Abstract
Background
Environmental exposures that occur in utero and during early life may contribute to the development of childhood asthma through alteration of the human microbiome. The objectives of this study were to estimate the cumulative effect and relative importance of environmental exposures on the risk of childhood asthma.
Methods
We conducted a population-based birth cohort study of mother-child dyads who were born between 1995 and 2003 and were continuously enrolled in the PRIMA (Prevention of RSV: Impact on Morbidity and Asthma) cohort. The individual and cumulative impact of maternal urinary tract infections (UTI) during pregnancy, maternal colonization with group B streptococcus (GBS), mode of delivery, infant antibiotic use, and older siblings at home, on the risk of childhood asthma were estimated using logistic regression. Dose-response effect on childhood asthma risk was assessed for continuous risk factors: number of maternal UTIs during pregnancy, courses of infant antibiotics, and number of older siblings at home. We further assessed and compared the relative importance of these exposures on the asthma risk. In a subgroup of children for whom maternal antibiotic use during pregnancy information was available, the effect of maternal antibiotic use on the risk of childhood asthma was estimated.
Results
Among 136,098 singleton birth infants, 13.29% developed asthma. In both univariate and adjusted analyses, maternal UTI during pregnancy (odds ratio [OR] 1.2, 95% confidence interval [CI] 1.18, 1.25; adjusted OR [AOR] 1.04, 95%CI 1.02, 1.07 for every additional UTI) and infant antibiotic use (OR 1.21, 95%CI 1.20, 1.22; AOR 1.16, 95%CI 1.15, 1.17 for every additional course) were associated with an increased risk of childhood asthma, while having older siblings at home (OR 0.92, 95%CI 0.91, 0.93; AOR 0.85, 95%CI 0.84, 0.87 for each additional sibling) was associated with a decreased risk of childhood asthma, in a dose-dependent manner. Compared with vaginal delivery, C-section delivery increased odds of childhood asthma by 34% (OR 1.34, 95%CI 1.29, 1.39) in the univariate analysis and 11% after adjusting for other environmental exposures and covariates (AOR 1.11, 95%CI 1.06, 1.15). Maternal GBS was associated with a significant increased risk of childhood asthma in the univariate analysis (OR 1.27, 95%CI 1.19, 1.35), but not in the adjusted analysis (AOR 1.03, 95%CI 0.96, 1.10). In the subgroup analysis of children whose maternal antibiotic use information was available, maternal antibiotic use was associated with an increased risk of childhood asthma in a similar dose-dependent manner in the univariate and adjusted analyses (OR 1.13, 95%CI 1.12, 1.15; AOR 1.06, 95%CI 1.05, 1.08 for every additional course). Compared with infants with the lowest number of exposures (no UTI during pregnancy, vaginal delivery, at least five older siblings at home, no antibiotics during infancy), infants with the highest number of exposures (at least three UTIs during pregnancy, C-section delivery, no older siblings, eight or more courses of antibiotics during infancy) had a 7.77 fold increased odds of developing asthma (AOR: 7.77, 95%CI: 6.25, 9.65). Lastly, infant antibiotic use had the greatest impact on asthma risk compared with maternal UTI during pregnancy, mode of delivery and having older siblings at home.
Conclusion
Early-life exposures, maternal UTI during pregnancy (maternal antibiotic use), mode of delivery, infant antibiotic use, and having older siblings at home, are associated with an increased risk of childhood asthma in a cumulative manner, and for those continuous variables, a dose-dependent relationship. Compared with in utero exposures, exposures occurring during infancy have a greater impact on the risk of developing childhood asthma.
Introduction
Asthma is the most common serious chronic disease of childhood [1]. The worldwide prevalence of asthma has increased during the past 30 years [2]. Increasing evidence suggests that the human microbiome plays an important role in the development of asthma [3].
The human microbiome is the collective genomes and gene products of microbes living in and on the human body [4,5]. It plays an important role in the development of protective immune responses [6]. The alteration of the microbiome, particularly during the prenatal, perinatal, and infant period, may lead to the development of asthma and atopic diseases [7–10]. Numerous studies have shown that exposures which modify the early-life microbiome (such as prenatal and postnatal antibiotic use [11–15], mode of delivery [16–22], early diet [23–34], and number of older siblings [35,36]) are associated with an increased risk of childhood asthma. However, the relative, dose-dependent and cumulative impact of these early-life exposures on the development of asthma is not known. Neither do we know which early-life exposures have the most influence on asthma inception, nor whether prenatal and postnatal exposures contribute equally. The objective of this study was to determine the relative impact of and cumulative effect of in utero, perinatal, and postnatal exposures that could be measured during pregnancy and infancy on the risk of developing early childhood asthma: maternal antibiotic use/urinary tract infection (UTI), mode of delivery, infant antibiotic use, and having older siblings. We also assessed the dose-dependent relationship of maternal antibiotic use/UTI, infant antibiotic use, and number of older siblings on the risk of developing early childhood asthma.
Materials and Methods
Study population
The study population is part of an existing large population-based birth cohort study, PRIMA (Prevention of RSV: Impact on Morbidity and Asthma) [37]. We included singleton birth mother-child dyads born between 1995–2003, birth weight of 500–6999 grams, gestational age between 24 0/7 and 45 5/7 weeks, and with continuous enrollment and birth in one of six Kaiser Permanente Northern California (KPNC) hospitals or enrollment and birth in the Tennessee Medicaid program (TennCare). We required continuous enrollment for both mothers and children. For mothers, continuous enrollment was defined as 60 or fewer days of disenrollment in either program during the period from 180 days before last menstrual period to date of delivery. For children, we defined continuous enrollment as 90 or fewer days of disenrollment in either program during the period from birth to 365 days of age and age 4.5 to 6 years. KPNC and TennCare provide health insurance for approximately 30% of all the deliveries and births each year among the insured population of Northern California and 50% in Tennessee, respectively. The rationale and detailed methods for the PRIMA cohort have been reported previously [37]. The study protocol was approved by the KPNC Institutional Board for the Protection of Human Subjects, the State of California Committee for the Protection of Human Subjects, and the Vanderbilt University Institutional Review Board.
Event ascertainment
Events or exposures during pregnancy, at delivery, and during infancy, included number of maternal urinary tract infections (UTI) during pregnancy, maternal colonization with group B streptococcus (GBS), mode of delivery, antibiotic use during infancy, and number of older siblings at home. Maternal UTI during pregnancy and maternal GBS captured using ICD-9-CM codes served as our indicator of maternal infection and antibiotic use during pregnancy. Mode of delivery (vaginal, assisted, and C-section delivery) and number of older siblings were determined from the linked birth certificates. Infant antibiotic use during the first 12 months of life was captured from medical claims prescription fill data of TennCare or electronic medical record data of KPNC. Lastly, we also directly captured maternal antibiotic use during pregnancy in subjects enrolled in TennCare.
Childhood asthma ascertainment
Childhood asthma was ascertained between age 4.5 to 6 years using healthcare encounters and pharmacy claims data. Children with an ICD-9-CM diagnosis of 493 in any diagnostic field for inpatient, other hospital care, emergency department visit, and/or outpatient physician visit claims (two outpatient visits separated by at least 30 days were required) were considered to have asthma. In addition, children with two prescriptions for any short-acting beta-agonist, two prescriptions for montelukast in a 365 day period prior to Food and Drug Administration approval of montelukast for allergic rhinitis, or a single prescription of any other asthma-specific medication were considered as having asthma. Our algorithm for asthma ascertainment has been previously validated and is similar to that used by other US Medicaid systems [38,39]. We identified asthma at age 4.5 years to 6 years to exclude the transient wheezing phenotype. Furthermore, we allowed 18 months ascertainment period to capture mild asthmatics [38].
Covariates
Maternal smoking during pregnancy, maternal asthma status, maternal age at delivery, maternal education level, gestational age at delivery, infant’s birth hospitalization length of stay, birth weight, infant’s race, gender, having chronic lung disease, having congenital heart disease, type of most severe bronchiolitis healthcare encounters experienced during infancy (hospitalization, emergence department visit, outpatient visit, and no visit), and birth year were determined from medical records or linked birth certificates. We determined maternal asthma status using a similar algorithm defined for childhood asthma (see above). All covariates, including study site (KPNC or TennCare) were chosen a priori based on clinical relevance.
Statistical analysis
We expressed descriptive statistics as frequencies and proportions for categorical variables, and as means and standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables, depending on the distribution of the variables.
To assess the relationship of early life exposures with the risk of childhood asthma, we performed univariate and multivariable logistic regression models. The main exposures of interest included pregnancy exposure (number of maternal UTI during pregnancy: 0 1, 2, and 3+), delivery exposures (GBS: yes/no; and mode of delivery: vaginal, assisted, and C-section delivery), infant exposures (antibiotic use: 0, 1, 2, 3, 4, 5, 6, 7, and 8+; and number of older siblings: 0, 1, 2, 3, 4, and 5+). Covariates listed above were adjusted in the multivariable regression models. Maternal UTI during pregnancy, infant antibiotic use, and number of older siblings were treated as categorical variables to estimate the specific effect at each dose level, as well as continuous variables to estimate the dose response effect. Relative contribution and the importance of each exposure on the asthma risk were assessed by the proportion of asthma outcome variability explained by each individual exposure on the Chi-square scale [40].
In children whose mothers were continuously enrolled in TennCare where specific maternal antibiotic prescription dispensing information was available, we directly estimated the effect of maternal antibiotic use during pregnancy on the risk of childhood asthma.
As prematurity and low birth weight are often associated with use of systemic antibiotics in practice. We conducted a subset analysis of term (≥37 weeks), non-low birth weight (≥2500 grams), and healthy (no chronic lung disease and no congenital heart disease) infants to evaluate the association between exposures of interest and childhood asthma.
Lastly, instead of using maternal UTI during pregnancy as markers of maternal infection and antibiotic use, we captured any type of infection and conducted a sensitivity analysis to determine if we could distinguish the effect of maternal infection during pregnancy versus maternal antibiotics on the risk of childhood asthma. Similar analyses using infant infection instead of infant antibiotic use were conducted. All above analyses were repeated separately for each study site.
All analyses were performed using R-software version 3.1.2 (www.r-project.org) [41] package rms [40] and data management using SAS version 9.4 (SAS Institutes; Cary, NC). We used a two-sided 5% significance level for all statistical analyses.
Results
There were 136,098 singleton birth mother-child dyads (33,142 from KPNC and 102,956 from TennCare) in this study. There were 18,081 (13.29%) infants (3,075 [9.28%] from KPNC and 15,006 [14.58%] from TennCare) who developed asthma at age 6 years. The average mothers’ age at delivery was 25 (SD: 6.34) years. The average gestational age was 39 (SD: 2.37) weeks and the average birth weight was 3,221 (SD: 603) grams. Compared with children who enrolled in KPNC, children enrolled in TennCare were more likely to be born prematurely, be low birth weight, have at least one infant bronchiolitis hospitalization, have a younger mother, have a mother who smoked during pregnancy, and have a less educated mother (p<0.001 for all comparisons) (Table 1).
Table 1. Maternal and infant characteristics by type of healthcare coverage among children enrolled in PRIMA cohort, 1995–2009 (n = 136,098).
| Characteristics | KPNC 33,142 (24.35%) | TennCare 102,956 (75.65%) |
|---|---|---|
| Maternal age at delivery (years) (n = 135,938), median (IQR^)* | 31.00 (27.00, 35.00) | 22.00 (19.00, 26.00) |
| Maternal smoking during pregnancy (n = 135,866), n (%)* | 1,324 (3.99) | 29,124 (28.29) |
| Maternal education (years) (n = 134,973), n (%)* | ||
| <12 | 2,610 (7.88) | 48,229 (46.84) |
| 12 | 7,663 (23.12) | 44,269 (43.00) |
| >12 | 21,972 (66.30) | 10,230 (9.94) |
| Maternal asthma, n (%) | 1,518 (4.58) | 4,628 (4.50) |
| Gestational age (weeks), median (IQR)* | 39.00 (38.00, 40.00) | 39.00 (38.00, 40.00) |
| Infant birth weight (grams), median (IQR)* | 3445.00 (3105.00, 3788.00) | 3200.50 (2835.00, 3515.00) |
| Infant male gender (n = 136,091), n (%) | 16,992 (51.27) | 52,772 (51.26) |
| Infant race (n = 136,047), n (%)* | ||
| White | 14586 (44.01) | 50,244 (48.80) |
| Black | 2,025 (6.11) | 46,734 (45.40) |
| Hispanic | 6,280 (18.95) | 1,041 (1.01) |
| Asian | 7,810 (23.57) | 600 (0.58) |
| Other | 2,390 (7.21) | 4,334 (4.21) |
| One or more infant bronchiolitis hospitalization(s), n (%)* | 4245 (12.81) | 22815 (22.16) |
^IQR: interquartile range
*p <0.001
There were 18,834 (13.84%) infants whose mothers had at least one UTI during pregnancy; 8,016 (6%) infants whose mothers had GBS at delivery, and 28,945 (21%) born via C-section. Thirty three percent (44,721) of infants had no older siblings at home, and 73% (99,054) of infants had at least one course of antibiotics during the first year of life (Table 2).
Table 2. In utero and early life exposures by type of healthcare coverage among children enrolled in the PRIMA cohort, 1995–2009 (n = 136,098).
| Characteristics | KPNC n = 33,142 (24.35%) | TennCare n = 102,956 (75.65%) |
|---|---|---|
| In utero and delivery exposures | ||
| Maternal UTI during pregnancy, n (%) | ||
| 0 | 31,984 (96.51) | 85,280 (82.83) |
| 1 | 1,034 (3.12) | 12,947 (12.58) |
| 2 | 104 (0.31) | 3,151 (3.06) |
| ≥3 | 20 (0.06) | 1,578 (1.53) |
| Maternal GBS, n (%) | ||
| No | 31,934 (96.36) | 96,148 (93.39) |
| Yes | 1,208 (3.64) | 6,808 (6.61) |
| Mode of delivery (n = 136,060), n (%) | ||
| Vaginal | 24,147 (72.86) | 73,644 (71.53) |
| Assisted | 2,159 (6.51) | 7,165 (6.96) |
| C-section | 6,836 (20.63) | 22,109 (21.47) |
| Infant exposures | ||
| Infant antibiotics use, n (%) | ||
| 0 | 16,328 (49.27) | 20,716 (20.12) |
| 1 | 8,774 (26.47) | 21,640 (21.02) |
| 2 | 4,392 (13.25) | 19,285 (18.73) |
| 3 | 2,148 (6.48) | 15,010 (14.58) |
| 4 | 920 (2.78) | 10,650 (10.34) |
| 5 | 382 (1.15) | 7,104 (6.90) |
| 6 | 122 0.37) | 4,251 (4.13) |
| 7 | 44 (0.13) | 2,300 (2.23) |
| ≥8 | 32 (0.10) | 2,000 (1.94) |
| Number of older siblings (n = 136,011), n (%) | ||
| 0 | 13,539 (40.85) | 31,182 (30.29) |
| 1 | 12,300 (37.11) | 35,596 (34.57) |
| 2 | 5,028 (15.17) | 20,413 (19.83) |
| 3 | 1,571 (4.74) | 8,947 (8.69) |
| 4 | 426 (1.29) | 3,638 (3.53) |
| ≥5 | 273 (0.82) | 3,098 (3.01) |
In utero and delivery exposures
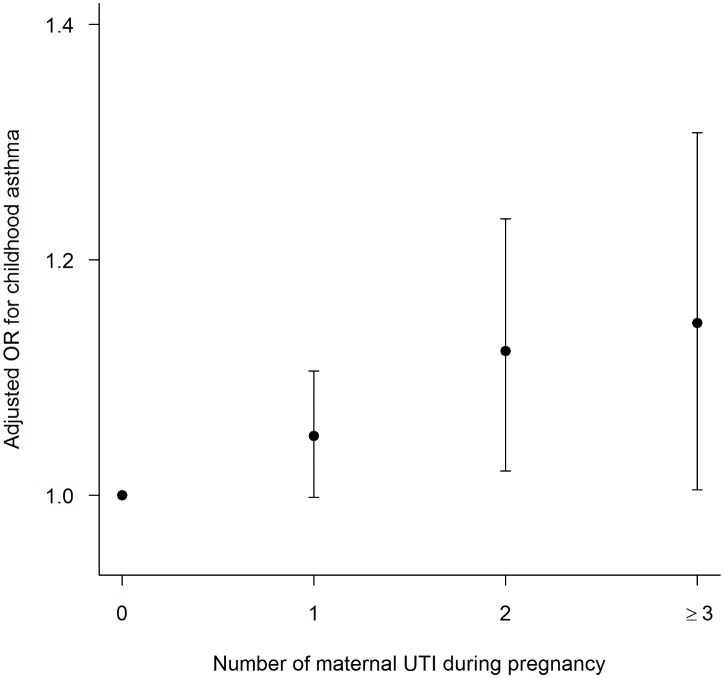
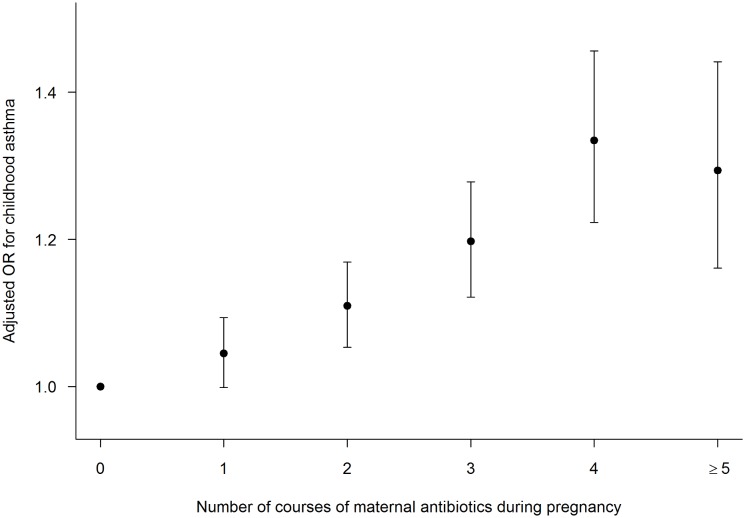
Maternal UTI during pregnancy significantly increased the risk of childhood asthma in both unadjusted and adjusted analyses. Compared with children whose mothers did not have a UTI during pregnancy, having at least one UTI during pregnancy increased the odds of having childhood asthma by 34% (Odds ratio [OR] 1.34, 95% confidence interval [CI] 1.28, 1.40). There was a significant dose-dependent relationship between increasing number of maternal UTIs and the risk of asthma development. For every additional prenatal UTI women experienced, the odds of their children developing asthma increased by 20% (OR 1.20, 95%CI 1.18, 1.25). The significant dose-dependent association between maternal UTI during pregnancy and the risk of childhood asthma persisted after adjusting for maternal GBS, mode of delivery, infant antibiotic use, number of older siblings at home, and other confounding covariates (Adjusted OR [AOR] 1.04, 95%CI 1.02, 1.07) (Fig 1). In the analysis with any type of maternal infection during pregnancy included, maternal infection increased the odds of childhood asthma in a similar dose response manner (one episode of infection: AOR 1.06, 95%CI 1.04, 1.08) (S1 Fig).
Fig 1. Adjusted odds ratio (AOR) for childhood asthma in relation to the number of maternal UTIs during pregnancy.
Infants of mothers who did not have a UTI during pregnancy served as the reference group.
GBS colonization at delivery, the least common of all exposures, increased the odds of childhood asthma by 27% (OR 1.27, 95%CI 1.19, 1.35) in the unadjusted analysis. However, the association was not significant after adjusting for other exposures and confounding covariates (AOR 1.03, 95%CI 0.96, 1.10). C-section was associated with an increased risk of childhood asthma in both univariate and multivariable analyses (p<0.001). Compared with vaginal delivery, C-section delivery increased the odds of having childhood asthma by 34% (OR 1.34, 95%CI 1.29, 1.39) in unadjusted analysis and 11% adjusting for other exposures of interest and additional confounding covariates (AOR 1.11, 95%CI 1.06, 1.15).
Infant exposures
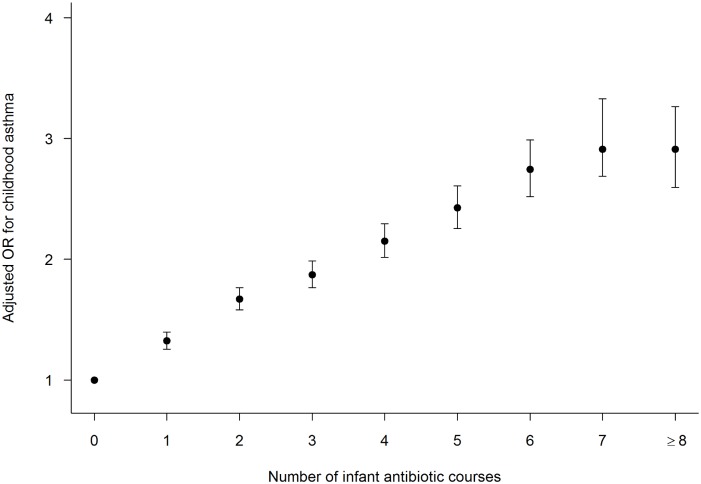
Compared with children who did not receive any antibiotics in the first year of life, receiving at least one course of antibiotics significantly increased a child’s risk of developing asthma in the unadjusted analysis (OR 2.06, 95%CI 1.98, 2.15). There was a significant dose-dependent relationship between increasing number of infant antibiotic courses and the risk of asthma development. For every additional course of antibiotics, the odds of developing asthma increased by 21% (OR 1.21, 95%CI 1.20, 1.22). This significant dose-dependent relationship between antibiotic use and the risk of childhood asthma persisted after adjusting for other exposures and confounding covariates (AOR 1.16, 95%CI 1.15, 1.17) (Fig 2). In a sensitivity analysis when number of infant infections was used in lieu of the number of antibiotic courses, the dose-response relationship between asthma risk and the number of infections during infancy was similar to that for the number of antibiotic courses (S2 Fig). For each additional infection infants experienced, the odds of having childhood asthma increased by 10% after adjusting for maternal UTI during pregnancy, mode of delivery, number of older siblings and other confounding covariates (AOR 1.10, 95%CI 1.07, 1.13).
Fig 2. Adjusted odds ratio (AOR) for childhood asthma in relation to the number of antibiotic courses used during infancy.
Infants who received no antibiotics during their first 12 months of life served as the reference group.
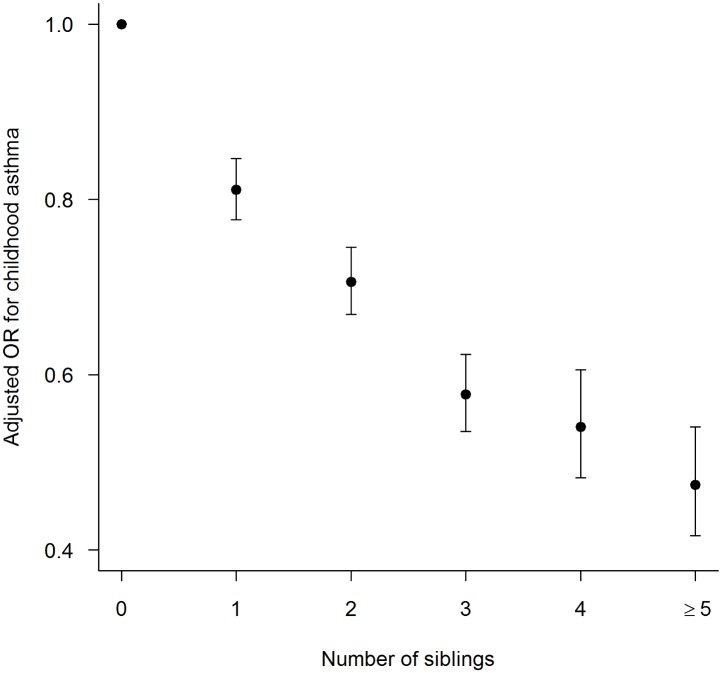
On the other hand, having older siblings at home significantly decreased the risk of childhood asthma in the unadjusted and after adjusting for other exposures and confounding covariates (Fig 3). There was also a significant dose response relationship between asthma and number of older siblings at home. With every additional sibling at home, the odds of childhood asthma at age 6 years decreased by 8% (OR 0.92, 95%CI 0.91, 0.93) in the unadjusted analysis and 15% after adjusting for other exposures and confounding covariates (AOR 0.85, 95%CI 0.84, 0.87).
Fig 3. Adjusted odds ratio (AOR) for childhood asthma in relation to the number of older siblings at home.
Infants with no older siblings at home served as the reference group.
Relative and combined contribution of exposures
The relative contribution and importance of exposures on asthma risk was calculated and plotted as the fraction of variability explained by each exposure (S3 Fig). Compared with known and studied risk factors of childhood asthma, infant antibiotic use conferred the greatest risk, similar to the risk of having a bronchiolitis healthcare visit during infancy. The variability of asthma explained by having older siblings at home ranked next with similar contribution and importance as infant gender and having a mother with asthma.
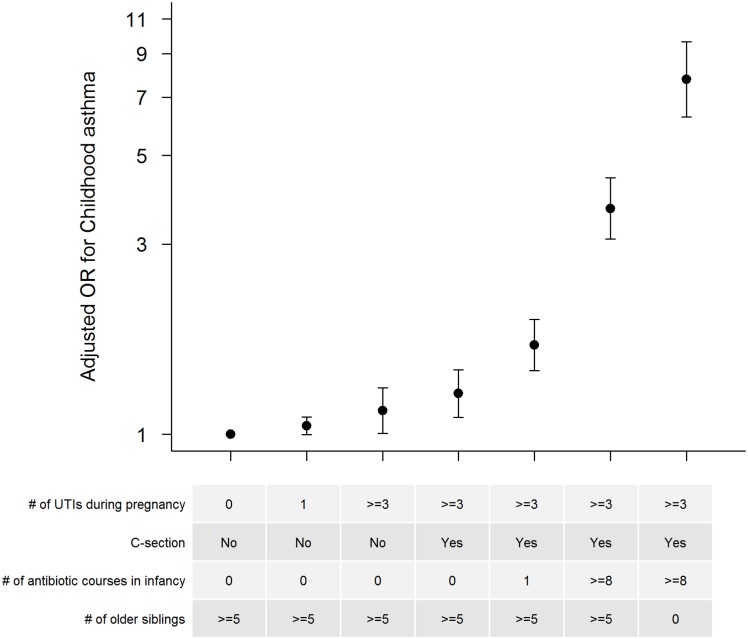
We further estimated the combined effects of these events (maternal UTI during pregnancy, mode of delivery, infant antibiotic use, and having older siblings at home) in several different scenarios (Fig 4). Children whose mothers did not have a UTI during pregnancy, were born by vaginal delivery, had at least five older siblings at home, and had not used antibiotics during infancy had the lowest risk of developing asthma and served as reference group. The risk of developing asthma increased with every additional risk factor (Fig 4). In extreme scenarios, children with mothers who had at least three UTIs during pregnancy, were born by C-section, had no older siblings at home, and had used at least eight courses of antibiotics during infancy had a 7.77 fold increase in the odds of developing asthma compared with children with none of these risk factors (AOR 7.77, 95%CI 6.25, 9.65) (Fig 4).
Fig 4. Adjusted odds ratios (AOR) for childhood asthma in various scenarios of cumulative exposures.
AOR for childhood asthma in various scenarios of maternal urinary tract infection (UTI) during pregnancy, mode of delivery, infant antibiotic courses, and having older siblings at home. Infants with mothers who did not have a UTI during pregnancy, were born via vaginal delivery, had zero antibiotics during infancy, and had at least five older siblings at home served as the reference group.
In the subgroup of infants enrolled in TennCare in whom maternal antibiotic dispensing information was available, the number of maternal antibiotic courses (0, 1, 2, 3, 4, 5+) was used in our models. Maternal antibiotic use increased the risk of childhood asthma in a similar dose-dependent manner as did maternal UTI (Fig 5). With each additional antibiotic course during pregnancy, the odds of childhood asthma increased by 13% (OR 1.13, 95%CI 1.12, 1.15) in the unadjusted analysis and 6% (AOR 1.06, 95%CI 1.05, 1.08) after adjusting for mode of delivery, infant antibiotic use, number of older siblings, and other confounding covariates. Lastly, the relationship between exposures of interest with childhood asthma was similar in subgroup analyses of term, non-low birth weight healthy infants and infants who were enrolled in either KPNC or TennCare (data not shown).
Fig 5. Adjusted odds ratios (AOR) for childhood asthma in relation to the number of antibiotic courses used during pregnancy.
Infants with mothers who did not use antibiotics during pregnancy served as the reference group. This is among the group of children who were enrolled in TennCare.
Discussion
In this large population-based cohort study, we reported that in utero and early life events, including maternal UTI during pregnancy, maternal antibiotic use, C-section delivery, infant antibiotic use, and having no older siblings at home were all associated with an increased risk of childhood asthma. In addition, there were strong dose-dependent relationships between number of maternal UTIs during pregnancy, maternal antibiotic use, infant antibiotic use, and older siblings at home with asthma risk. Infant exposures including antibiotic use and absence of older siblings at home conferred the greatest risk of childhood asthma development, while maternal exposures such as maternal antibiotic use, UTI, and GBS had less impact on childhood asthma development. Individuals with extremes of multiple exposures had a nearly eight-fold increased odds of developing asthma by age 6 years.
The increased risk of maternal UTI during pregnancy, maternal antibiotic use, mode of delivery, infant antibiotic use, and number of older siblings at home on the development of childhood asthma may act through various mechanisms, one of which may be through altering the maternal and infant microbiome. The human microbiome plays an important symbiotic role in our health. It influences the development of the immune system, and if altered, may lead to an increased risk of diseases such as asthma [3,42]. Recent studies support initial colonization starting in the womb, with supplemental early colonization influenced via delivery route and breast feeding [43]. The maternal genital tract (including the placenta) is likely the main source of the initial microbial colonization, which we and others have demonstrated to significantly differ among infants born via vaginal delivery compared with C-section [19,44,45]. Additionally, the microbiome can be altered by infections such as UTI, and the antibiotics used to treat those infections [46]. Maternal antibiotics are likely to alter the in utero microbiome as well as the maternal skin and vaginal flora that provides the sequential colonization at birth [19,47]. Antibiotics can also cross the placenta and stay in the fetal blood stream at high levels for several hours after administration to the mother, which could affect both the in utero colonization and that determined by mode of delivery for antibiotics administered perinatally [48]. In our study, maternal UTI during pregnancy and maternal antibiotic use were both associated with an increased risk of childhood asthma in a dose-dependent manner. It is likely that both the infection itself as well as antibiotic use play a separate and additive role in altering the microbiome. In addition, we adjusted for maternal asthma status in the multivariable regression models, demonstrating that antibiotics and infections have an effect that is independent of inherited risk.
As mentioned previously, the mode of delivery leads to divergent bacterial colonization in the infant, which can progress to an altered adult microbiome. If an infant is born via C-section, he or she is largely colonized by skin flora such as Staphylococcus and Corynebacterium. In contrast, infants’ born via vaginal delivery are colonized with vaginal and fecal flora including Lactobacillus and Prevotella [19,47]. In agreement with other studies, we found that delivery via C-section, compared to vaginal delivery, increases the risk of developing childhood asthma [49–53].
We also found a significant dose response protective effect of having older siblings on childhood asthma. This significant protective effect may be a reflection of the hygiene hypothesis (as older siblings may share protective bacteria with their infant sibling). Azad and colleagues recently published data from the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort in which 24 healthy term infants’ intestinal microbiome was measured, demonstrating that having older siblings does alter the microbiome [54].
Our large study population makes it possible for us to tease out the relative impact of early-life exposures in two diverse populations, demonstrating their comparative role, with infant antibiotic use and having fewer older siblings at home having the greatest impact on childhood asthma risk. Infancy is a period of time when infants establish their own microbiome, which influences the longitudinal development of the microbiome, the immune system, and susceptibility to infection, among other roles. While the impact of these risk factors is not likely mediated exclusively through altering the early life microbiome, exposures that potentially alter the microbiome at this period of time may have the greatest impact on both subsequent microbial diversity as well as risk of childhood asthma.
Our study has considerable strengths. First, unlike other studies that have examined all these risk factors individually, we examined the combined effect of relevant exposures in a large a population-based study. Second, we established the effect size of each risk factor, which may help us to understand the most vulnerable period (prenatal, perinatal, or postnatal), and the most important modifiable risk factor in the development of asthma. Third, we were able to demonstrate the dose-dependent association of these exposures and the additive effect of these early life events on asthma risk. Fourth, we measured these exposures prior to the asthma diagnosis. Lastly, we were able to ascertain and adjust for maternal asthma status, an important marker of genetic predisposition to childhood asthma. This study also has important limitations that should be considered. Although we propose that one of the mechanisms through which the exposures measured in this study have an effect on asthma risk is through altering the microbiome, we did not directly measure the microbiome. However, all exposures measured in our study have been previously demonstrated to alter the microbiome [42,44,55–59]. All exposures ascertained and considered in our study have also been demonstrated to be associated with the development of childhood asthma [35,36,49,60–63]. Future studies using next-generation sequencing of the bacterial genome will be important in identifying patterns and components of the microbiome that may be protective or detrimental. To date, studies using these new technologies have had small sample sizes that, unlike ours, have not allowed assessment of the relative effect size, establishment of a dose-dependent relationship, nor ability to assess the combined effect of relevant exposures. Second, we were unable to include several other factors known to alter the early-life microbiome or known to be associated with asthma risk, including the impact of infant’s diet (breast feeding, formula feeding, time of weaning to solids), growing up with pets, early daycare attendance, and other environment exposures [25–28,32,33,35,64–67].
In conclusion, our data show that early-life exposures, maternal antibiotic use /maternal UTI infection, mode of delivery, infant antibiotic use, and number of older siblings at home are associated with childhood asthma development in a cumulative manner, and for those continuous variables, a dose-dependent relationship. Measured infant exposures also had a greater effect than in utero and at delivery exposures. The strong association of these exposures with asthma risk, the dose-dependent relationship, and the additive effect suggest that testing interventions to decrease these exposures, perhaps mediated through establishing a protective microbiome, could prevent the development of asthma in children.
Supporting Information
Infants with mothers who did not have an infection during pregnancy served as the reference group.
(PDF)
Infants with no coded infections during infancy served as the reference group.
(PDF)
Relative contribution and importance of maternal and infant risk factors as well as other known risk factors for childhood asthma in full penalized model, as judged by the fraction of variability of asthma explained by each exposure.
(PDF)
Acknowledgments
The authors are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, the Tennessee Department of Health, Office of Policy, Planning & Assessment, and Kaiser Permanente Northern California for providing the data.
Data Availability
Due to privacy restrictions imposed by Vanderbilt University and Kaiser Permanente Medical Care Program, the study data cannot be made publicly available. A fully de-identified version can be requested from the principal investigator Tina V. Hartert, MD, MPH, Vanderbilt University, TN, USA (tina.hartert@vanderbilt.edu).
Funding Statement
This work was supported by a grant from the Agency for Healthcare Research and Quality [R01 HS018454; to TVH] and NIH [K24 AI 077930; to TVH]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Malveaux FJ. The state of childhood asthma: introduction. Pediatrics 2009. March; 123 Suppl 3: S129–S130 10.1542/peds.2008-2233B [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ,von Mutius E. The asthma epidemic. N.Engl.J.Med. 2006. November 23; 355: 2226–2235 [DOI] [PubMed] [Google Scholar]
- 3.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N.Engl.J Med 2007. October 11; 357: 1487–1495 [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R,Gordon JI. The human microbiome project. Nature 2007. October 18; 449: 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science 2006. June 2; 312: 1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazmanian SK, Liu CH, Tzianabos AO,Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005. July 15; 122: 107–118 [DOI] [PubMed] [Google Scholar]
- 7.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 2007. May; 56: 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YJ. Asthma Microbiome Studies and the Potential for New Therapeutic Strategies. Curr.Allergy Asthma Rep. 2013. May 25; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS.One. 2010; 5: e8578 10.1371/journal.pone.0008578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J.Allergy Clin.Immunol. 2011. February; 127: 372–381 10.1016/j.jaci.2010.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dethlefsen L and Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc.Natl.Acad.Sci.U.S.A 2011. March 15; 108 Suppl 1: 4554–4561 10.1073/pnas.1000087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jernberg C, Lofmark S, Edlund C,Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010. November; 156: 3216–3223 10.1099/mic.0.040618-0 [DOI] [PubMed] [Google Scholar]
- 13.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK,Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS.One. 2010; 5: e9836 10.1371/journal.pone.0009836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alm B, Erdes L, Mollborg P, Pettersson R, Norvenius SG, Aberg N, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics 2008. April; 121: 697–702 10.1542/peds.2007-1232 [DOI] [PubMed] [Google Scholar]
- 15.Sjolund M, Wreiber K, Andersson DI, Blaser MJ,Engstrand L. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann.Intern.Med. 2003. September 16; 139: 483–487 [DOI] [PubMed] [Google Scholar]
- 16.Isolauri E. Development of healthy gut microbiota early in life. J.Paediatr.Child Health 2012. June; 48 Suppl 3: 1–6 10.1111/j.1440-1754.2012.02489.x [DOI] [PubMed] [Google Scholar]
- 17.Valles Y, Gosalbes MJ, de Vries LE, Abellan JJ,Francino MP. Metagenomics and development of the gut microbiota in infants. Clin.Microbiol.Infect. 2012. July; 18 Suppl 4: 21–26 10.1111/j.1469-0691.2012.03876.x [DOI] [PubMed] [Google Scholar]
- 18.Palmer C, Bik EM, DiGiulio DB, Relman DA,Brown PO. Development of the human infant intestinal microbiota. PLoS.Biol. 2007. July; 5: e177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc.Natl.Acad.Sci.U.S.A 2010. June 29; 107: 11971–11975 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006. August; 118: 511–521 [DOI] [PubMed] [Google Scholar]
- 21.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E,Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum.Dev. 2010. July; 86 Suppl 1: 13–15 10.1016/j.earlhumdev.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Vael C and Desager K. The importance of the development of the intestinal microbiota in infancy. Curr.Opin.Pediatr. 2009. December; 21: 794–800 10.1097/MOP.0b013e328332351b [DOI] [PubMed] [Google Scholar]
- 23.Murgas TR and Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J.Perinatol. 2011. April; 31 Suppl 1: S29–S34 10.1038/jp.2010.172 [DOI] [PubMed] [Google Scholar]
- 24.Vaishampayan PA, Kuehl JV, Froula JL, Morgan JL, Ochman H,Francino MP. Comparative metagenomics and population dynamics of the gut microbiota in mother and infant. Genome Biol.Evol. 2010; 2: 53–66 10.1093/gbe/evp057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barile D and Rastall RA. Human milk and related oligosaccharides as prebiotics. Curr.Opin.Biotechnol. 2013. April; 24: 214–219 10.1016/j.copbio.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J.Pediatr.Gastroenterol.Nutr. 2000. January; 30: 61–67 [DOI] [PubMed] [Google Scholar]
- 27.Hanson LA, Korotkova M,Telemo E. Breast-feeding, infant formulas, and the immune system. Ann.Allergy Asthma Immunol. 2003. June; 90: 59–63 [DOI] [PubMed] [Google Scholar]
- 28.Ward RE, Ninonuevo M, Mills DA, Lebrilla CB,German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl.Environ.Microbiol. 2006. June; 72: 4497–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Backhed F, Ley RE, Sonnenburg JL, Peterson DA,Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005. March 25; 307: 1915–1920 [DOI] [PubMed] [Google Scholar]
- 30.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R,Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci.Transl.Med. 2009. November 11; 1: 6ra14 10.1126/scitranslmed.3000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011. October 7; 334: 105–108 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De FC, Cavalieri D, Di PM, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc.Natl.Acad.Sci.U.S.A 2010. August 17; 107: 14691–14696 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA,Datta SK. Parental Dietary Fat Intake Alters Offspring Microbiome and Immunity. J.Immunol. 2013. August 9; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moeller AH and Ochman H. Factors that drive variation among gut microbial communities. Gut Microbes. 2013. August 9; 4: 10.4161/gmic.26039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD,Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med 2000. August 24; 343: 538–543 [DOI] [PubMed] [Google Scholar]
- 36.Midodzi WK, Rowe BH, Majaesic CM, Saunders LD,Senthilselvan A. Early life factors associated with incidence of physician-diagnosed asthma in preschool children: results from the Canadian Early Childhood Development cohort study. J.Asthma 2010. February; 47: 7–13 10.3109/02770900903380996 [DOI] [PubMed] [Google Scholar]
- 37.Escobar GJ, Gebretsadik T, Carroll K, Li SX, Walsh EM, Wu P, et al. Adherence to Immunoprophylaxis Regimens for Respiratory Syncytial Virus Infection in Insured and Medicaid Populations. J Pediatric Infect.Dis.Soc. 2013. September; 2: 205–214 10.1093/jpids/pit007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am.J.Respir.Crit Care Med. 2008. December 1; 178: 1123–1129 10.1164/rccm.200804-579OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakefield DB and Cloutier MM. Modifications to HEDIS and CSTE algorithms improve case recognition of pediatric asthma. Pediatr.Pulmonol. 2006. October; 41: 962–971 [DOI] [PubMed] [Google Scholar]
- 40.Harrell FEJ. Regression modeling strategies. 2015; 2: [Google Scholar]
- 41.R Core Team. R: A language and environment for statistical computing. 2015; [Google Scholar]
- 42.Huang YJ and Boushey HA. The microbiome in asthma. J.Allergy Clin.Immunol. 2015. January; 135: 25–30 10.1016/j.jaci.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funkhouser LJ and Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS.Biol. 2013; 11: e1001631 10.1371/journal.pbio.1001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006. August; 118: 511–521 [DOI] [PubMed] [Google Scholar]
- 45.Shilts MH, Rosas-Salazar C, Tovchigrechko A, Larkin EK, Torralba M, Akopov A, et al. Minimally Invasive Sampling Method Identifies Differences in Taxonomic Richness of Nasal Microbiomes in Young Infants Associated with Mode of Delivery. Microb.Ecol. 2015. September 14; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J,Versalovic J. The placenta harbors a unique microbiome. Sci.Transl.Med. 2014. May 21; 6: 237ra65 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isolauri E, Rautava S,Salminen S. Probiotics in the development and treatment of allergic disease. Gastroenterol.Clin.North Am. 2012. December; 41: 747–762 10.1016/j.gtc.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 48.Colombo DF, Lew JL, Pedersen CA, Johnson JR,Fan-Havard P. Optimal timing of ampicillin administration to pregnant women for establishing bactericidal levels in the prophylaxis of Group B Streptococcus. Am.J.Obstet.Gynecol. 2006. February; 194: 466–470 [DOI] [PubMed] [Google Scholar]
- 49.Thavagnanam S, Fleming J, Bromley A, Shields MD,Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin.Exp.Allergy 2008. April; 38: 629–633 10.1111/j.1365-2222.2007.02780.x [DOI] [PubMed] [Google Scholar]
- 50.Bager P, Wohlfahrt J,Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin.Exp.Allergy 2008. April; 38: 634–642 10.1111/j.1365-2222.2008.02939.x [DOI] [PubMed] [Google Scholar]
- 51.Magnus MC, Haberg SE, Stigum H, Nafstad P, London SJ, Vangen S, et al. Delivery by Cesarean section and early childhood respiratory symptoms and disorders: the Norwegian mother and child cohort study. Am.J.Epidemiol. 2011. December 1; 174: 1275–1285 10.1093/aje/kwr242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roduit C, Scholtens S, de Jongste JC, Wijga AH, Gerritsen J, Postma DS, et al. Asthma at 8 years of age in children born by caesarean section. Thorax 2009. February; 64: 107–113 10.1136/thx.2008.100875 [DOI] [PubMed] [Google Scholar]
- 53.Tollanes MC, Moster D, Daltveit AK,Irgens LM. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J.Pediatr. 2008. July; 153: 112–116 10.1016/j.jpeds.2008.01.029 [DOI] [PubMed] [Google Scholar]
- 54.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Sears MR, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin.Immunol. 2013; 9: 15 10.1186/1710-1492-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dethlefsen L and Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc.Natl.Acad.Sci.U.S.A 2011. March 15; 108 Suppl 1: 4554–4561 10.1073/pnas.1000087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK,Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS.One. 2010; 5: e9836 10.1371/journal.pone.0009836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sjolund M, Wreiber K, Andersson DI, Blaser MJ,Engstrand L. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann.Intern.Med. 2003. September 16; 139: 483–487 [DOI] [PubMed] [Google Scholar]
- 58.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc.Natl.Acad.Sci.U.S.A 2010. June 29; 107: 11971–11975 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E,Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum.Dev. 2010. July; 86 Suppl 1: 13–15 10.1016/j.earlhumdev.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 60.Stensballe LG, Simonsen J, Jensen SM, Bonnelykke K,Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J.Pediatr. 2013. April; 162: 832–838 10.1016/j.jpeds.2012.09.049 [DOI] [PubMed] [Google Scholar]
- 61.Risnes KR, Belanger K, Murk W,Bracken MB. Antibiotic exposure by 6 months and asthma and allergy at 6 years: Findings in a cohort of 1,401 US children. Am.J.Epidemiol. 2011. February 1; 173: 310–318 10.1093/aje/kwq400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kozyrskyj AL, Ernst P,Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest 2007. June; 131: 1753–1759 [DOI] [PubMed] [Google Scholar]
- 63.Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 2009. March; 123: 1003–1010 10.1542/peds.2008-1146 [DOI] [PubMed] [Google Scholar]
- 64.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R,Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci.Transl.Med. 2009. November 11; 1: 6ra14 10.1126/scitranslmed.3000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011. October 7; 334: 105–108 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ownby DR, Johnson CC,Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA 2002. August 28; 288: 963–972 [DOI] [PubMed] [Google Scholar]
- 67.Huang YJ and Boushey HA. The microbiome and asthma. Ann.Am.Thorac.Soc. 2014. January; 11 Suppl 1: S48–S51 10.1513/AnnalsATS.201306-187MG [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Infants with mothers who did not have an infection during pregnancy served as the reference group.
(PDF)
Infants with no coded infections during infancy served as the reference group.
(PDF)
Relative contribution and importance of maternal and infant risk factors as well as other known risk factors for childhood asthma in full penalized model, as judged by the fraction of variability of asthma explained by each exposure.
(PDF)
Data Availability Statement
Due to privacy restrictions imposed by Vanderbilt University and Kaiser Permanente Medical Care Program, the study data cannot be made publicly available. A fully de-identified version can be requested from the principal investigator Tina V. Hartert, MD, MPH, Vanderbilt University, TN, USA (tina.hartert@vanderbilt.edu).