Abstract
♦ Introduction:
Chronic exposure to conventional peritoneal dialysis (PD) solutions has been related to peritoneal function alterations in PD patients, and associated with mesothelial cell loss, submesothelial fibrosis, vasculopathy, and angiogenesis. In vitro and ex vivo analyses, as well as studies with animal models, have demonstrated that biocompatible PD solutions attenuate these morphological alterations. Our aim was to confirm the morphological benefits of biocompatible solutions in PD patients.
♦ Methods:
We analyzed biopsies from 23 patients treated with biocompatible solutions (study group, SG), and compared them with a control group (n = 23) treated with conventional solutions (CG), matched for time on PD.
♦ Results:
A total of 56.5% of SG patients showed total or partial preservation of mesothelial cells monolayer, in contrast with 26.1% of patients in CG (p = 0.036). Peritoneal fibrosis was not significantly less frequent in SG patients (47.8% SG vs 69.6% CG; p = 0.13). In patients without previous peritonitis, a significantly lower prevalence of fibrosis was present in SG patients (41.7% SG vs 77.8% CG; p = 0.04). Hyalinizing vasculopathy (HV) was significantly lower in SG (4.3% SG vs 30.4% CG; p = 0.02). Cytokeratin-positive fibroblast-like cells were detected in 10 patients (22%), but the prevalence was not significantly lower in SG. In the univariate regression analysis, the use of biocompatible solutions was associated with mesothelial monolayer integrity (p = 0.04) and an absence of vasculopathy (p = 0.04).
♦ Conclusion:
The present study demonstrates in vivo in human biopsies that biocompatible solutions are better tolerated by the peritoneum in the medium and long term than conventional solutions.
Keywords: Peritoneal biopsy, biocompatible dialysis solutions, mesothelial cell integrity, hyalinizing vasculopathy, submesothelial fibrosis
Peritoneal membrane failure is among the main limitations for long-term peritoneal dialysis (PD). Chronic exposure to conventional PD solutions and peritoneal inflammation have been described as the main factors associated with peritoneal function alterations in PD patients (1). Loss of ultrafiltration has been related to peritoneal morphological changes (mesothelial cell loss, submesothelial fibrosis, vasculopathy and angiogenesis) (2), although the exact morpho-functional correlation has not yet been established. It is expected that some peritoneal alterations could be prevented or delayed with more biocompatible PD solutions. Indeed, the use of these newer solutions has been associated with some clinical benefits, such as delayed onset of anuria (3). However, there are conflicting results regarding their favorable effects on peritonitis rates (4), patient and technical survival, residual renal function, and peritoneal membrane function. In vitro and ex vivo analyses, as well as experimental animal studies, have demonstrated that biocompatible PD solutions attenuate some of the morphological abnormalities seen with conventional solutions (5–7). Due to the difficulties in obtaining peritoneal biopsies in clinical practice, we still lack confirmatory in vivo morphological evidence of the possible benefits of biocompatible solutions. The histological information regarding peritoneal morphological changes induced by this type of fluid in humans is scarce (8–10). In the present study, we analyzed biopsies from 23 patients treated for variable periods of time with biocompatible solutions, and we compared the morphological findings with a control group treated with conventional solutions matched for time on PD.
Patients and Methods
Study Design and Patients
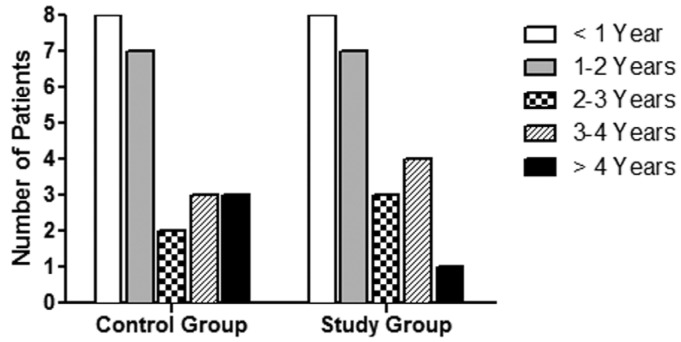
A comparative case-control matched study of biopsies from 46 PD patients was performed; 23 of them were treated with conventional solutions (control group, CG) and 23 with biocompatible solutions (study group, SG). We analyzed all available biocompatible peritoneal biopsies collected up to September 2013 in 4 centers, and we compared them with peritoneal biopsies from patients under conventional solutions in the same time period, matched for time on PD (mean 24.2 ± 18 months in CG vs 22.7 ± 16 months in SG, p = 0.8) (Figure 1). All SG patients used biocompatible solutions from the beginning of PD. No biopsy was taken during an inflammatory episode or during the following 30 days. In 78% of cases (17 patients in CG, 19 in SG), the peritoneal biopsy was performed during kidney transplant surgery. In the remaining 22%, the biopsy was obtained during incidental abdominal programmed surgery (in CG: 3 nephrectomies, 1 omentectomy, 1 cholecystectomy, and 1 necropsy; in SG: 2 catheter removals not related to peritoneal problems, 1 omentectomy, and 1 polypeptomy).
Figure 1 —
Distribution of patients according to time on PD, expressed in years, in both groups.
Clinical characteristics are shown in Table 1. The causes of end-stage renal disease were not significantly different between the 2 groups (data not shown). Age, gender, and diabetes prevalence were similar in both groups (Table 1). Patients in SG used automated PD (APD) less frequently than CG patients (43.5% SG vs 91% CG, p = 0.001). In CG, 5 patients suffered 8 episodes of peritonitis whereas in SG, 11 patients had 23 episodes.
TABLE 1.
Patient Characteristics at the Time of Peritoneal Biopsy
Subgroup Analysis
We separately performed an analysis according to diabetes and peritonitis episodes, designating 3 subsets of patients:
Patients with no peritonitis episodes (n = 30) (12 SG, 18 CG)
Patients with previous peritonitis episodes (n = 16) (11 SG, 5 CG)
Non-diabetic patients (n = 41) (20 SG, 21 CG)
PD Solutions
In SG, 7 patients used Physioneal (Baxter Healthcare Corporation, Deerfield, IL, USA), 5 Balance (Fresenius Medical Care, Bad Homburg, Germany), 6 BicaVera (Fresenius Medical Care, Bad Homburg, Germany) and 5 Selutrio (Fresenius Medical Care, Bad Homburg, Germany). In CG, 20 used Dianeal (Baxter Healthcare Corporation, Deerfield, IL, USA) and 3 stay•safe (Fresenius Medical Care, Bad Homburg, Germany). Icodextrin (Baxter Healthcare Corporation, Deerfield, IL, USA) was used by 14 patients (61%) in CG and by 5 patients (22%) in SG (p = 0.007).
Peritoneal Samples
Parietal peritoneal samples were obtained after opening the anterior abdominal wall. Biopsy collection and processing was done as previously reported (12). Samples were evaluated by 2 pathologists without knowledge of clinical data. The morphological parameters analyzed were:
Mesothelial cell integrity. This was measured using a semi-quantitative scale (grade 3 = normal cell density; grade 0 = complete denudation), as described by Plum et al. (13).
Submesothelial thickness. The thickness of the compact zone was measured with a micrometer ocular. The mean of 3 different measures of representative zones was obtained. A measurement of less than 150 μm was considered normal (Grade 1). A measurement of 150 – 350 μm was considered as a moderate thickening (Grade 2). Results greater than 350 μm were regarded as intense thickening (Grade 3). Fibrosis was defined as a submesothelial compact zone measuring more than 150 μm (the highest value recorded for normal subjects) (10).
Hyalinizing vasculopathy (HV). This was measured using the 4-grade system described by Honda et al. (14): grade 0 = no abnormalities; grade 1 = mild thickening without stenosis of the lumen; grade 2 = moderate thickening with partial luminal stenosis; and grade 3 = intense thickening with marked stenosis and luminal distortion or complete occlusion.
In vivo evidence of epithelial-to-mesenchymal transition (EMT) (defined by the presence of submesothelial fibroblast-like cells expressing cytokeratin) was also evaluated as present or absent.
Ethical Issues
The procedures were in accordance with the ethical standards of the institutional committee on human experimentation and with the Helsinki Declaration of 1975 (revised in 1983). All patients gave their informed consent.
Statistical Analysis
Results are given as means ± standard deviation (SD). A p value < 0.05 was considered statistically significant. We used the non-parametric Mann–Whitney U-test for comparisons of means between groups and Fischer's exact test for comparisons of proportions. Univariate and multivariate logistic regression analyses were employed to investigate the factors associated with the presence of mesothelial cell integrity, submesothelial fibrosis, HV, and EMT on peritoneal biopsy. All statistical analyses were done using SPSS 14.5 (Chicago, IL, USA).
Results
Mesothelial Cell Integrity
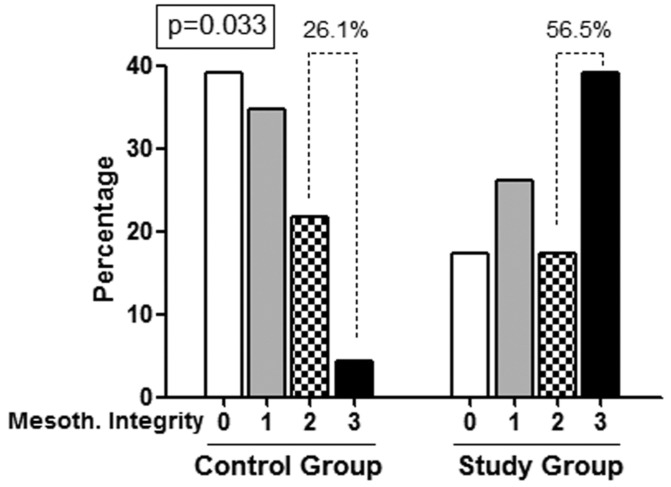
Patients in SG had a significantly greater mesothelial cell preservation score than those in CG (mean values 1.78 ± 1.16 vs 0.91 ± 0.9, p = 0.007). In SG, 56.5% of patients showed total or partial preservation of mesothelial cells (score 2 – 3) in contrast with 26.1% of patients in CG (p = 0.036) (Figure 2).
Figure 2 —
Frequency of patients with different mesothelial preservation score in the 2 groups. Patients using biocompatible solutions presented high scores of mesothelial layer preservation much more frequently.
To rule out a negative effect of prior inflammation on mesothelial preservation, patients without previous peritonitis were examined. These patients under biocompatible solutions had scores of 2 – 3 more frequently than CG patients (67% SG vs 28% CG, p = 0.035). However, patients with peritonitis antecedents (n = 16; 11 SG and 5 CG) showed non-significant differences (45.5% SG vs 20% CG, p = 0.33), probably due to the low number of patients.
Non-diabetic patients, who represent the majority of the series, also showed significantly better preservation of mesothelial layer among SG (60%) than CG (29%) (p = 0.043).
Submesothelial Thickness
Peritoneal fibrosis was less frequent in biocompatible solution patients, although it did not reach statistical significance (47.8% SG vs 69.6% CG, p = 0.13). The average score confirmed the lack of significant differences (1.09 ± 1.1 SG vs 1.7 ± 0.97 CG, p = 0.06). Extreme values of submesothelial thickness (> 350 μm) were less common in SG (8.7% vs 17.4%), but again differences were not significant.
However, when patients with previous peritonitis were excluded, a significant lower prevalence of fibrosis was present in SG patients (41.7% SG vs 77.8% CG) (p = 0.04). In contrast, these differences did not appear between both groups in patients with previous peritonitis (p = 0.59) and in non-diabetic patients (p = 0.28).
Hyalinizing Vasculopathy
The prevalence of HV was significantly lower in the group treated with biocompatible solutions (4.3% SG vs 30.4% CG, p = 0.02). The average score was also lower in SG (0.09 ± 0.41 SG vs 0.48 ± 0.8 CG, p = 0.05). When patients with peritonitis were removed, no patients from SG showed HV, in contrast with 33.3% from CG (p = 0.025). Patients with prior peritonitis showed similar HV prevalence with both types of solution (p = 0.54) (Figure 3).
Figure 3 —
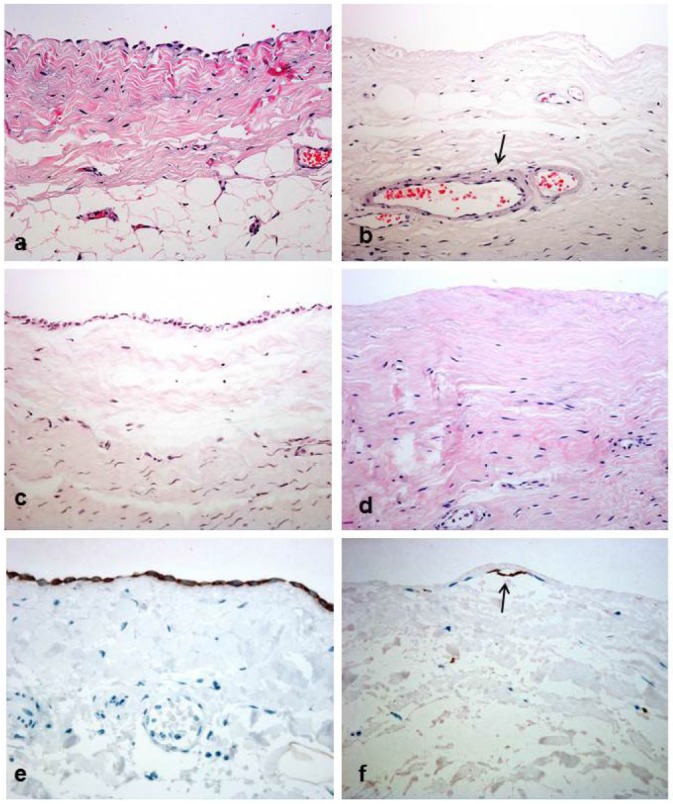
Peritoneal biopsies from patients receiving biocompatible solutions (a,c,e) showed better mesothelial cell preservation, less submesothelial thickness and hyalinizing vasculopathy when compared with patients treated with conventional fluids (b,d,f). Grade 1 hyalinizing vasculopathy lesions are seen on image b (arrow). A clear contrast among mesothelial cell preservation is evident on all images (a,b,c,d: hematoxylin and eosin, ×200). Immunohistochemistry for cytokeratins reveals a modified, superficial mesothelial cell (arrow) that contrasts with the well preserved layer seen on a biocompatible patient (e,f: immunoperoxidase, ×400).
Among non-diabetic patients, HV also showed a lower prevalence with biocompatible solutions (5% SG vs 28.6% CG, p = 0.045).
Epithelial-to-Mesenchymal Transition (Submesothelial Cytokeratin Expression)
Evidence of EMT was present in 10 patients (22%), with a lower prevalence in SG, but this did not reach statistical significance, probably due to the low number of patients (13% SG vs 30.4% CG, p = 0.15). Interestingly, when EMT was present, mesothelial layer integrity (scores 2 – 3) was never present (for both solutions). The incidence of early EMT (less than 2 years on PD) did not reach a statistically significant difference between the 2 groups (13.3 % SG showed EMT in contrast with 33.3% from CG, p = 0.19).
We also observed a higher prevalence of EMT, although not statistically significant, in patients with previous peritonitis in both groups (data not shown). Among non-diabetics, a lower but also non-significant prevalence of EMT in SG was present (p = 0.3).
Logistic Regression Analysis
In the univariate regression analysis, the use of biocompatible solutions was associated with the presence of mesothelial cell integrity (odds ratio 3.68; confidence interval 1.06 – 12.7; p = 0.04), and continued to be significant after adjusting for age, time on PD, and diabetes in the multivariate analysis. The significance was lost when accumulated glucose load and accumulated days of peritonitis were added to the model.
In the univariate logistic regression analysis, the use of conventional solutions was the only factor that predicted the presence of HV (odds ratio 9.62; confidence interval 1.07 – 86.17; p = 0.04). In the multivariate analysis, its significance was lost after adjusting for glucose load.
Discussion
This study represents the first evidence in vivo of the benefit of biocompatible solutions on peritoneal morphology in a short series of non-selected PD patients. Peritoneal biopsies from patients on PD with biocompatible solutions showed better mesothelial cell preservation and lower prevalence of hyalinizing vasculopathy when compared with biopsies from patients treated with conventional solutions during a similar period of time. In addition, when patients with prior peritonitis episodes were excluded, we also found a lower induction of submesothelial fibrosis with biocompatible PD fluids. Improvement of these morphological variables (mesothelial integrity, fibrosis, and vasculopathy) demonstrates better peritoneal protection with the new PD solutions.
These in vivo findings on PD patients confirm previous in vitro and ex vivo studies and experiments in animal models showing that biocompatible solutions result in better viability of the peritoneal membrane (6–8,15–18), particularly of mesothelial cells. Bajo et al. (6) described that in vitro exposure of mesothelial cells to conventional fluids resulted in a non-epithelioid shape and downregulation of E-cadherin, indicative of EMT, and in a strong induction of vascular endothelial growth factor (VEGF) expression. In contrast, cells in vitro exposed to low-GDP solution did not develop these phenotype changes. They also observed ex vivo that the prevalence of a non-epithelioid phenotype in the conventional group was significantly higher with increasing PD duration but, in contrast, mesothelial cells from PD effluent of patients treated with low-GDP fluids had fewer signs of EMT. Fernández-Perpén et al. (18) also showed that ex vivo mesothelial cells grown from effluent of patients treated with bicarbonate/low-GDP fluid showed a trend to acquire an epithelial phenotype, with lower production of pro-inflammatory cytokines and chemokines than those from patients treated with a lactate-buffered conventional PD solution. These 2 studies were released by the same group of investigators and are complementary, but very consistent. Other studies describing the anatomical alterations with biocompatible solutions are scant. In 2005, Do et al. (8) reported less ex vivo EMT induction and faster remesothelialization with low-GDP fluids in a randomized prospective controlled study in 60 PD patients, when compared with high-GDP solutions. Ayuzawa et al. (9) showed less peritoneal fibrosis and vasculopathy with the use of hybrid therapy, combining PD with biocompatible solutions and hemodialysis, in patients on PD longer than 3 years. More recently, Kawanishi et al. (10) described 12 patients treated with low-GDP neutral solutions who had less peritoneal fibrosis, vascular sclerosis, and advanced glycation end-product accumulation, but an increased blood capillary density, when compared with 12 patients treated with acidic high-GDP solutions. However, the low number of patients in the study and the fact that peritoneal specimens were obtained during catheter removal, probably due to peritoneal membrane problems, make further studies necessary.
Animal studies have also shown attenuation of peritoneal fibrosis and vascularization when biocompatible solutions are used. Hekking et al. (19) reported, for the first time, better preservation of peritoneal morphology in rats after long-term exposure to a bicarbonate/lactate-buffered solution compared with a standard solution. This study showed better preservation of the mesothelial layer and a tendency to lower matrix thickness in the biocompatible group. More recently, Mortier et al. (20) reported that low-GDP bicarbonate/lactate-buffered fluids showed greater peritoneal integrity in rats when compared with conventional PD fluids.
Another observation from our study is that these morphological benefits may be neutralized by repeated episodes of peritonitis. When patients with peritonitis are removed from the comparative study, all the morphological variables, including fibrosis, show significant differences. It seems plausible that peritoneal infections causing inflammation modify the peritoneal response to PD fluids, masking their potential benefits. In our series, the biocompatible group had higher peritonitis prevalence and more accumulated days of peritoneal inflammation. Differences in peritonitis may be partially related to the higher proportion of patients treated with continuous ambulatory PD in the biocompatible group. Continuous ambulatory PD implies more manual exchanges and a greater risk of peritoneal infection. However, despite a greater number of peritonitis episodes, patients treated with biocompatible solutions showed less peritoneal damage. We found no peritoneal morphological differences among APD and continuous ambulatory PD patients.
Epithelial-to-mesenchymal transition of mesothelial cells (conversion into myofibroblasts) has emerged as an important contributor to peritoneal damage (21). Part of the protective effect of biocompatible solutions depends on its greater mesothelial preservation and therefore attenuation of EMT. In our study, EMT evidence was more prevalent (3 times) among patients using conventional solutions, although with a limited significance (probably due to the low number of patients, the low sensitivity of EMT markers in tissue, and the potential confounding factor represented by peritonitis).
Other limitations of the study concern the matching procedure. Control group subjects were younger than SG subjects and used APD more frequently; these 2 factors are intrinsically associated, since younger patients use APD more often. The case-control design according to time on PD permits the comparisons at equivalent PD duration, a well-recognized factor implicated in the morphological alterations of peritoneal membrane in the medium to long term. The main strength of our study is the large number of peritoneal biopsy samples collected, despite the difficulties in obtaining them.
In conclusion, the present study offers in vivo evidence in human biopsies that biocompatible solutions are better tolerated by the peritoneum in the medium and long term than conventional solutions. As a result, we suggest that PD should be offered with biocompatible solutions.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
This work was supported by grant SAF2010-21249 from the “Ministerio de Economia y Competitividad” to ML-C, by grant S2010/BMD-2321 from “Comunidad Autónoma de Madrid” to ML-C and RS, by grants from “Fondo de Investigaciones Sanitarias” (PI 09/0641, 12/0204) to RS and PI 14/01035 to MAB, and from REDinREN (RETICS 12/0021, Fondos FEDER, EU) to RS. This work was also partially supported by Fresenius Medical Care and Baxter Healthcare Corporation (The Baxter Extramural Grant Program 2007).
REFERENCES
- 1. Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int 2000; 20:S22–42. [PubMed] [Google Scholar]
- 2. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9. [DOI] [PubMed] [Google Scholar]
- 3. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23 (6):1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho Y, Badve SV, Hawley CM, McDonald SP, Brown FG, Boudville N, et al. Association of biocompatible peritoneal dialysis solutions with peritonitis risk, treatment, and outcomes. Clin J Am Soc Nephrol 2013; 8(9):1556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim CD, Kwon HM, Park SH, Oh EJ, Kim MH, Choi SY, et al. Effects of low glucose degradation products peritoneal dialysis fluid on the peritoneal fibrosis and vascularization in a chronic rat model. Ther Apher Dial 2007; 11(1):56–64. [DOI] [PubMed] [Google Scholar]
- 6. Bajo MA, Pérez-Lozano ML, Albar-Vizcaino P, del Peso G, Castro MJ, Gonzalez-Mateo G, et al. Low-GDP peritoneal dialysis fluid (“balance”) has less impact in vitro and ex vivo on epithelial-to-mesenchymal transition (EMT) of mesothelial cells than a standard fluid. Nephrol Dial Transplant 2011; 26:282–91. [DOI] [PubMed] [Google Scholar]
- 7. Aroeira LS, Lara-Pezzi E, Loureiro J, Aguilera A, Ramírez-Huesca M, González-Mateo G, et al. Cyclooxygenase-2 mediates dialysate-induced alterations of the peritoneal membrane. J Am Soc Nephrol 2009; 20:582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Do JY, Kim YL, Park JW, Cho KH, Kim TW, Yoon KW, et al. The effect of low glucose degradation product dialysis solution on epithelial-to-mesenchymal transition in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 2005; 25(Suppl 3):S22–5. [PubMed] [Google Scholar]
- 9. Ayuzawa N, Ishibashi Y, Takazawa Y, Kume H, Fujita T. Peritoneal morphology after long-term peritoneal dialysis with biocompatible fluid: recent clinical practice in Japan. Perit Dial Int 2012; 32:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawanishi K, Honda K, Tsukada M, Oda H, Nitta K. Neutral solution low in glucose degradation products is associated with less peritoneal fibrosis and vascular sclerosis in patients receiving peritoneal dialysis. Perit Dial Int 2013; 33(3):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Selgas R, Fernandez-Reyes MJ, Bosque E, Bajo MA, Borrego F, Jiménez C, et al. Functional longevity of the human peritoneum: how long is continuous peritoneal dialysis possible? Results of a prospective medium long–term study. Am J Kidney Dis 1994; 23:64–73. [DOI] [PubMed] [Google Scholar]
- 12. Del Peso G, Jiménez-Heffernan JA, Bajo MA, Aroeira LS, Aguilera A, Fernández-Perpén A, et al. Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney Int Suppl 2008; 108:S26–33. [DOI] [PubMed] [Google Scholar]
- 13. Plum J, Hermann S, Fusshöller A, Schoenicke G, Donner A, Röhrborn A, et al. Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int 2001; 59(Suppl 78):42–7. [DOI] [PubMed] [Google Scholar]
- 14. Honda K, Nitta K, Horita S, Yumura W, Nihei H, Nagai R, et al. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol Dial Transplant 1999; 14:1541–9. [DOI] [PubMed] [Google Scholar]
- 15. Witowski J, Korybalska K, Ksiazek K, Wisniewska-Elnur J, Jörres A, Lage C, et al. Peritoneal dialysis with solutions low in glucose degradation products is associated with improved biocompatibility profile towards peritoneal mesothelial cells. Nephrol Dial Transplant 2004; 19:917–24. [DOI] [PubMed] [Google Scholar]
- 16. Jones S, Holmes CJ, Krediet RT, Mackenzie R, Faict D, Tranaeus A, et al. Bicarbonate/Lactate Study Group. Bicarbonate/lactate-based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney Int 2001; 59:1529–38. [DOI] [PubMed] [Google Scholar]
- 17. Rippe B, Simonsen O, Heimbürger O, Christensson A, Haraldsson B, Stelin G, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59:348–57. [DOI] [PubMed] [Google Scholar]
- 18. Fernández-Perpén A, Pérez-Lozano ML, Bajo MA, Albar-Vizcaino P, Sandoval Correa P, del Peso G, et al. Influence of bicarbonate/low-GDP peritoneal dialysis fluid (BicaVera) on in vitro and ex vivo epithelial-to-mesenchymal transition of mesothelial cells. Perit Dial Int 2012; 32:292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hekking LH, Zareie M, Driesprong BA, Faict D, Welten AG, de Greeuw I, et al. Better preservation of peritoneal morphologic features and defense in rats after long-term exposure to a bicarbonate/lactate-buffered solution. J Am Soc Nephrol 2001; 12(12):2775–86. [DOI] [PubMed] [Google Scholar]
- 20. Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int 2004; 66:1257–65. [DOI] [PubMed] [Google Scholar]
- 21. Loureiro J, Aguilera A, Selgas R, Sandoval P, Albar-Vizcaíno P, Pérez-Lozano ML, et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol 2011; 22(9):1682–95. [DOI] [PMC free article] [PubMed] [Google Scholar]