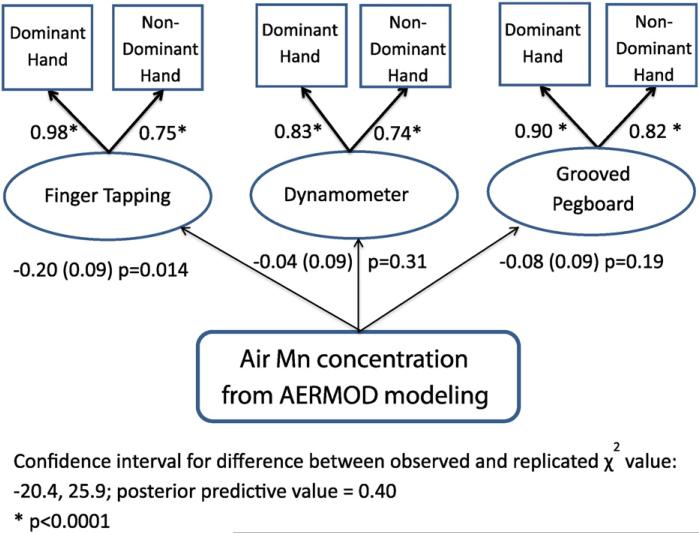
Exposure to high modeled air-Mn concentrations was associated with reduced Finger Tapping speed, dominant and non-dominant hands, in a Bayesian structural equation model (Table 4 and Fig. 2). Although Hand Dynamometer and Grooved Pegboard effects did not reach significance, the Bayesian model showed a better fit when they were included. In a model adjusted for household income, a slight reduction in the effect size between Finger Tapping scores and air-Mn was observed, but remained statistically significant. Household income was significantly associated with the factors derived for all motor function tests (Finger Tapping, Dynamometer and Grooved Pegboard).
Table 4.
Results of unadjusted and income-adjusted Bayesian structural equation model for the associations between three motor function domains and manganese air concentration from AERMOD surface air modeling (combined Marietta and East Liverpool, OH) averaged over 2003-2013.
| Associations and model fit statistics | Unadjusted effect estimate β ± SE (p-value) | Adjusted effect estimate β ± SE (p-value) |
|---|---|---|
| Air-Mn and finger tapping, both hands combined | –0.22 ± 0.09 (0.006) | –0.20 ± 0.09 (0.014) |
| Air-Mn and dynamometer, both hands combined | –0.06 ± 0.09 (0.26) | –0.04 ± 0.09 (0.31) |
| Air-Mn and grooved pegboard, both hands combined | –0.07 ± 0.09 (0.23) | –0.08 ± 0.09 (0.19) |
| Income and Finger tapping | 0.08 ± 0.03 (0.001) | |
| Dynamometer | NA | 0.06 ± 0.03 (0.003) |
| Grooved pegboard | 0.07 ± 0.03 (0.005) | |
| Model fit | ||
| Posterior predictive value (PPV) | 0.406 | 0.399 |
| 95% CI for difference between observed and replicated χ2 values | (–18.1,24.6) | (–20.4, 25.9) |
| Potential scale reduction (PSR) | 1.015 | 1.017 |
Fig. 2.
Structural equation model of motor function tests and Mn air concentration estimated using Bayesian regression.
4. Discussion
Tremor and motor function were examined in 186 residents from Marietta and East Liverpool, Ohio; two towns located near sources of airborne Mn pollution. Although Mn exposure in previous occupational studies has been associated similarly with motor and tremor findings, assessment of low level (compared to occupational studies) chronic Mn exposures require highly sensitive tests of motor function and tremor, and experienced examiners. Our findings suggest that sensitive clinical tests of motor and tremor function can detect the type of subtle changes that might be expected from low level chronic environmental Mn exposure. Of the existing environmental studies, few used either the Finger Tapping or Grooved Pegboard tests, sensitive tests used by clinically trained neuropsychologists.
The earlier Quebec community-based studies by Mergler et al. (1999) involving 273 people used the less clinically sensitive computerized SPES test of finger tapping speed which has less height between the board and tapper. Fine psychomotor speed, measured by Finger Tapping, may be an earlier effect of Mn exposure, whereas impairment on tests of tactile function and grip strength may be later effects, more similar to the clinical Parkinsonism seen in occupational studies.
Several factors could explain why Finger Tapping performance may have been vulnerable to low level chronic Mn exposure in our sample, while Grooved Pegboard and Dynamometer performances were not. The literature shows variability in performance among these three measures of motor function, across both participants with brain lesions and normal controls (Bowler and Lezak, 2015). Finger tapping requires less complex coordination, psychomotor speed, and cognition compared to the Grooved Pegboard Test, which, in addition to psychomotor speed, requires visuomotor coordination, sequencing, and tactile manipulative ability. Finger Tapping has been associated with cortical areas and pathways to the dorsolateral prefrontal area (Johnson and Prigatano, 2000). These pathways are affected in Mn exposure, but no specific association with these pathways have been identified for Grooved Pegboard or Dynamometer (Strauss et al., 2006). Furthermore, Finger Tapping performance is associated with the dopaminergic system (Yang et al., 2003), which is also affected by Mn exposure and in Parkinson's disease. In low level Mn exposure, Grooved Pegboard and Dynamometer performance may be less affected (Strauss et al., 2006), but deficits become more pronounced in later stages of Parkinsonism and Parkinson's disease.
Mn neurotoxicity and Parkinson's disease (PD) have similar clinical presentations. Both disease processes are known to target the basal ganglia and result in motor, cognitive, and mood dysfunction (Lezak et al., 2012). Although Mn neurotoxicity and PD may be distinguished by early onset in workers and patients exposed to Mn and by certain clinical features (e.g. postural tremor in Mn toxicity, resting tremor in PD; L-Dopa ineffectiveness in Mn toxicity), Mn neurotoxicity is often misdiagnosed as PD, even by experienced neurologists. In PD, dopamine levels are depleted through nigrostriatal dopamine neuron degeneration; with Mn overexposure, pathology appears to be related to postsynaptic impairment to the nigrostriatal system (Calne et al., 1994). In a current paper addressing this issue, Guilarte and Gonzales (2015) suggest the damage from Mn may not be linked to midbrain dopaminergic neuron cell loss, which is the pathophysiological process in PD. Despite these differences, the functional result of neuropathology in both disorders is considered similar and involves disruption of the pathways connecting the basal ganglia to the prefrontal cortex, which govern purposeful movement and behavioral and motor inhibition.
The literature is inconsistent as to whether performance on tests of motor function is related to educational and mental ability factors. However, higher income, which is directly related to education, is reported to be associated with overall better scores on the Wechsler Scales (Strauss et al., 2006). Years of education do not reflect the quality of the education, but income is considered a “proxy for quality of education and health status” by some authors (Strauss et al., 2006).
The current findings are not surprising considering environmental-polluting industries are often located in neighborhoods with lower education and subsequently lower incomes (Fan et al., 2010; Johnson and Coulberson, 1993; Mohai et al., 2009; Norton et al., 2007; Payne-Sturges and Gee, 2006). This may be a contributing factor in more adverse health outcomes and contribute to health disparities in some communities. A shorter distance from the Mn exposure point source was associated with fewer years of education (r = 0.32, p < 0.001) and a lower household income (r = 0.20, p = 0.008). Using hierarchical linear regressions and squared multiple partial correlations (R2) as a description of effect size (Cohen, 1992), distance from the point source accounted for a large amount of variance in CATSYS center frequency non-dominant (R2 = 27%) and dominant hand (R2 = 27%) scores, and CATSYS harmonic index non-dominant (R2 = 43%) and dominant hand (R2 = 45%) scores. Only small effects were present for associations between distance and neuropsychological motor tests. This indicates that while tremor intensity does not have an effect on distance, proximity to the Mn exposure point source has a large effect on the frequency and acceleration of the tremor oscillations.
Diet is the major route of Mn exposure for humans. As discussed above, a detailed self-report diet history questionnaire was constructed which used established reported Mn levels in certain foods such as vegetables, grains, beans, nuts and tea from the National Nutrient Database for Standard Reference (Release 27) (U.S. Department of Agriculture). Additionally, under normal exposure levels the majority of Mn consumed is quickly excreted, as the body works to maintain homeostasis and thereby prevents toxicity/over-absorption of Mn (Schroeter et al., 2011; Yoon et al., 2011). Dietary Mn intake between the towns on total dietary Mn was within reported normal levels (ATSDR, 2012) for total Mn consumption (0.7–10.9 mg of Mn daily in Western diets). Also, dietary Mn was not related to Mn air or any outcome variables.
The AERMOD modeling provided estimates of inhaled Mn, which was associated with several motor function deficits and tremor. Inhaled Mn enters through the lungs and nose, reaching the brain areas that are involved in motor abilities. It would be worthwhile for future studies to use MRI to examine Mn deposition in the brain in the relation to motor function deficits.
In all, the findings of both the motor and tremor results in terms of clinical impairment are in the mild range in both towns but are slightly greater in East Liverpool. The authors used the extensive normative data designating impaired and unimpaired scores for clinical evaluations as reported by Lezak et al. (2012); Mitrushina et al. (2005), and by Bowler and Lezak (2015). Additionally, available normative data from the norming program developed by Heaton et al. (2004) for the motor tests was utilized because it also has relevant stratified adjustments for age, sex and education. The chosen motor tests all are typically used in sensitive clinical evaluations and have shown to have good reliability (Mitrushina et al., 2005). It is notable that residents in East Liverpool, more of whom are engaged in skilled trade jobs, have lower scores than the residents of Marietta. This is contrary to expectation, as skilled workers would typically have higher scores on motor tests due to the practice effects in their work. This may also be a result of their somewhat higher levels of air-Mn exposure.
The CATSYS tests for hand tremor are stratified by age and sex and also have good reliability (Després et al., 2000). The normative data reported by Després et al. (2000) have been used for the detection of Mn neurotoxicity and are used by neurologists as a more sensitive quantifiable psychometric instrument than the qualitative assessment in the UPDRS described by Fahn et al. and the members of the UPDRS Development Committee (Fahn and Elton, 1987). Although the results of the motor scale of UPDRS test for the first group studied by Kim et al. (2011) showed some associations between the UPDRS motor scale and air-Mn, these relationships were of a lower magnitude than the results presented here when examining the results of the larger group of the 2 exposed towns combined. Kim et al. (2011) reported subclinical qualitative findings for motor and bradykinesia.
We found a higher center frequency (~8 Hz) and intensity of tremor in participants of East Liverpool than those of Marietta (~4 Hz). We also found a decreased harmonic index in participants in East Liverpool than those in Marietta. High frequency or intensity of tremor indicates increased tremor, but a decreased harmonic index indicates that the tremor is composed of many oscillations (Després et al., 2000). Moreover, there was a correlation between the center frequency and intensity and harmonic index in both hands, and the air Mn concentrations. This means that the higher the air Mn levels, the more frequent, stronger, and irregular the tremor. The present study found a change in postural tremor and bilaterality in tremor, which is more frequent in manganism than in Parkinson's disease (Calne et al., 1994). In addition, being more exposed to air Mn would increase tremor frequencies which become progressively distant from the typical tremor frequency of Parkinson's disease, for example (4–6 Hz) (Hughes et al., 1992). Further studies will be needed to clarify a different neurophysiological mechanism of tremor between manganism and Parkinson's disease.
Many methodological strengths are associated with this study. The study's design included stringent selection and inclusion criteria and well trained testing administrators. The two communities were relatively stable as far as residence, and thus we were able to recruit sufficient number of participants who resided in the community a minimum of 10 years, with mean years of residency 36 years for Marietta and 47 years for East Liverpool.
Selection criteria required exclusion of any prior or present work exposures and other illnesses affecting motor efficiency. Environmental studies with lower exposures than typically found in occupational settings have lower and more subtle deficits which are more difficult to detect, making controlling for possible confounding sources of motor efficiency problems essential and an important strength of this study.
The different particle size fractions of Mn in both towns, which necessitated a difference in the distance for recruitment, is a limitation of this study. However, the distance from the exposure source was based on an estimate of the distance for the airborne Mn dispersion (Marietta: 12 air miles; East Liverpool: 2 air miles). In addition, differing Mn release characteristics in both towns and air dispersion modeling uncertainties may affect Mn inhalation exposure estimates. The current findings do not suggest that specific concentrations of Mn lead to impairment in motor function over a set period of time; instead, chronic, long-term Mn exposure at the level documented in East Liverpool and Marietta is implicated.
Although 186 participants were sufficient to detect associations between modeled air-Mn and motor speed, as measured by Finger Tapping test scores, a larger sample size would further substantiate these findings. Additionally, replication of the study design is needed to evaluate the generalizability of the findings, especially in light of the limited literature of environmental Mn exposures in adults.
In any epidemiological study of environmental exposures there is the potential of uncontrolled confounding. Although the questionnaire covered an exhaustive list of neurological conditions, it is possible that latent neurological disorders may be present in study participants, and it is possible that these residents were of lower household income and had higher air Mn exposures.
Study data were collected in 2009 and 2011, by the same testers/interviewers using the same methodology. Due to funding considerations, it was not possible to study both towns at the same time. However, no significant regional political and economic events occurred during the intervening time. In addition, potentially confounding factors (i.e., diet, behavior, etc.) are not expected to be influenced by dates of data collection. The possibility for a self-selection bias was minimized by the strict methodological criteria. The demographic characteristics of both East Liverpool (EL) and Marietta (M) study sample (SS) were mostly representative of the 2008–2012 U.S. Census (C) data (with the exception of education) for the respective towns in Ohio (East Liverpool, Marietta and Census samples): percent white: EL: SS = 95.3%, C = 94.3% and M: SS = 94.0%, C = 96.7%; percent aged 62 years and older: EL: SS = 32.6%, C = 17.4% and M: SS = 25.0%, C = 22.2%; percent with education of high school and above: EL: SS = 88.5%, C = 79.5% and M: SS = 96.0%, C = 86.9%.
5. Conclusions
In summary, this study found evidence for an association of long-term chronic environmental Mn exposure with impairment in motor function. Household income affected motor ability in the context of Mn exposure. This finding raises questions about important health disparities which would benefit from future research. Slowing of Finger Tapping speed seems to be a precursor of impaired psychomotor efficiency, in basal ganglia disease (PD) as is impaired performance on Grooved Pegboard, Hand Dynamometer and other Parkinson's disease signs related to dopaminergic loss. The motor and tremor effects of environmental Mn exposure in adults represent a relatively new area of environmental research, and one that requires sensitive methods to detect the subtle changes that are expected at the lower environmental (compared to occupational) exposure levels of interest. Further studies with similar methodology are needed to continue to characterize the effects of chronic, low-dose environmental Mn exposure.
HIGHLIGHTS.
Adult residents of two towns environmentally exposed to manganese in air
10 year modeled air-Mn exposures range from 0.01 to 6.32 μg/m3
Air-Mn associated with CATSYS tremor intensity, center frequency, and harmonic index
Center frequency showed strongest associations with air-Mn exposure.
Increased air-Mn levels also associated with lower psychomotor speed.
Acknowledgments
Special thanks are given to Dr. Harry Roels for his extensive input and assistance with planning and carrying out this study. Also acknowledged for their valuable support are: Ohio Department of Health, Bureau of Environmental Health (Greg Stein, Robert Frey); East Liverpool City Health District (Ms. Jelayne Dray, Health Commissioner); East Liverpool City Council and the mayor of East Liverpool, Mr. James P. Swogger; Marietta City Health Department (Michael Brockett, Health Commissioner); and Washington County Health Department (Kathleen Meckstroth, Health Commissioner). We additionally thank Dr. Donna Mergler, Université du Québec à Montréal, for her generous consultation throughout this project and Stanley Durkee and William Boyes (EPA) for review of this manuscript. The research described here has been funded wholly (or in part) by the U.S. Environmental Protection Agency through cooperative agreement number 83416001 to San Francisco State University (Marietta study) and EPA Contracts EP-11-D-000424 and EP-13-D-000146 (East Liverpool study).
We thank the doctoral students assisting in testing and scoring: Dr. Beth Stutsman, Ms. Jessica Warren, Ms. Katherine Brown, Dr. Katherine Wilson, Dr. Linda Mora, Mr. Matthew Beristianos, Dr. Matthew Harris, Mr. Ralph Rasalan and Ms. Tori Strong. Special thanks are also given to the late Dr. Roxanne Burns of Kent State University for her assistance with recruiting and arranging space at Kent State University for the study feedback meeting in East Liverpool, Ms. Stephanie Halfhill and Ms. Megan Rodgers who volunteered their time to assist us.
Most of all, we thank the study participants in both East Liverpool and Marietta for their invaluable contribution to the knowledge gained from this investigation.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA or ATSDR.
References
- ATSDR . Health Consultation: Washington County Air Quality. Marietta, Washington County, Ohio: 2007. [Aug 30 2010]. ([Online]. Available: http://www.atsdr.cdc.gov/HAC/pha/marietta3/ATSDRHealthConsultation2007.pdf.) [Google Scholar]
- ATSDR . Health Consultation: Marietta Area Air Investigation. Marietta, Ohio: 2009. [Aug 30 2010]. ([Online]. Available: http://www.atsdr.cdc.gov/HAC/pha/marietta3/ATSDRMariettaHealthConsultationIII2009Final.pdf.) [Google Scholar]
- ATSDR [May 5 2011];Health Consultation: East Liverpool Air Quality. 2010 ([Online]. Available: http://www.atsdr.cdc.gov/HAC/pha/EastLiverpoolHC/EastLiverpoolHealthConsultation11210.pdf)
- ATSDR . Toxicological Profile for Manganese. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA.: 2012. [Google Scholar]
- Baldwin M, Mergler D, Larribe F, et al. Bioindicator and exposure data for a population based study of manganese. Neurotoxicology. 1999;20:343–354. [PubMed] [Google Scholar]
- Bowler RM, Lezak MD. Neuropsychological evaluation and exposure to neurotoxicants. In: Lotti M, Ml B, editors. Occupational Neurology. Elsevier; Amsterdam: 2015. [Google Scholar]
- Bowler RM, Nakagawa S, Drezgic M, et al. Sequelae of fume exposure in con-fined space welding: a neurological and neuropsychological case series. Neurotoxicology. 2007a;28:298–311. doi: 10.1016/j.neuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup. Environ. Med. 2007b;64:167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Gocheva V, Harris M, et al. Prospective study on neurotoxic effects in manganese-exposed bridge construction welders. Neurotoxicology. 2011;32:596–605. doi: 10.1016/j.neuro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Harris M, Gocheva V, et al. Anxiety affecting parkinsonian outcome and motor efficiency in adults of an Ohio community with environmental airborne manganese exposure. Int. J. Hyg. Environ. Health. 2012;215:393–405. doi: 10.1016/j.ijheh.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Kornblith ES, Gocheva VV, et al. Environmental exposure to manganese in air: associations with cognitive functions. Neurotoxicology. 2015;49:139–148. doi: 10.1016/j.neuro.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler RM, Mergler D, Sassine MP, et al. Neuropsychiatric effects of manganese on mood. Neurotoxicology. 1999;20:367–378. [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, et al. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Colledge MA, Julian JR, Gocheva VV, et al. Characterization of air manganese exposure estimates for residents in two Ohio towns. J. Air Waste Manage. Assoc. 2015;65:948–957. doi: 10.1080/10962247.2015.1040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Product Development . CATSYS 7.0 User's Manual. Danish Product Development; Snekkersten, Denmark: 1996. [Google Scholar]
- Després C, Lamoureux D, Beuter A. Standardization of a neuromotor test battery: the CATSYS system. Neurotoxicology. 2000;21:724–735. [PubMed] [Google Scholar]
- Edwards R, Beuter A. Indices for identification of abnormal tremor using computer tremor evaluation systems. IEEE Trans. Biomed. Eng. 1999;46:895–898. doi: 10.1109/10.771207. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Unified Parkinson's disease rating scale. In: Fahn S, Cd M, Goldstein M, et al., editors. Recent Developments in Parkinson's Disease. MacMillan Healthcare Information; Florham Park, NJ.: 1987. [Google Scholar]
- Fan AM, Alexeeff G, Harris SB. Cumulative risks and cumulative impacts of environmental chemical exposures. Int. J. Toxicol. 2010;29:57. doi: 10.1177/1091581809344224. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carlin JB, Stern HD, et al. Bayesian Data Analysis. Chapman & Hall; Boca Raton: 2004. [Google Scholar]
- Guilarte TR, Gonzales KK. Manganese-induced parkinsonism is not idiopathic Parkinson's disease: environmental and genetic evidence. Toxicol. Sci. 2015;146:204–212. doi: 10.1093/toxsci/kfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes E, Heckel P, Ryan P, et al. Environmental manganese exposure in residents living near a ferromanganese refinery in southeast Ohio: a pilot study. Neurotoxicology. 2010;31:468–474. doi: 10.1016/j.neuro.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, et al. Psychological Assessment Resources. Lutz, Fla, USA: 2004. Revised Comprehensive Norms for an Expanded Halstead–Reitan Battery: Demographically Adjusted Norms for African American and Caucasian Adults. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM Statistics for Windows, Version 21.0. Version 9.1 ed. IBM Corp.; Armonk, NY.: 2012. [Google Scholar]
- Johnson BL, Coulberson SL. Environmental epidemiologic issues and minority health. Ann. Epidemiol. 1993;3:175–180. doi: 10.1016/1047-2797(93)90133-o. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Prigatano GP. Functional MR imaging during finger tapping. BNI Quaterly. 2000;16:37–41. [Google Scholar]
- Kim Y, Bowler R, Abdelouahab N, et al. Motor function in adults of an Ohio community with environmental manganese exposure. Neurotoxicology. 2011;32:606–614. doi: 10.1016/j.neuro.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Kløve H. Clinical neuropsychology. Med. Clin. N. Am. 1963;47:1647–1658. [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, et al. Neuropsychological Assessment. NY, Oxford University Press; New York: 2012. [Google Scholar]
- MacLehose RF, Dunson DB, Herring AH, et al. Bayesian methods for highly correlated exposure data. Epidemiology. 2007;18:199–207. doi: 10.1097/01.ede.0000256320.30737.c0. [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, Novaes CO, Moreira JC, et al. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ. Res. 2011;111:156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Bélanger S, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–342. [PubMed] [Google Scholar]
- Meyer-Baron M, Schaper M, Knapp G, et al. The neurobehavioral impact of manganese: results and challenges obtained by a meta-analysis of individual participant data. Neurotoxicology. 2013;36:1–9. doi: 10.1016/j.neuro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrushina MN, Boone KB, Razani J, et al. Handbook of Normative Data for Neuropsychological Assessment. Oxford University Press; New York: 2005. [Google Scholar]
- Mohai P, Lantz PM, Morenoff J, et al. Racial and socioeconomic disparities in residential proximity to polluting industrial facilities: evidence from the Americans' Changing Lives Study. Am. J. Public Health. 2009;99:S646–S656. doi: 10.2105/AJPH.2007.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO, Asparouhov T. Bayesian structural equation modeling: a more flexible representation of substantive theory. Psychol. Methods. 2012;17:313–335. doi: 10.1037/a0026802. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Sixth Edition. Muthén & Muthén; Los Angeles, CA.: 1998-2010. [Google Scholar]
- Myers J, Thompson ML, Ramushu S, et al. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003;24:885–894. doi: 10.1016/S0161-813X(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Norton JM, Wing S, Lipscomb HJ, et al. Race, wealth, and solid waste facilities in North Carolina. Environ. Health Perspect. 2007;115:1344–1350. doi: 10.1289/ehp.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne-Sturges D, Gee GC. National environmental health measures for minority and low-income populations: tracking social disparities in environmental health. Environ. Res. 2006;102:154–171. doi: 10.1016/j.envres.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Rodriquez-Agudelo Y, Riojas-Rodriguez H, Rios C, et al. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci. Total Environ. 2006;368:542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Schroeter JD, Nong A, Yoon M, et al. Analysis of manganese tracer kinetics and target tissue dosimetry in monkeys and humans with multi-route physiologically-based pharmacokinetic models. Toxicol. Sci. 2011;120:481–498. doi: 10.1093/toxsci/kfq389. [DOI] [PubMed] [Google Scholar]
- Solís-Vivanco R, Rodríguez-Agudelo Y, Riojas-Rodríguez H, et al. Cognitive impairment in an adult Mexican population non-occupationally exposed to manganese. Environ. Toxicol. Pharmacol. 2009;28:172–178. doi: 10.1016/j.etap.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York: 2006. [Google Scholar]
- [July 15, 2015];U.S. Department of Agriculture National Nutrient Database for Standard Reference Release 27 [Online] Available: http://ndb.nal.usda.gov/ndb/search/list.
- EPA US. Compendium of Methods for the Determination of Inorganic Compounds in Ambient Air [Online] Washington, DC: 2009. [October 15 2014]. Available: http://www.epa.gov/ttnamti1/files/ambient/inorganic/iocompen.pdf. [Google Scholar]
- EPA US. [March 16 2011];SAT Initiative: The Ohio Valley Education Center (Marietta, OH), Warren Elementary School (Marietta, OH), and Neale Elementary School (Vienna, WV) [Online] 2010a (Available: http://epa.gov/schoolair/pdfs/MariettaTechReport.pdf)
- U.S. EPA (U.S. Environmental Protection Agency) [November 13 2010];SAT Initiative: East Elementary School (East Liverpool, OH) [Online] 2010b (Available: http://www.epa.gov/schoolair/pdfs/EastTechReport.pdf)
- Yang YK, Chiu NT, Chen CC, et al. Correlation between fine motor activity and striatal dopamine D2 receptor density in patients with schizophrenia and healthy controls. Psychiatry Res. 2003;123:191–197. doi: 10.1016/s0925-4927(03)00066-0. [DOI] [PubMed] [Google Scholar]
- Yoon M, Renganathan S, Kim K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Am. Chem. Soc. 2011;112:1196–1231. doi: 10.1021/cr2003147. [DOI] [PubMed] [Google Scholar]