Abstract
Sodium sieving in peritoneal dialysis (PD) occurs in a situation with high osmotically-driven ultrafiltration rates. This dilutional phenomenon is caused by free water transport through the water channel aquaporin-1. It has recently been described that encapsulating peritoneal fibrosis is associated with impaired free water transport, despite normal expression of aquaporin-1. In this review, it will be argued that free water transport can be used for assessment of fibrotic peritoneal alterations, due to the water-binding capacity of collagen. Finally, the consequences for clinical practice will be discussed.
Keywords: Free water transport, small pore fluid transport, peritoneal fibrosis, encapsulating peritoneal sclerosis, sodium, peritoneal dialysis
Nolph et al. were the first who described in 1969 the presence of a decrease in the peritoneal effluent Na+ concentration during peritoneal dialysis (PD) exchanges of about 1 hour with a 7% glucose-based dialysis solution, as used for treatment of overhydration in virtually anuric patients (1). They named the phenomenon sodium sieving and postulated that this uncoupling of the transport of water and Na+ was caused by peritoneal water transport without concomittant equal solute diffusion, because plasma Na+ concentration showed an increase. They tried to explain this unexpected finding by hindrance of the peritoneal membrane to sodium diffusion.
Ten years later, this observation was confirmed by the same group in 4.25% glucose exchanges, as used in continuous ambulatory PD (CAPD) (2). The minimum dialysate Na+ concentration was found after about 60 minutes. The interpretation of sodium sieving in these days was that transperitoneal transport of Na+ was hindered in the presence of high osmotically-induced fluid flows, but the possibility that some transcellular water transport might occur was also mentioned, albeit in one sentence. Probably as a consequence of the inability to give a good explanation for the observed discrepancy between peritoneal sodium and water transport, it tended to be forgotten and in the peritoneal equilibrium test (PET) developed by the same authors, a 2.27/2.25% glucose solution was advocated, thereby neglecting Na+ kinetics (3).
Another 10 years later, Rippe et al. published the results of their peritoneal transport simulations in CAPD, showing that solute transport characteristics could be explained by assuming transport of small solutes through a large number of small interendothelial pores, in combination with a small quantity of large pores involved in peritoneal transport of macromolecules. In addition, the authors simulated water transport to occur through ultrasmall transcellular pores that were impermeable to solutes (4). They further refined their model of solute and fluid transport (5,6) and used this 3-pore model of peritoneal transport for a description of the transfer of fluid and solutes during PD (7).
In the same years that the assumption of ultrasmall, water-selective transcellular pores was made, Agre et al. discovered a 26 kD protein in the cell membrane of red blood cells (8), that later appeared to be a water channel (9). It was also found in renal proximal tubule cells. Shortly thereafter, this water channel appeared to be present in many non-fenestrated endothelia (10) and was called aquaporin-1 (AQP-1). Using immunochemistry, it was found that this protein also appeared to be present in the endothelial cells of peritoneal capillaries and venules (11–13). In 2006, a study in aquaporin (AQP)-1 knock-out mice proved that AQP-1 was the ultrasmall water selective pore of the 3-pore model, because these animals had no sodium sieving in the presence of otherwise normal peritoneal transport characteristics (14). It implies that a part of transendothelial fluid transport during PD is free water transport through AQP-1.
Clinical Assessment of Free Water Transport
The above mentioned studies assumed that AQP-1 is an archetypal water channel that is expressed constitutively and functions in the presence of an osmotic pressure gradient, as is the case for red blood cells. Its function can be temporarily inhibited by the administration of mercury chloride as has been shown in rats (15) and rabbits (16). This raised the question of whether impaired AQP-function could also be caused by endothelial damage as might occur in some long-term PD patients. This was already suggested in 1995 by an observation of 6 CAPD patients who developed otherwise unexplained ultrafiltration failure (UFF) and appeared to have no sodium sieving at all (17). A few years later, a similar patient was described with apparently impaired AQP-1 function, but with normal expression of the protein (18).
Based on these observations on the development of reduced free water transport, as summarized in Krediet et al. (19), the International Society for Peritoneal Dialysis (ISPD) published a guideline, stating the importance of assessment of sodium sieving in peritoneal function tests, like the PET (20). Therefore this “modified PET” should be performed with the most hypertonic dialysis solution (3.86/4.25% glucose) and a dialysate and plasma sample should be obtained after 60 minutes for assessment of the D/P Na+ dip. Furthermore, the committee defined UFF by the 3x4 rule, meaning net ultrafiltration (UF) < 400 mL after a 4-h dwell with a 3.86/4.25% glucose solution.
Using the above methodology, Smit et al. found a lower dip of D/P Na+ in long-term patients with UFF compared with a similar group without this complication (21), and also when compared to patients with early UF (22). However, the D/P ratio only provides a semiquantitative assessment of free water transport. Knowledge of the intraperitoneal volume after 60 minutes as well makes it possible to calculate sodium removal during this initial period of a dwell when UF has its maximum. Assuming that Na+ only passes through the small pores makes it possible to calculate small pore fluid transport (SPFT) as the peritoneal Na+ clearance. Free water transport is then quantitatively assessed as the difference between net UF and SPFT after 60 minutes. This suggestion by La Milia was subsequently employed in studies by Smit et al. (23) and by La Milia et al. who named it the “mini PET” (24).
Computer simulations indicated that water transport through AQP-1 could account for about 40% of total UF (4). In 4 cross-sectional studies with the La Milia method for FWT assessment, remarkably similar values were reported for FWT0-60 min (23–26). Medians or means ranged from 35 to 44%, but showed a large interindividual variability. Median/mean absolute values were 164 mL (23), 215 mL (24), 180 mL (25), and 152 mL (26). Free water transport was related to the P/D Na+ dip. A relationship was present between FWT and SPFT in the majority of patients, but not in some with UFF (26). This relationship between FWT and SPFT makes sense because both are dependent on the osmotic gradient. Accordingly, both FWT and SPFT rates decreased with the duration of the dwell due to the decrease of the osmotic gradient, but the SPFT rate leveled off after 2 hours while the FWT rate showed a continuous decline (25).
Fluid Transport in Encapsulating Peritoneal Sclerosis
The duration of PD is the most important risk factor for the development of encapsulating peritoneal sclerosis (EPS), as shown in a number of studies (27,28). Peritoneal transport assessment has not been done frequently, but EPS is always associated with UFF and often, but not always, with fast transport of small solutes (29,30). Also, the presence of a reduced osmotic conductance to glucose has been described (31). The old finding that the potential to induce UF is especially impaired for the most hypertonic solution also points to a decrease in FWT (29).
The largest study on the time course of peritoneal transport in patients who eventually developed EPS was published in 2011 (32). It concerned 417 PD patients who were admitted to the PD program since 1995, and from whom the results of standard peritoneal permeability analyses with 3.86% glucose-based dialysate were available (32). Encapsulating peritoneal sclerosis developed in 12 of these patients (3%) after a median PD duration of 8 years. They were compared with the 21 patients who had late UFF, but no EPS, after a duration of 6 years, and 26 controls without UFF and a PD duration of 5.5 years. It appeared that small solute transport was lower in the normal UF group, compared to both UFF and EPS patients; no difference was present for peritoneal protein clearances, while net UF was similar in the UFF and EPS group. Further analysis of fluid transport in the 3 patient groups using receiver operating curves showed that only FWT was a predictor of EPS with an area under the receiver operating curve of 0.82. This value was markedly higher than for the mass transfer coefficient of creatinine, SPFT, the osmotic conductance to glucose, LpA (product of hydraulic permeability and surface area), the osmotic reflection coefficient and net UF (33). An absolute value of 75 mL/60 min had the best discriminative power with a sensitivity of 100% and a specificity of 81%, much better than when FWT was expressed as 45% or 35% of the total ultrafiltered volume (Lopes Barreto et al., unpublished).
Recently, a study was published that confirmed the above discussed findings (34). The analysis was done in 234 PD patients, 6 of whom developed EPS (3%) after a mean period of 4.8 years. Although no free water transport was calculated, the authors found lower sodium sieving in EPS than in 28 controls matched for PD duration, but not divided for the presence or absence of UFF. The EPS patients had normal AQP-1 expression. Taking the studies by Sampimon et al. (33) and Morelle et al. (34) together now shows for the first time, in 19 EPS patients from different countries, that impaired FWT is the most important predictor of EPS, but that AQP-1 is unlikely to be the culprit.
The Time Course of Free Water Transport
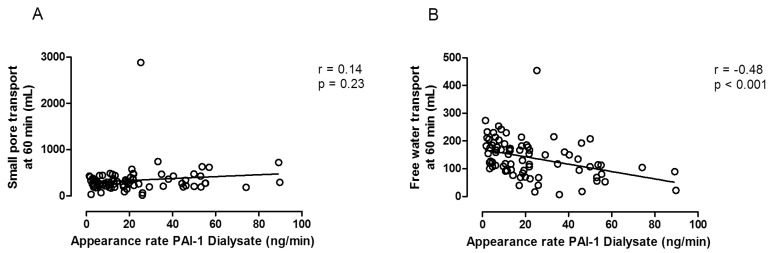
The results in EPS patients suggest that extensive peritoneal fibrosis is an important cause of impaired FWT. As peritoneal fibrosis develops with time on PD, it can be speculated whether the amount of FWT can be used as an approximation of peritoneal fibrosis severity. This contention is supported by the relationship that is present in PD patients between effluent markers of peritoneal fibrosis and FWT, as shown in Figure 1 (35).
Figure 1 —
Left panel: the absence of a relationship between small pore fluid transport (SPFT) and the dialysate appearance rate of plasminogen activator inhibitor-1 (PAI-1); Right panel: the obvious negative correlation between free water transport (FWT) and the appearance rate of PAI-1. Calculated from data published in Lopes Barreto et al. (35).
Remarkably few longitudinal studies on FWT have been performed, probably because in most of these a PET with 2.27/2.5% glucose-based dialysate was used, despite the recommendations by the ISPD, published in 2000 (20). The prospective longitudinal single-center study by Coester et al. of 138 incident PD patients treated with conventional dialysis solutions for a maximum of 5 years showed that FWT remained stable during the first 4 years of treatment, but that it decreased after that time (36). This decrease was not present for patients treated with a “biocompatible” solution (unpublished). No correction was made for peritonitis incidence. In a subsequent study by the same group, a marked difference was found for FWT between patients with any episode of peritonitis and those who never suffered this complication (37). Patients with 1 or more peritonitis episodes showed a gradual decrease from the start of PD onwards.
Frequent peritonitis is associated with fibrotic peritoneal alterations (38). Even after the first peritonitis episode, discrete signs of peritoneal interstitial changes are present such as an increase of the restriction coefficient to macromolecules (39). This makes it conceivable that the decrease of FWT with the duration of PD can be explained by fibrotic alterations in peritoneal interstitial tissues. Also the reported beneficial effect of corticosteroid treatment on FWT in patients with a kidney transplant (40) may have nothing to do with AQP-1 function, but could have been caused by the well-known beneficial effect of these drugs on fibrotic processes.
Sodium Sieving or Water Sieving in Peritoneal Fibrosis?
Here, a possible pathogenetic explanation will be discussed. Lumped models for peritoneal transport that consider the peritoneum as a single membrane similar to a hemodialysis one, cannot be used, because of the interstitial compartment. The addition of a fiber matrix to the 3-pore model has been used for estimations of effects of peritoneal fibrosis on the mass transfer area of glucose, but not for estimations of effects on FWT (41). Therefore, a simple qualitative explanation will be given. It is illustrated in Figure 2.
Figure 2 —
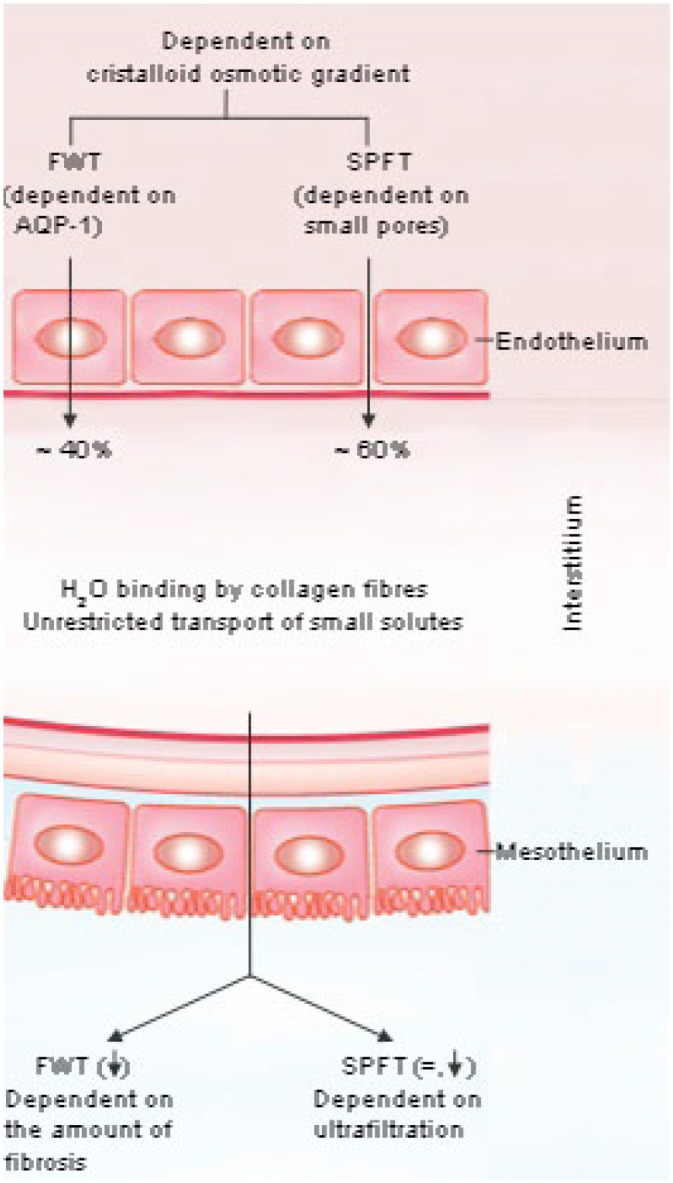
A schematic representation of the hypothesis on pathways of filtered capillary fluid and peritoneal fibrosis. Note that measurements of free water transport in drained effluent are not necessarily representative of capillary and venular aquaporin-1 function, because of the peritoneal interstitial barrier. FWT = free water transport; SPFT = small pore fluid transport; AQP-1 = aquaporin-1.
Fluid from the circulation is filtered during PD through small pores in the microvascular endothelial layer by hydrostatic and cristalloid pressure gradients, and water is filtered by cristalloid osmosis through AQP-1, which is especially localized in the capillary and venular walls. It is likely, as seen in computer simulations and from observations in newly started patients, who are unlikely to have marked peritoneal fibrosis, that the total endothelial filtrate consists for on average 60% of fluid that has passed through the small interendothelial pores, and for 40% through AQP-1. The 2 are likely to mix in the interstitial compartment. Assuming a plasma Na+ concentration of 138 mmol/L, the Na+ concentration in the filtrate after mixing will average 82.8 mmol/L. However, despite this, the interstitium is still hypertonic, because of the dialysate glucose, present in the peritoneal cavity, which probably diffuses freely into the interstitial compartment. When a patient is treated with 4-hour dwells of 3.86% glucose-based dialysate only, it can be calculated that the interstitial glucose concentration may average 125 mmol/L.
Collagen fibers bind water. Most research on this has been done with tissue from bovine paw tendons that are normally dehydrated. Hydration causes a linear expansion (42). At physiological levels of hydration, type 1 collagen fibers consist of about 30% collagen and 70% water by volume (42). In vitro experiments using gel filtration over a column packed with collagen 1 showed that small solutes like glucose pass without binding (43). This is in contrast to proteins; electrolytes were not investigated. The capacity of hydrated collagen 1 fibers to bind water in well-hydrated tissue like the peritoneal interstitium is not known, but may be substantial because of its hyperosmolality. Interstitial retention of water, but not of Na+ would result in an increased Na+ concentration in the filtered volume that reaches the peritoneal cavity, the location where the measurements of D/P ratios and FWT are performed. This would lead to the spurious conclusion of impaired FWT, while it only means interstitial water retention in collagen fibers. This assumption is only valid in the absence of interstitial sodium retention. An excessive intake of sodium leads to storage of osmotically inactive Na+, for instance in the extracellular matrix of the skin (44). It is unknown if such storage can also happen in the peritoneal tissues, but is not very likely, because the filtrate Na+ concentration in the peritoneal interstitial compartment is lower than plasma Na+. It can be concluded that the absence of sodium sieving is replaced by water sieving in long-term UFF.
Consequences for Clinical Practice
Assessment of peritoneal function should be done at regular time intervals in every PD patient, for instance once yearly. The PET in its present form only provides information on small solute transport and net UF, and these parameters can only guide prescriptions of the dialysis dose. They cannot be used to monitor long-term peritoneal alterations and the development of fibrosis and EPS. In the present review, it is argued that determination of FWT by sodium kinetics may be used for assessment of the severity of peritoneal fibrosis and the development of EPS. As both FWT and SPFT are dependent on the osmotic gradient, situations that influence this gradient in an early phase of PD, like inflammation and endothelial-to-mesenchymal transition of mesothelial cells (45), interpretation of the results will be difficult. However the development of peritoneal fibrosis takes some years, meaning that the predictive value of FWT may only become evident after more than 2 years of dialysis.
Two tests can be used in clinical practice. The Double Mini-PET is simple, but requires 2 tests (46), the Modified PET with temporary drainage after 60 min and final drainage after 240 min provides a combination for assessment of FWT and solute transport (47). This test has later been called the Two-in-one protocol of the modified PET (26). These tests are simple to perform and do not generate additional costs.
Disclosures
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Nolph KD, Hano JE, Teschan PE. Peritoneal sodium transport during hypertonic peritoneal dialysis. Ann Intern Med 1969; 70:931–41. [DOI] [PubMed] [Google Scholar]
- 2. Nolph KD, Twardowsky ZJ, Popovich RP, Rubin J. Equilibration of peritoneal dialysis solutions during long-dwell exchanges. J Lab Clin Med 1979; 93:246–56. [PubMed] [Google Scholar]
- 3. Twardowsky ZJ, Nolph KD, Khanna R, Prowant BF, Ryan LR, Moore HL, et al. Peritoneal equilibration test. Perit Dial Bull 1987; 7:138–47. [Google Scholar]
- 4. Rippe B, Stelin G. Simulations of peritoneal solute transport during CAPD. Application of two pore formalism. Kidney Int 1989;35:1234-44. [DOI] [PubMed] [Google Scholar]
- 5. Stelin G, Rippe B. A phenomenological interpretation of the variation in dialysate volume with dwell time in CAPD. Kidney Int 1990; 38:465–72. [DOI] [PubMed] [Google Scholar]
- 6. Rippe B, Stelin G, Haraldsson B. Computer simulations of peritoneal fluid transport in CAPD. Kidney Int 1991;40: 315–25. [DOI] [PubMed] [Google Scholar]
- 7. Rippe B, Venturoli D, Simonsen O, DeArtega J. Fluid and electrolyte trtansport across the peritoneal membrane during CAPD according to the three-pore model. Perit Dial Int 2004; 24:10–27. [PubMed] [Google Scholar]
- 8. Agre P, Saboori AM, Asimos A, Smith BL. Purification and partial characterizatioin of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem 1987; 262:17497–503. [PubMed] [Google Scholar]
- 9. Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem 1988; 263:15634–42. [PubMed] [Google Scholar]
- 10. Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, et al. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol 1993; 265: F463–76. [DOI] [PubMed] [Google Scholar]
- 11. Pannekeet MM, Mulder JB, Weening JJ, Struijk DG, Zweers MM, Krediet RT. Demonstration of aquaporin-CHIP in peritoneal tissue of uremic and CAPD patients. Perit Dial Int 1996; 16(Suppl 1):S54–7. [PubMed] [Google Scholar]
- 12. Devuyst O, Nielsen S, Cosyns J-P, Smith BL, Agre P, Squifflet JP, et al. Aquaporin-1 and endothelial nitric oxide synthase expression in capillary endothelia of human peritoneum. Am J Physiol 1998; 275:H234–42. [DOI] [PubMed] [Google Scholar]
- 13. Schoenicke G, Diamant R, Donner A, Roehrborn A, Grabensee B, Plum J. Histochemical distribution and expression of aquaporin-1 in the peritoneum of patients undergoing peritoneal dialysis: relation to peritoneal transport. Am J Kidney Dis 2004; 44:146–54. [DOI] [PubMed] [Google Scholar]
- 14. Ni J, Verbavatz J-M, Rippe A, Boisde I, Moulin P, Rippe B, et al. Aquaporin-1 plays an essential role in water permeability and ultrafiltration during peritoneal dialysis. Kidney Int 2006; 69:1518–25. [DOI] [PubMed] [Google Scholar]
- 15. Carlsson O, Nielsen S, Zakaria ER, Rippe B. In vivo inhibition of transcellular water channels (aquaporin-1) during acute peritoneal dialyisis in rats. Am J Physiol 1996; 271:H2254–62. [DOI] [PubMed] [Google Scholar]
- 16. Zweers MM, Douma CE, DeWaart DR, Korevaar J, Krediet RT, Struijk DG. Amphotericin B, mercury chloride and peritoneal transport in rabbits. Clin Nephrol 2001; 56:60–8. [PubMed] [Google Scholar]
- 17. Monquil MCJ, Imholz ALT, Struijk DG, Krediet RT. The contribution of transcellular water transport in net ultrafiltration failure during CAPD. Perit Dial Int 1995; 15:42–8. [PubMed] [Google Scholar]
- 18. Goffin E, Combet S, Jamar F, Cosyns J-P, Devuyst O. Expression of aquaporin-1 in a long-term peritoneal dialysis patient with impaired transcellular water transport. Am J Kidney Dis 1999; 33:383–8. [DOI] [PubMed] [Google Scholar]
- 19. Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int 2000; 20(Suppl 4):S22–42. [PubMed] [Google Scholar]
- 20. Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, et al. Evaluation and management of ultrafiltration problems in peritoneal dialysis. Perit Dial Int 2000; 20(Suppl 4):S5–21. [PubMed] [Google Scholar]
- 21. Smit W, Schouten N, Van den Berg N, Langedijk MJ, Struijk DG, Krediet RT. Analysis of the prevalence and causes of ultrafiltration failure during long-term peritoneal dialysis; a cross-sectional study. Perit Dial Int 2004; 24:562–70. [PubMed] [Google Scholar]
- 22. Smit W, Parikova A, Krediet RT. Ultrafiltration failure in peritoneal dialysis; causes and consequences. Minerva Urol Nephrol 2005; 51:165–7. [PubMed] [Google Scholar]
- 23. Smit W, Struijk DG, Ho-dac-Pannekeet MM, Krediet RT. Quantification of free water transport in peritoneal dialysis. Kidney Int 2004; 66:849–54. [DOI] [PubMed] [Google Scholar]
- 24. La Milia V, Di Fillipo S, Crepaldi M, Dell Vecchio L, DellÓro C, Andrulli S, et al. Mini-peritoneal equilibration test: a simple and fast method to assess free water transport and small solute transport across the peritoneal membrane. Kidney Int 2005; 68:840–6. [DOI] [PubMed] [Google Scholar]
- 25. Parikova A, Smit W, Struijk DG, Zweers MM, Krediet RT. The contribution of free water transport and small pore transport to the total fluid removal in peritoneal dialysis. Kidney Int 2005; 68:1849–56. [DOI] [PubMed] [Google Scholar]
- 26. Bernardo AP, Bajo MA, Santos O, Del Peso G, Carvalho MJ, Cabrita A, et al. Two-in-one protocol: simultaneous small-pore and ultrasmall-pore peritoneal transport quantification. Perit Dial Int 2012; 32:537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rigby RJ, Howley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 1998; 13:154–9. [DOI] [PubMed] [Google Scholar]
- 28. Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CWN, Zietse R, et al. Risk factors associated with increased incidence of encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 2011; 31:269–78. [DOI] [PubMed] [Google Scholar]
- 29. Krediet RT, Boeschoten EW, Koomen GCM, Stouthart JML, Hoek FJ, Arisz L. The time course of peritoneal dialysis patients who develop sclerosing peritonitis. Am J Kidney Dis 1989; 13:299–307. [DOI] [PubMed] [Google Scholar]
- 30. Hendriks PMEM, Ho-dac-Pannekeet, Van Gulik TM, Struijk DG, Phoa SSKS, Sie L, et al. Peritoneal sclerosis in chronic dialysis patients: analysis of clinical presentation, risk factors and peritoneal transport kinetics. Perit Dial Int 1997; 17:136–43. [PubMed] [Google Scholar]
- 31. Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ. The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int 2010; 78:611–8. [DOI] [PubMed] [Google Scholar]
- 32. Sampimon DE, Coester AM, Struijk DG, Krediet RT. The time course of peritoneal transport parameters in peritoneal dialysis patients who develop encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2011; 26:291–8. [DOI] [PubMed] [Google Scholar]
- 33. Sampimon DE, Lopes Barreto D, Coester AM, Struijk DG, Krediet RT. The value of osmotic conductance and free water transport in the prediction of encapsulating peritoneal sclerosis. Adv Perit Dial 2014; 30:21–6. [PubMed] [Google Scholar]
- 34. Morelle J, Sow A, Hautem N, Bouzin C, Crott R, Devuyst O, et al. Interstitial fibrosis restricts osmotic water transport in encapsulating peritoneal sclerosis. J Am Soc Nephrol 2015; 26:2521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopes Barreto D, Coester AM, Struijk DG, Krediet RT. Can effluent matrix metalloproteinase-2 and plasminogen activator inhibitor-1 be used as biomarkers of peritoneal alterations in peritoneal dialysis patients? Perit Dial Int 2013; 33:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coester AM, Smit W, Struijk DG, Parikova A, Krediet RT. Longitudinal analysis of peritoneal fluid transport and its determinants in a cohort of incident peritoneal dialysis patients. Perit Dial Int 2014; 34:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Esch S, Struijk DG, Krediet RT. The natural time-course of membrane alterations during peritoneal dialysis is partly altered by peritonitis. Perit Dial Int, accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yung S, Chan TM. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: the role of mesothelial cells. Mediators Inflamm 2012; 2012:484167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Diepen ATN, Van Esch S, Struijk DG, Krediet RT. The first peritonitis episode alters the natural course of peritoneal membrane characteristics in peritoneal dialysis patients. Perit Dial Int 2015; 35:324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Arteaga J, Ledesma F, Garay G, Chiurchiu C, de la Fuente J, Douthat W, et al. High-dose steroid treatment increases free water transport in peritoneal dialysis patients. Nephrol Dial Transplant 2011; 26:4142–5. [DOI] [PubMed] [Google Scholar]
- 41. Rippe B, Venturoli D. Simulations of osmotic ultrafiltration failure in CAPD using a serial three-pore membrane/fiber matrix model. Am J Physiol Renal Physiol 2007; 292:F1035–43. [DOI] [PubMed] [Google Scholar]
- 42. Fullerton GD, Amurao MR. Evidence that collagen and tendon have monolayer water coverage in the native state. Cell Biol Int 2006; 30:56–65. [DOI] [PubMed] [Google Scholar]
- 43. Toroian D, Lim JE, Price PA. The size exclusion characteristics of type 1 collagen. J Biol Chem 2007; 31:22437–47. [DOI] [PubMed] [Google Scholar]
- 44. Tilze J. Water-free Na+ retention: interaction with hypertension and tissue hydration. Blood Purif 2008; 26:95–9. [DOI] [PubMed] [Google Scholar]
- 45. La Milia V, Limardo M, Virga G, Crepaldi M, Locatelli F. Simultaneus measurement of peritoneal glucose and free water osmotic conductances. Kidney Int 2007; 72:643–50. [DOI] [PubMed] [Google Scholar]
- 46. Cnossen TT, Smit W, Konings CJAM, Kooman JP, Leunissen KM, Krediet RT. Quantification of free water transport during the peritoneal equilibration test. Perit Dial Int 2009; 29:523–7. [PubMed] [Google Scholar]
- 47. La Milia V, Limardo M, Virga G, Crepaldi M, Locatelli F. Simultaneous measurement of peritoneal glucose and free water osmotic conductances. Kidney Int 2007; 72:643–50. [DOI] [PubMed] [Google Scholar]