Abstract
As an initial step toward targeting cartilage tissue for potential therapeutic applications, we sought cartilage-binding peptides using phage display, a powerful technology for selection of peptides that bind to molecules of interest. A library of phage displaying random 12-amino acid peptides was iteratively incubated with cultured chondrocytes to select phage that bind cartilage. The resulting phage clones demonstrated increased affinity to chondrocytes by ELISA, when compared to a wild-type, insertless phage. Furthermore, the selected phage showed little preferential binding to other cell types, including primary skin fibroblast, myocyte and hepatocyte cultures, suggesting a tissue-specific interaction. Immunohistochemical staining revealed that the selected phage bound chondrocytes themselves and the surrounding extracellular matrix. FITC-tagged peptides were synthesized based on the sequence of cartilage-binding phage clones. These peptides, but not a random peptide, bound cultured chondrocytes, and extracelluar matrix. In conclusion, using phage display, we identified peptide sequences that specifically target chondrocytes. We anticipate that such peptides may be coupled to therapeutic molecules to provide targeted treatment for cartilage disorders.
Keywords: phage display, peptide, cartilage, chondrocytes, targeted therapy
INTRODUCTION
Cartilage is a resilient connective tissue that serves important roles in many structures, including articular surfaces, growth plates, bronchial airways, and external ear. Within cartilage tissue, chondrocytes secrete a specialized extracellular matrix that confers the tensile strength and flexibility characteristic of the tissue.1 Because articular cartilage is avascular and composed of chondrocytes with low proliferative activity, it has limited reparative capacity after traumatic injury and is prone to the degenerative disease, osteoarthritis.
Multiple polypeptide growth factors, including bone morphogenetic proteins, insulin-like growth factors, and fibroblast growth factors2,3 can promote chondrogenesis and thus may have therapeutic potential to treat osteoarthritis and articular injuries. However, these regulatory molecules are produced locally and act locally as paracrine signals, and thus do not lend themselves to systemic therapeutic approaches. If these locally acting growth factors, however, could be linked to cartilage-binding peptides and then administered systemically, the molecules might specifically target cartilage tissue, thereby increasing efficacy and decreasing off-target side effects. A similar approach has been adopted to target bone using a short peptide that binds hydroxyapatite to allow delivery of therapeutic molecules to bone tissue.4,5
As an initial step toward targeting cartilage tissue for potential therapeutic application, we employed phage display to identify peptides with affinity for cartilage. Phage display is an effective means for isolation of specific peptides that have affinity for various targets, including protein molecules,6,7 isolated cells,8–12 and tissue-specific vascular beds,13–16 in vitro and in vivo. The technique uses a phage display library, in which peptide variants encoded by random DNA sequences are expressed and displayed on the surface of bacteriophage. The phage library is allowed to incubate with the target of interest, and the bound phage are then eluted and amplified. This process is reiterated to enrich the phage pool in favor of target binding sequences. In order to select phage that bind cartilage tissue, we chose not to use a single, purified cartilage protein as target, but rather use primary cultured chondrocytes, which secrete a large number of matrix proteins in native conformation to provide a rich array of possible targets for peptide binding.
MATERIALS AND METHODS
Animal Care
All animal procedures were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee.
Cell Culture
Epiphyseal cartilage was dissected from the distal femurs and proximal tibias of 4-day-old C57BL/6 male mice. Primary mouse chondrocytes were isolated by 0.125% trypsin (Gibco, Carlsbad, CA) digestion for 15 min, followed by 0.3% collagenase (Sigma- Aldrich, St. Louis, MO) digestion for 60 min at 37°C, and subsequently resuspended in DMEM/F12 medium (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (FCS) (Gibco), 1% penicillin–streptomycin (PS) (Gibco), 0.1% fungizone (Gibco), and 0.1% ascorbic acid. 2.5 × 105 cells per well were seeded in 6-well plates and cultured at 37°C in 5% CO2 for 4 days to reach 80–90% confluence for panning with phage display library. 1 × 104 cells per well were seeded in 96-well plates and cultured for 48 h to reach 80–90% confluence for ELISA.
To assess phage binding specificity, skin fibroblasts and myocytes were also isolated from 4-day-old C57BL/6 male mice. Skin and muscles were digested in 0.25% trypsin for 30 min at 37°C and resuspended in DMEM medium (Invitrogen) containing 10% FCS, 1% PS, 0.1% fungizone. 1 × 104 cells per well were seeded in 96-well plates and cultured for 48 h to reach 80–90% confluence for ELISA.
For isolation of osteoblasts, calvaria were excised from 6- week-old C57BL/6 male mice and washed three times with PBS. The calvaria were then cut into small pieces (approximately 1 mm2) and incubated in Liberase digestion solution (Roche, Branchburg, NJ) for 120 min at 37°C. After that, bone chips were cultured in DMEM medium containing 10% FCS, 1% PS, 0.1% fungizone. Cells gradually migrated out from the bone chips and grew until they reached 80–90% confluence. 1 × 104 cells per well were then seeded in 96- well plates and cultured overnight for ELISA.
For isolation of hepatocytes, liver was excised from 4-week-old C57BL/6 male mice and perfused with Liver Perfusion Medium (Invitrogen) until the effluent was no longer blood tinged, followed by Liver Digest Medium (Invitrogen) for 5 min. The liver capsule was then disrupted to release the hepatocytes into the liver perfusion medium. Isolated cells were resuspended in supplemented William’s medium (Invitrogen) as described elsewhere.17 5 × 104 cells per well were seeded in 6-well collagen-coated plate (BD Biosciences, Franklin Lakes, NJ) and cultured for 48 h to reach 80–90% confluence for ELISA.
Phage Display Panning
Ph.D.-12 phage display peptide library was purchased from New England BioLabs (Ipswich, MA), and the panning procedures were performed as described in the instruction manual with the following modifications. Prior to panning, culture medium was removed and cells were rinsed twice with PBS. Thereafter, 5% BSA (Sigma-Aldrich) in PBS (Gibco) was added to the culture for non-specific blocking at 37°C for 2 h. The first round of panning was carried out by incubating 1 × 1011 plaque-froming units (pfu) of phage library with cultured chondrocytes at room temperature for 2 h with gentle shaking. The cells were washed five times with PBS. Bound phage was eluted using elution buffer (0.2 M glycine–HCl, pH 2.2, 1 mg/ml BSA) (Sigma-Aldrich) at room temperature for 10 min and then neutralized with 1 M NaCl (pH 9.1) (Sigma-Aldrich). Eluted phage were titered by adding 10 ml of eluate to 200 ml of E. coli ER2738 and placed in melted LB top agar (7 g agarose/100 ml LB agar medium) on IPTG/X-Gal (Fermentas, Glen Burnie, MD) LB agar plates. The residual eluate was then amplified and taken through two additional binding/amplification cycles, at a fixed phage input of 1 × 1011 pfu.
DNA Sequencing and Sequence Analysis
After three rounds of panning, E. coli ER2738 was infected with the recovered phage and plated onto LB agar plates. Twenty-three single phage plaques were randomly picked and amplified. DNA was then purified for DNA sequencing, using the ---------------96 gIII sequencing primer provided: 50-CCC TCA TAG TTA GCG TAA CG-30 (New England BioLabs).
Enzyme-Linked Immunosorbent Assay (ELISA)
To assess binding to cells, chondrocytes, skin fibroblasts, myocytes, osteoblasts, and hepatocytes were cultured as described above. To investigate binding to abundant cartilage matrix molecules, 100 ml of aggrecan (1 mg/ml, Sigma- Aldrich) or 100 ml of collagen II (1 mg/ml, Sigma-Aldrich) were used to coat 96-well plates at 4°C overnight. Each phage clone was tested in triplicate wells. After blocking with 5% BSA, 1 × 1010 pfu of each selected phage, resuspended in 5% BSA, was introduced to each well and incubated at 37°C for 2 h. Wild-type M13 phage (New England BioLabs), which did not bear any peptide insert, was used as a negative control. The wells were washed three times with PBS, then horseradish peroxidase (HRP)-conjugated anti-M13 antibody (GE Healthcare, Piscataway, NJ) (diluted 1:5,000 in 5% BSA) was added and incubated at room temperature for 1 h. Tetramethybezidine (TMB) substrate reagent (eBioscience, San Diego, CA) was added for color development and absorbance was read at 450 nm.
Immunohistochemical Staining of Phage Bound to Cultured Chondrocytes
3 × 104 cells per chamber were seeded on an 8-chamber tissue culture chamber slide (Lab-Tek, Scotts Valley, CA). Subsequent to blocking with 5% BSA for 1 h at room temperature, educated phage clones, insertless M13KE phage, and naive phage library were added to chondrocytes and incubated at 37°C for 1 h. Unbound phage was removed by three washes of PBS, and then HRP-conjugated anti- M13 antibody was added and incubated at 37°C for another hour. Diaminobenzidine (DAB) staining solution (Vector, Burlingame, CA) was introduced for visualization.
Fluorescence Microscopy
The FITC-labeled C1 (RLDPTSYLRTFWGGGK-FITC), C19 (HDSQLEALIKFMGGGK-FITC) and random (NDELQCH CTHSRGGGK-FITC) peptides were synthesized and purified by Peptide 2.0, Inc. (Chantilly, VA). The FITC moiety was attached to the peptide at the C-terminus via a lysine residue after the triple glycine (GGG) linker. The sequence of the random peptide was generated using a random number generator program.
Chondrocytes were cultured as for immunohistochemistry. Culture medium was removed and cells were gently rinsed with PBS twice and then blocked with 5% BSA for 1 h at 37°C. FITC-labeled C1, C19 and random peptides in 5% BSA were added to the cells and allowed to stand at room temperature for 1 h. Unbound peptide was washed away with PBS three times. Subsequently, cells were mounted with cover-slips using DAPI mounting medium (Vector). Fluorescent images were visualized using a Leica microscope DMI 3000.
RESULTS
Selection and Identification of Chondrocyte-Binding Phage Clones
A library of phage displaying random 12-amino acid peptides was iteratively selected for binding to cultured primary chondrocytes during three rounds of panning. The ratio of output to input phage only showed minimal increase over three rounds of selection (first round: 8.05 × 10−5; second round: 1.17 × 10−4; third round: 1.20 × 10−4).
After the third round of panning, 23 single phage plaques were randomly picked and sequenced. Alignment analysis revealed that the sequences of phage clones C1 and C9 exhibited some amino acid sequence similarity, while the remaining sequences showed no clear similarity to each other (Supplementary Table 1). Analysis using BLAST did not identify any convincing sequence similarity between the peptides and known murine protein sequences (data not shown).
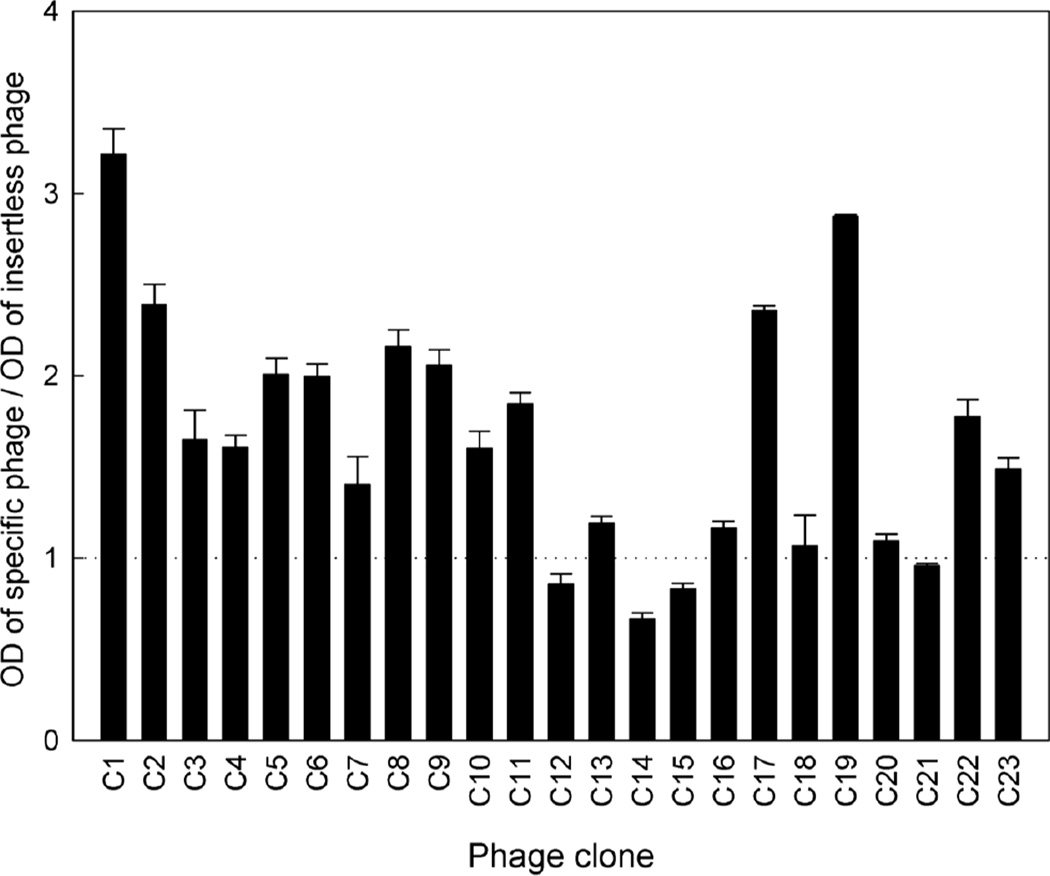
ELISA was performed to assess the cartilage-binding capability of the identified phage. Among the 23 phage clones obtained, 8 demonstrated at least a twofold increase in binding to cultured chondrocytes, compared to insertless phage (Fig. 1). We focused on phage C1 and C19, which display peptide sequences RLDPTSYLRTFW and HDSQLEALIKFM, respectively, for further characterization because these two clones exhibited the greatest binding to chondrocytes.
Figure 1.
Binding ability of the 23 selected phage clones to cultured chondrocytes by ELISA. Individual phage clones were incubated with mouse primary epiphyseal chondrocyte culture, washed and then detected using anti-M13 antibody. Optical density of each clone was normalized to that of the insertless phage. Data represent mean ± SEM from three independent experiments.
Tissue Specificity of Phage Clone Binding
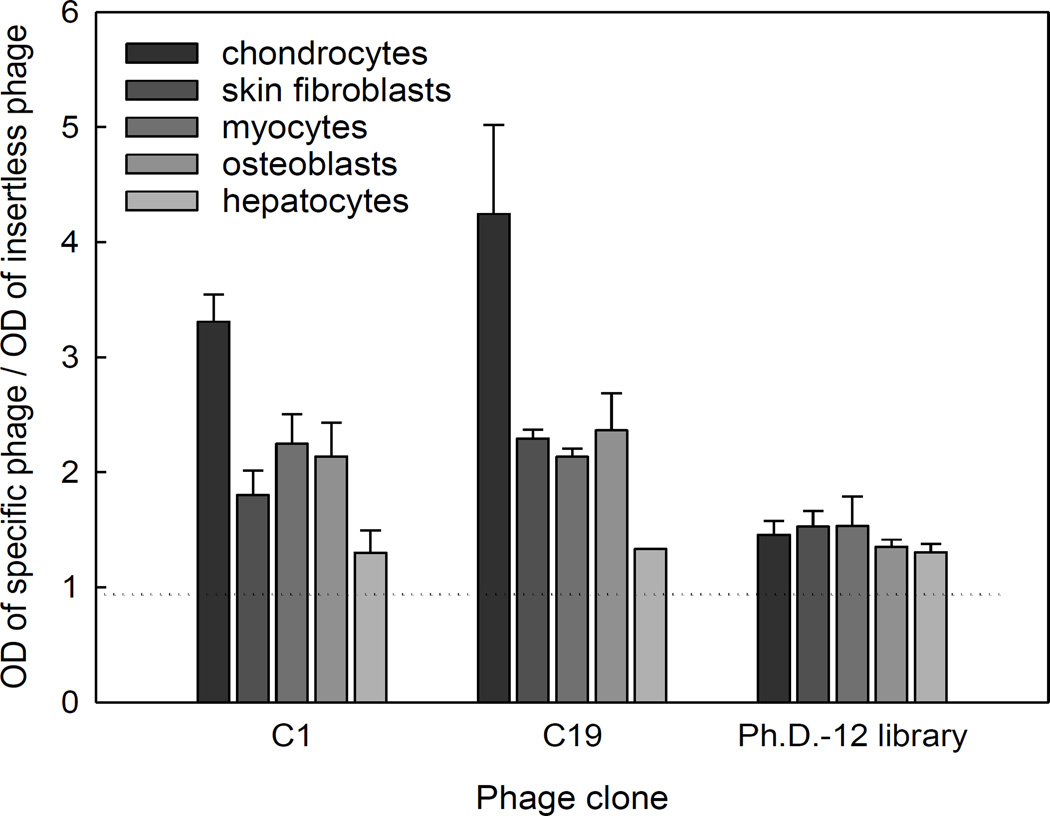
In order to investigate tissue specificity, murine chondrocytes, skin fibroblasts, myocytes, osteoblasts, and hepatocytes were cultured and incubated with phage clones C1 and C19. Binding of specific phage to the target was expressed as a ratio relative to binding of insertless phage. C1 and C19 phage exhibited preferential binding to chondrocytes compared to other cell types, suggesting a tissue-specific interaction (Fig. 2). In contrast, naive phage library did not demonstrate increased binding to any cell type tested.
Figure 2.
Assessment of the tissue binding specificity of two phage clones, C1 and C19, by ELISA. Individual phage clones were incubated with primary cultures of mouse epiphyseal chondrocytes, skin fibroblasts, myocytes, osteoblasts, and hepatocytes, then washed and detected using anti-M13 antibody. Optical density of each clone was normalized to that of the insertless phage. Data represent mean ± SEM from three independent experiments.
Immunohistochemical Localization of Phage Binding to Chondrocytes
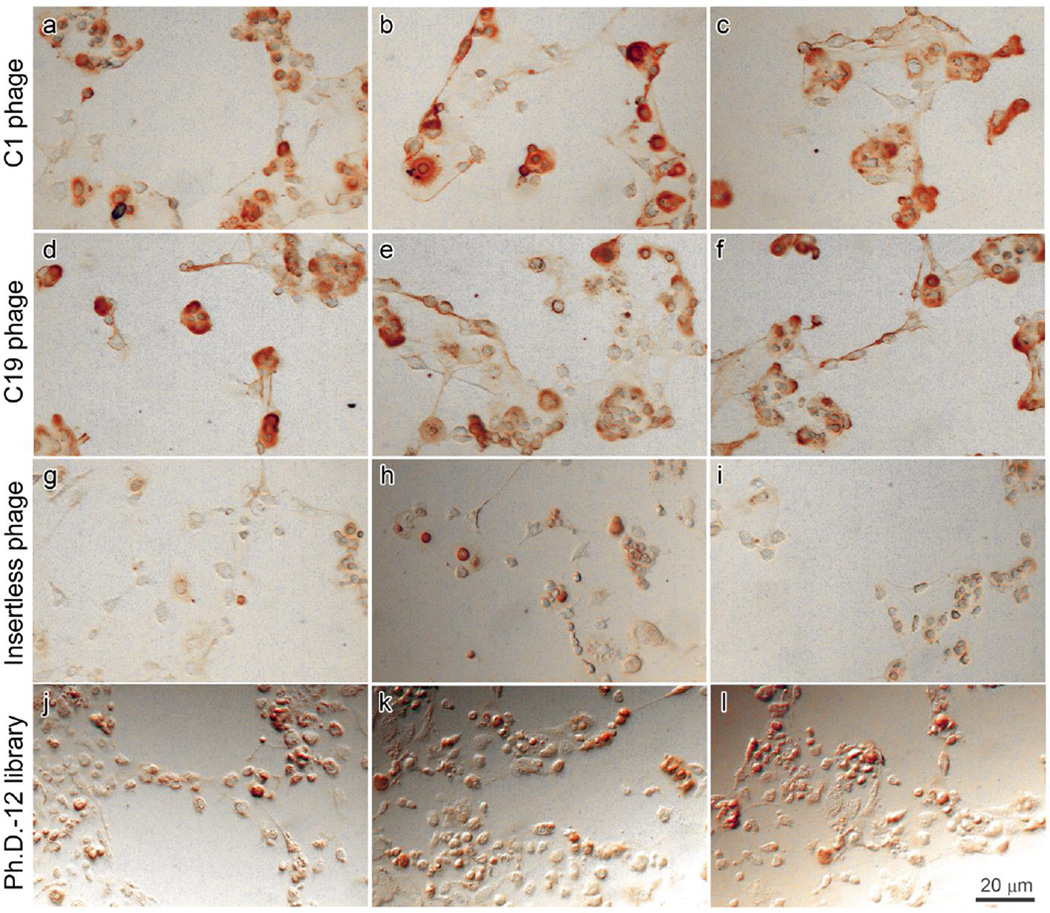
Phage clones C1 and C19 were incubated with cultured chondrocytes and washed, after which bound phage was detected by HRP-conjugated anti-M13 antibody. The pattern of staining suggested that both phage C1 and C19 bound at the cell surface and extracellular matrix of cultured chondrocytes (Fig. 3a– f). In contrast, insertless phage and naive phage library bound poorly to chondrocytes (Fig. 3g–l).
Figure 3.
Localization of phage binding to cultured chondrocytes by immunohistochemistry. C1 phage (a–c), C19 phage (d–f), insertless phage (g–i), and naive phage library (j–l) were incubated with primary culture of mouse epiphyseal chondrocytes, detected by HRP-conjugated anti-M13 antibody and stained using DAB substrate to produce a brown color. Images were taken from three representative fields.
Binding of Synthetic Peptides to Chondrocytes
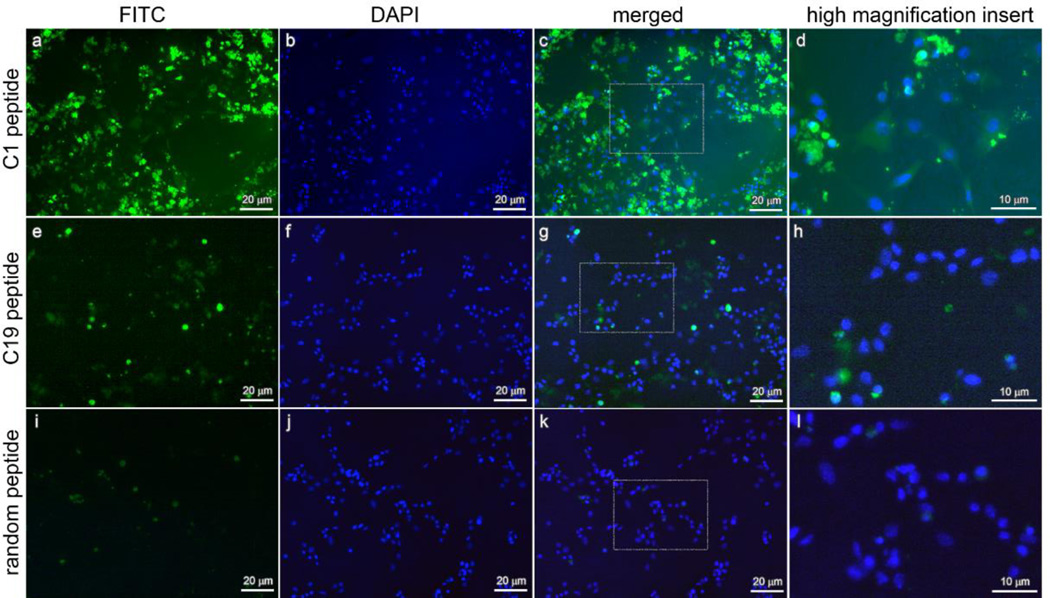
To confirm that the binding of phage to chondrocytes was due to the displayed peptides, synthetic C1, C19, and random peptides, conjugated with FITC, were incubated with cultured chondrocytes. Using fluorescence microscopy, we found that both C1 and C19 peptides bound chondrocytes, whereas the random peptide showed little binding (Fig. 4a,e,i). Greater fluorescence was seen for peptide C1 than peptide C19. Concurrent staining of nuclei with DAPI (Fig. 4b, f,j) revealed that the peptides labeled the same sites (chondrocyte cell surface and extracellular matrix) (Fig. 4c,d,g,h) as they did when they were linked to the phage (Fig. 3).
Figure 4.
Localization of peptide binding to cultured chondrocytes by immunofluorescence. FITC-labeled C1 peptide (a–d), C19 peptide (e–h), and random peptide (i–l) were incubated with primary cultures of mouse epiphyseal chondrocytes and detected by fluorescence microscopy. Green channel (a,e,i) shows signal from bound FITC peptides, and blue channel shows staining of nuclei in the same field with DAPI (b,f,j). Merged images show both channels superimposed (c,g,k). Higher magnification inserts show the binding of C1 peptide (d) and C19 peptide (h), but not the random peptide (l), to extracellular matrix.
Binding of Phage Clones to Cartilage Matrix Molecules
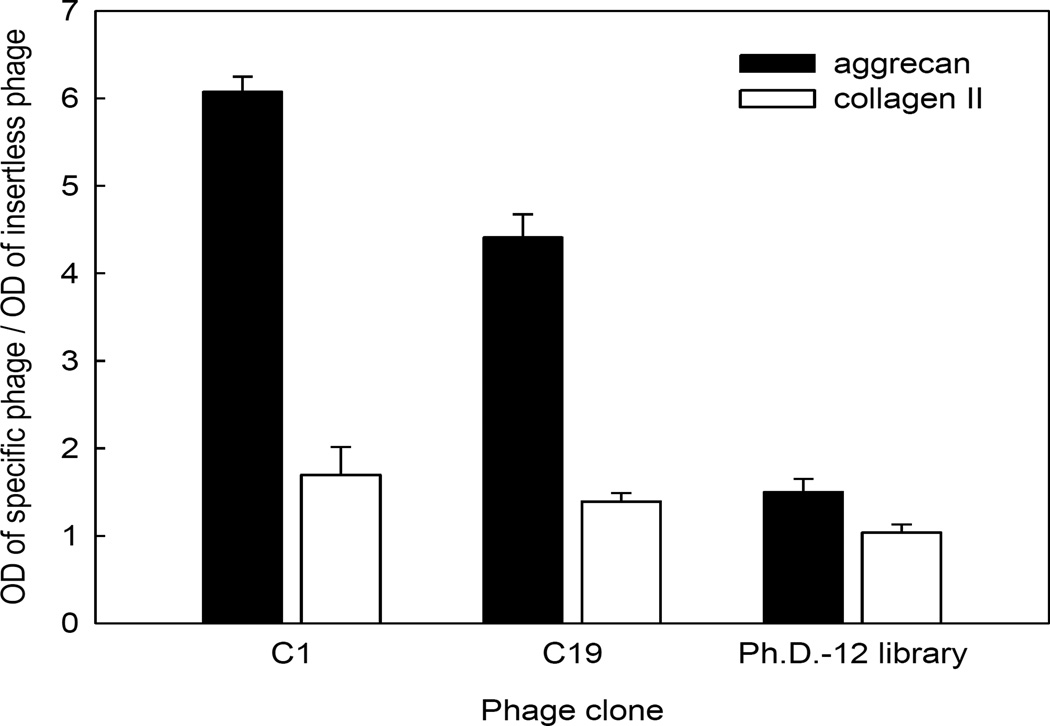
We next sought to determine whether C1 and C19 phage bound to specific molecules that are abundant components of cartilage extracellular matrix. ELISA was used to measure binding of specific phage clones to plastic wells coated with aggrecan and collagen II purified from bovine articular cartilage. C1 and C19 phage showed increased binding to aggrecan by an average of 6- and 4.5-fold, respectively, compared to insertless phage (Fig. 5). In contrast, binding of C1, C19, and insertless phage to collagen II were largely similar (Fig. 5). These findings suggest that the binding C1 and C19 phage to cultured chondrocytes is due in part to affinity of the peptide for aggrecan.
Figure 5.
Binding ability of two phage clones C1 and C19 towards aggrecan and collagen II proteins by ELISA. The selected phage clones, naive library and insertless phage were incubated with aggrecan and collagen II proteins and then detected with anti-M13 antibody. Optical density of each clone was normalized to that of the insertless phage. Data represent mean ± SEM from three independent experiments.
DISCUSSION
The long-term goal of this study is to target therapeutic molecules to cartilage tissue. As a first step toward this goal, we sought to develop short peptides that specifically bind cartilage. We employed a phage display library, in which linear peptides comprising 12 random amino acids were inserted into coat proteins of bacteriophage, and iteratively selected for binding to cultured chondrocytes. With this approach, we obtained phage presenting a diverse panel of peptides, as reflected by the low level of sequence similarity among individual clones. This sequence diversity may be explained by the wide variety of molecular targets available in the primary chondrocyte culture. Many of these selected phage clones demonstrated an increased ability to bind cultured chondrocytes compared to insertless phage by ELISA. This increased binding to chondrocytes by educated phage clones compared to insertless phage likely reflects the affinity of the inserted peptide, because, in principle, the educated phage differs from the insertless phage only by the presence of the insert. However, the denominator of this ratio depends on the affinity of the entire insertless phage, which contains many copies of different coat proteins. Therefore, this ratio does not quantitatively equal the fold of increase in the specific peptide insert compared to random peptide, which could be much greater in magnitude. Indeed, twoto four-fold increases in phage binding over insertless phage are often reported in similar applications of phage display.18,19 Among the 23 clones identified, phage C1 and C19 exhibited the greatest ability to bind chondrocytes. In addition to binding affinity, specificity for chondrocytes over other cell types is also an important characteristic for a peptide designed to specifically target therapeutic molecules to cartilage tissue in vivo. Here, both of the two selected phage were found to show substantially less ability to bind fibroblasts, myocytes, osteoblasts, and hepatocytes.
Immunohistochemical staining suggested that phage C1 and C19 bound both extracellular matrix surrounding the cultured chondrocytes and the chondrocytes themselves, although the latter appearance might result from binding of phage to matrix immediately surrounding the cells. A similar pattern was obtained when fluorescently labeled free peptides (peptides displayed on C1 and C19) were used to stain chondrocytes, confirming that the ability of the phage to bind chondrocytes was due to the affinity of the inserted peptide itself and not due to a conformational change in the remainder of the phage coat protein.
For the phage selection, we utilized cultured chondrocytes rather than a specific cartilage protein immobilized on a solid phase for two reasons. First, cultured chondrocytes present a diverse array of possible targets, including proteins and glycosylated proteins, potentially increasing the likelihood of finding a peptide with high affinity. Second, a purified protein immobilized on a solid phase may show a non-physiological confirmation and thus peptides that bind to the purified protein may not bind well to the native protein in its complex physiological environment. Therefore, we employed a cellular system to better mimic the complex micro-environment of chondrocytes and extracellular matrix, in an attempt to identify peptides that would have specific targeting ability for effective treatment of cartilage disorders.
The feasibility of using cartilage-targeting peptides therapeutically is supported by analogous studies targeting bone. Yokogawa et al.4 conjugated estradiol to a short peptide of six aspartate residues. When administered systemically, the peptide selectively delivered estradiol to bone, where it was retained for an extended period of time. Like free estradiol, this targeted preparation increased bone mineral density, but, unlike free estradiol, it did not significantly affect uterine or liver weight, suggesting effective therapeutic targeting. Similarly, a ten-aspartate peptide has been used to target the enzyme alkaline phosphatase to bone, thereby treating hypophosphatasia in mice.20 This approach was then successfully applied, in a recent clinical trial, to treat children with severe hypophosphatasia.21
There have been several studies identifying peptides or polypeptides that target articular cartilage. Rothenfluh et al.22 identified a linear six-amino acid peptide that bound the cartilage matrix component collagen IIaI of denuded bovine cartilage grafts. This peptide could target nanoparticles to articular cartilage after intra-articular injection. Testing for systemic application was not performed. Hughes et al.23 identified a single-chain variable fragment (scFv) that recognized reactive oxygen species-modified type II collagen, which is present in inflamed joints. When administered systemically, this antibody fragment targeted inflamed articular cartilage in mice and, when fused to soluble tumor necrosis factor receptor II, it successfully reduced inflammation in a murine arthritis model. Both of these previous studies report promising results. However, to develop safe and effective targeted therapies for clinical articular disorders using this approach, it will be important to have a wide menu of peptides or polypeptides, which can be conjugated or fused to specific biologically active molecules and then tested in laboratory animals and ultimately in humans. Toward this end, we employed phage display to generate a large number of independent cartilage-binding clones. To minimize side effects on non-cartilaginous tissues, the targeting peptide or polypeptide must show low affinity for tissues other than cartilage. We therefore screened the phage clones that emerged from our selection and found clones that possessed a binding preference for chondrocytes over other cell types tested. Thus, our study provides evidence that phage display or similar techniques can be used to find peptides with affinity and specificity for cartilage matrix. In subsequent studies designed to identify peptides or polypeptides for actual clinical use, it may be useful to perform the selection against human cultured chondrocytes, rather than murine chondrocytes and to screen large numbers of clones for those with the highest affinity and specificity.
In summary, we used phage display to identify peptides that bind cultured chondrocytes and show less binding to other cell types. Using this approach has the potential to provide a large number of possible cartilage-targeting peptides and polypeptides, which can then be screened for tissue specificity and, when conjugated or fused to bioactive substances, tested for therapeutic efficacy and safety.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
REFERENCES
- 1.Umlauf D, Frank S, Pap T, et al. Cartilage biology, pathology, and repair. Cell Mol Life Sci. 2010;67:4197–4211. doi: 10.1007/s00018-010-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortier LA, Barker JU, Strauss EJ, et al. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Z, Willers C, Xu J, et al. The chondrocyte: biology and clinical application. Tissue Eng. 2006;12:1971–1984. doi: 10.1089/ten.2006.12.1971. [DOI] [PubMed] [Google Scholar]
- 4.Yokogawa K, Miya K, Sekido T, et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–1233. doi: 10.1210/endo.142.3.8024. [DOI] [PubMed] [Google Scholar]
- 5.Kasugai S, Fujisawa R, Waki Y, et al. Selective drug delivery system to bone: small peptide (Asp)6 conjugation. J Bone Miner Res. 2000;15:936–943. doi: 10.1359/jbmr.2000.15.5.936. [DOI] [PubMed] [Google Scholar]
- 6.Park HY, Kim J, Cho JH, et al. Phage display screen for peptides that bind Bcl-2 protein. J Biomol Screen. 2011;16:82–89. doi: 10.1177/1087057110385816. [DOI] [PubMed] [Google Scholar]
- 7.Hong HW, Lee SW, Myung H. Selection of peptides binding to HCV e2 and inhibiting viral infectivity. J Microbiol Biotechnol. 2010;20:1769–1771. [PubMed] [Google Scholar]
- 8.Zhao S, Zhao W, Ma L. Novel peptide ligands that bind specifically to mouse embryonic stem cells. Peptides. 2010;31:2027–2034. doi: 10.1016/j.peptides.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Galili N, Devemy E, Raza A. Isolation of specific and biologically active peptides that bind cells from patients with acute myeloid leukemia (AML) J Hematol Oncol. 2008;1:8. doi: 10.1186/1756-8722-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo N, Akita N, Shimizu A, et al. Identification of oligopeptide binding to colon cancer cells separated from patients using laser capture microdissection. J Drug Target. 2008;16:396–404. doi: 10.1080/10611860802088796. [DOI] [PubMed] [Google Scholar]
- 11.Lee L, Buckley C, Blades MC, et al. Identification of synovium-specific homing peptides by in vivo phage display selection. Arthritis Rheum. 2002;46:2109–2120. doi: 10.1002/art.10464. [DOI] [PubMed] [Google Scholar]
- 12.Nicklin SA, White SJ, Watkins SJ, et al. Selective targeting of gene transfer to vascular endothelial cells by use of peptides isolated by phage display. Circulation. 2000;102:231–237. doi: 10.1161/01.cir.102.2.231. [DOI] [PubMed] [Google Scholar]
- 13.Lee NK, Kim M, Choi JH, et al. Identification of a peptide sequence targeting mammary vasculature via RPLP0 during lactation. Peptides. 2010;31:2247–2254. doi: 10.1016/j.peptides.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Cao S, Zhang Y, et al. A novel peptide (GX1) homing to gastric cancer vasculature inhibits angiogenesis and cooperates with TNF alpha in anti-tumor therapy. BMC Cell Biol. 2009;10:63. doi: 10.1186/1471-2121-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arap W, Haedicke W, Bernasconi M, et al. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci USA. 2002;99:1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruoslahti E. Targeting tumor vasculature with homing peptides from phage display. Semin Cancer Biol. 2000;10:435–442. doi: 10.1006/scbi.2000.0334. [DOI] [PubMed] [Google Scholar]
- 17.Lui JC, Forcinito P, Chang M, et al. Coordinated postnatal down-regulation of multiple growth-promoting genes: evidence for a genetic program limiting organ growth. FASEB J. 2010;24:3083–3092. doi: 10.1096/fj.09-152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin B, Tai W, Shukla RS, et al. Identification of a LNCaP-specific binding peptide using phage display. Pharm Res. 2011;28:2422–2434. doi: 10.1007/s11095-011-0469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Xu L, Wang Z. Screening serum biomarkers for early primary hepatocellular carcinoma using a phage display technique. J Clin Lab Anal. 2011;25:402–408. doi: 10.1002/jcla.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millán JL, Narisawa S, Lemire I, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav MC, Lemire I, Leonard P, et al. Dose response of bone-targeted enzyme replacement for murine hypophosphatasia. Bone. 2011;49:250–256. doi: 10.1016/j.bone.2011.03.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothenfluh DA, Bermudez H, O’Neil CP, et al. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 23.Hughes C, Faurholm B, Dell’Accio F, et al. Human single-chain variable fragment that specifically targets arthritic cartilage. Arthritis Rheum. 2010;62:1007–1016. doi: 10.1002/art.27346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.