Abstract
♦ Background:
In peritoneal dialysis (PD) patients, one of the major risk factors for cardiovascular disease is lipid abnormalities. This study was designed to investigate the effects of ginger supplementation on serum lipids and lipoproteins in PD patients.
♦ Methods:
In this randomized, double-blind, placebo-controlled trial, 36 PD patients were randomly assigned to either the ginger or the placebo group. The patients in the ginger group received 1,000 mg ginger daily for 10 weeks, while the placebo group received corresponding placebos. At baseline and at the end of week 10, 7 mL of blood were obtained from each patient after a 12- to 14-hour fast, and serum concentrations of triglyceride, total cholesterol, low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), and lipoprotein (a) [Lp (a)] were measured.
♦ Results:
Serum triglyceride concentration decreased significantly up to 15% in the ginger group at the end of week 10 compared with baseline (p < 0.01), and the reduction was significant in comparison with the placebo group (p < 0.05). There were no significant differences between the 2 groups in mean changes of serum total cholesterol, LDL-C, HDL-C, and Lp (a).
♦ Conclusion:
This study indicates that daily administration of 1,000 mg ginger reduces serum triglyceride concentration, which is a risk factor for cardiovascular disease, in PD patients.
Keywords: Ginger, peritoneal dialysis, lipids, lipoproteins, cardiovascular disease
The most important cause of mortality in patients with chronic renal failure, including peritoneal dialysis (PD) patients, is cardiovascular disease (CVD). A marked increase in CVD incidence and death rates has been reported in patients on dialysis compared with an age-matched general population (1,2). In PD patients, one of the major risk factors for CVD is lipid abnormalities, including high serum concentrations of triglyceride, total cholesterol, low density lipoprotein-cholesterol (LDL-C), and lipoprotein (a) [Lp (a)]; and low serum concentration of high density lipoprotein-cholesterol (HDL-C) (3–12).
Ginger (Zingiber officinale) is a non-toxic spice with negligible side effects and is generally recognized as safe by the United States Food and Drug Administration (13,14). Some studies have shown that ginger consumption could reduce serum triglyceride, total cholesterol, and LDL-C in patients with diabetes type 2 (15–17). According to the literature, such studies have not been performed on PD patients. Moreover, to date, no research has examined the effect of ginger consumption on serum Lp (a). Therefore, the present study was designed to investigate the effects of ginger supplementation on serum lipids and lipoproteins in PD patients.
Methods
Subjects and Ethical Aspects
The minimum sample size estimation for each group was 18 at a power (1-β) of 80% and α = 0.05 for a 2-arm parallel study with 2-tailed testing to detect a difference of 80 mg/dL (0.9 mmol/L) in serum triglyceride concentration with a pooled standard deviation of 88 mg/dL (0.99 mmol/L), obtained from Arablou et al.'s study (15).
Thirty-eight patients (23 men and 15 women) undergoing continuous ambulatory peritoneal dialysis (CAPD) aged 29 – 79 years were recruited from the Peritoneal Dialysis Unit of Shafa Clinic in Tehran, Iran. Patients enrolled in this study did not have inflammatory diseases, infectious diseases (especially peritonitis), gastrointestinal diseases, thyroid disorders, and none of them received steroidal or nonsteroidal anti-inflammatory drugs, levothyroxine, omega-3 fatty acid supplements, or warfarin. In addition, subjects who had regularly used ginger within 1 month prior to the start of the study were excluded. The study protocol was approved by the Ethics Committee of the National Nutrition and Food Technology Research Institute of Iran. The study was in adherence with the Declaration of Helsinki. Written, informed consent was obtained from all patients before initiating the study. This clinical trial was registered at Iranian Registry of Clinical Trials (IRCT) with number IRCT201312062716N2.
Protocol
This study was a randomized, double-blind, placebo-controlled trial. The patients, after stratification based on diabetes, were randomly allocated to either a ginger or placebo group by blocked randomization. Patients in the ginger group received 1,000 mg ginger as 4 capsules, daily for 10 weeks, while the placebo group received 4 corresponding placebo capsules containing starch. Ginger capsules and corresponding placebos were produced by the Gol Daru Pharmaceutical Company, Esfahan, Iran. Subjects were advised not to change their dietary habits, physical activities, and drug regimens. At baseline and at the end of week 10, 7 mL of blood were obtained from each patient after a 12- to 14-hour fast. Blood samples were kept at room temperature (20 – 25°C) for 20 minutes. After clotting, the samples were centrifuged at 2,000 rpm for 10 minutes. The samples of serum were separated into small aliquots and frozen at −70°C until they were used.
Measurements
Serum concentrations of triglyceride, total cholesterol, HDL-C, creatinine and urea were assessed using various colorimetry methods by commercial kits (Pars Azemoon, Tehran, Iran) with the aid of a Selectra 2 Autoanalyzer (Vital Scientific, Spankeren, The Netherlands). Intra-assay coefficients of variation (CVs) for serum triglyceride, total cholesterol, HDL-C, creatinine, and urea were 3.9%, 1.5%, 4.5%, 5.6%, and 3.7%, respectively. As serum triglyceride concentration in all participating patients was less than 400 mg/dL, serum LDL-C was estimated using the Friedwald equation (18). Serum concentration of interleukin-6 (IL-6) was determined by enzyme-linked immunosorbent assay kits (Diaclone, Besancon, France) with an intra-assay CV of 7.1%.
Patients were weighed at baseline and at the end of weeks 5 and 10. In addition, the dietary intake of subjects was assessed using a 3-day dietary recall (2 weekdays and 1 weekend day) at baseline and at the end of weeks 5 and 10. Patients' diets were analyzed by Nutritionist IV software (N Squared Computing, San Bruno, CA, USA).
Dialysis adequacy (as total Kt/V per week) was determined for each patient based on blood urea concentration, 24-hour urine volume, urine urea concentration, 24-hour dialysate drain volume, dialysate urea concentration, weight, height, and age, using a Kt/V calculator (19). The peritoneal equilibration tests (PET) for glucose, creatinine and urea were performed for each patient based on a 2-L 4.25% dextrose dwell with dialysate samples at 0 and 4 hours and a blood sample during the dwell period. The ratio of dialysate glucose concentration at time 4 to dialysate glucose level at time 0 (D4/D0) was determined, and then the percent of glucose absorbed from the dialysate was calculated based on the 1-D4/D0 formula (20,21). In addition, the ratio of dialysate to plasma creatinine and urea was determined (21).
Compliance
To ascertain the patients' compliance, we provided each patient with a fixed number of capsules and instructions to return the unused capsules at the end of the study. The degree of compliance for each patient was determined according to the number of returned capsules. The compliance of all patients was more than 90 % and no adverse events were reported.
Statistical Analysis
Statistical analysis of data was performed using the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, USA) for Windows version 21.0. A χ2 test was used to compare qualitative variables between the 2 groups. Since all quantitative parameters according to the Kolmogorov-Smirnov test had normal distributions, we used a t-test and paired t-test to compare parameters between and within groups, respectively. Because dietary and anthropometric parameters were measured 3 times during the study, analysis of variance for repeated measurements was used to compare data among these time points. In addition, since there was a significant reduction in dietary energy intake in the ginger group at the end of week 10 in comparison with week 5, we removed the effect of this confounding factor on serum concentrations of biochemical parameters by analysis of variance for repeated measurements and then compared serum concentrations of biochemical parameters between baseline and the end of week 10. The results are expressed as mean ± standard error (SE), and differences were considered statistically significant at p ≤ 0.05.
Results
Of the 38 CAPD patients initially enrolled, 1 subject in the ginger group and 1 patient in the placebo group were withdrawn due to lack of cooperation.
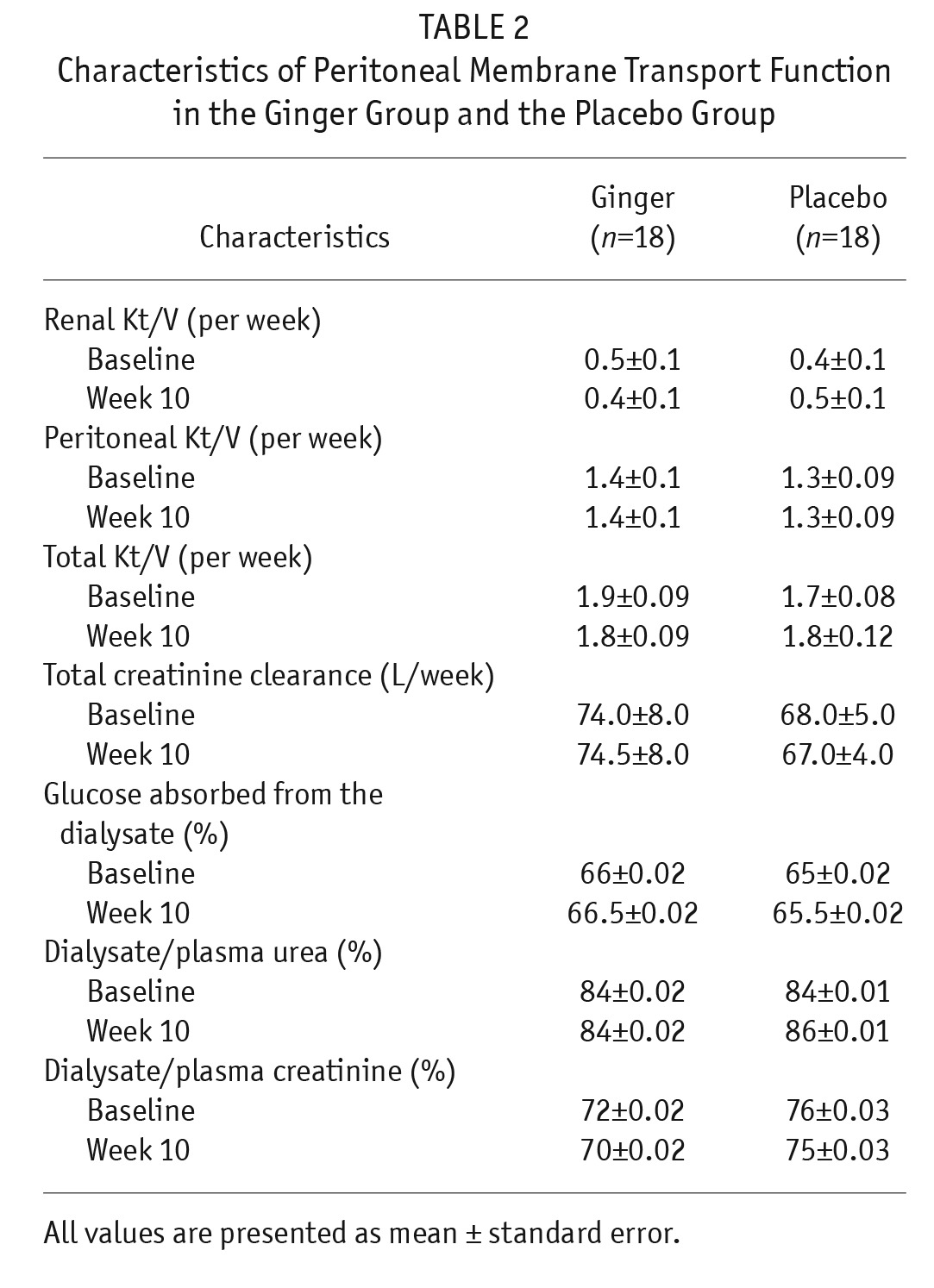
The baseline characteristics of the patients did not differ significantly between the 2 groups (Table 1). In addition, there were no significant differences in characteristics of peritoneal membrane transport function between the 2 groups at baseline and at the end of week 10 (Table 2).
TABLE 1.
Baseline Characteristics of Patients in the Ginger Group and the Placebo Group
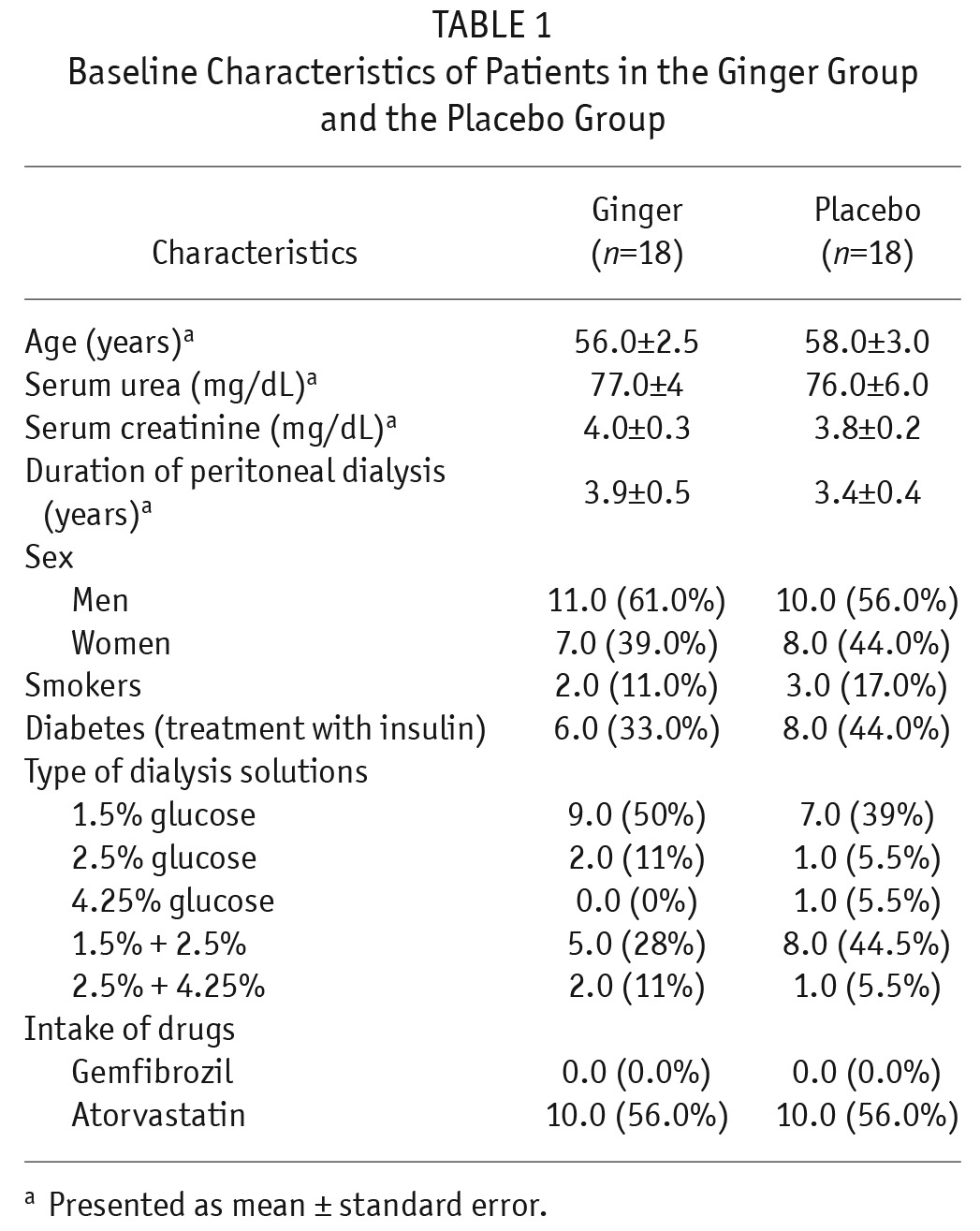
TABLE 2.
Characteristics of Peritoneal Membrane Transport Function in the Ginger Group and the Placebo Group
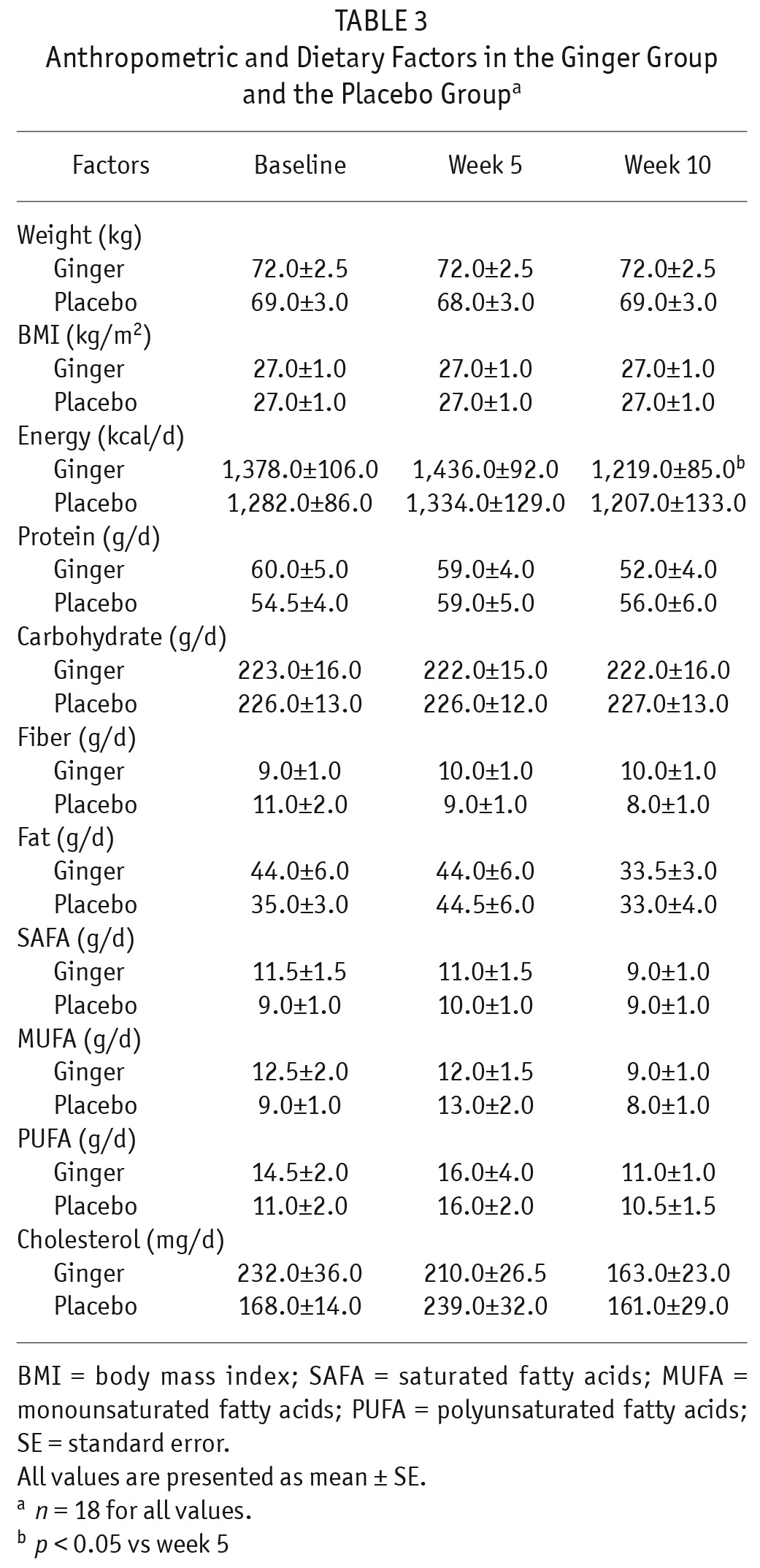
There were no significant differences in mean dietary intake of energy, protein, carbohydrate, fiber, total fat, saturated fatty acids (SAFAs), monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), and cholesterol between the 2 groups at baseline and at the end of weeks 5 and 10. In addition, these factors did not significantly change within each group during the study; in the ginger group only, dietary energy intake reduced significantly at the end of week 10 compared with week 5 (p < 0.05; Table 3).
TABLE 3.
Anthropometric and Dietary Factors in the Ginger Group and the Placebo Groupa
Serum triglyceride concentration was significantly reduced in the ginger group at the end of week 10 compared with baseline (p < 0.01), whereas no statistically significant change was observed in the placebo group. The reduction of serum triglyceride concentration in the ginger group was significant in comparison with the placebo group (p < 0.05; Table 4).
TABLE 4.
Serum Concentrations of Biochemical Parameters in the Ginger Group and the Placebo Groupa
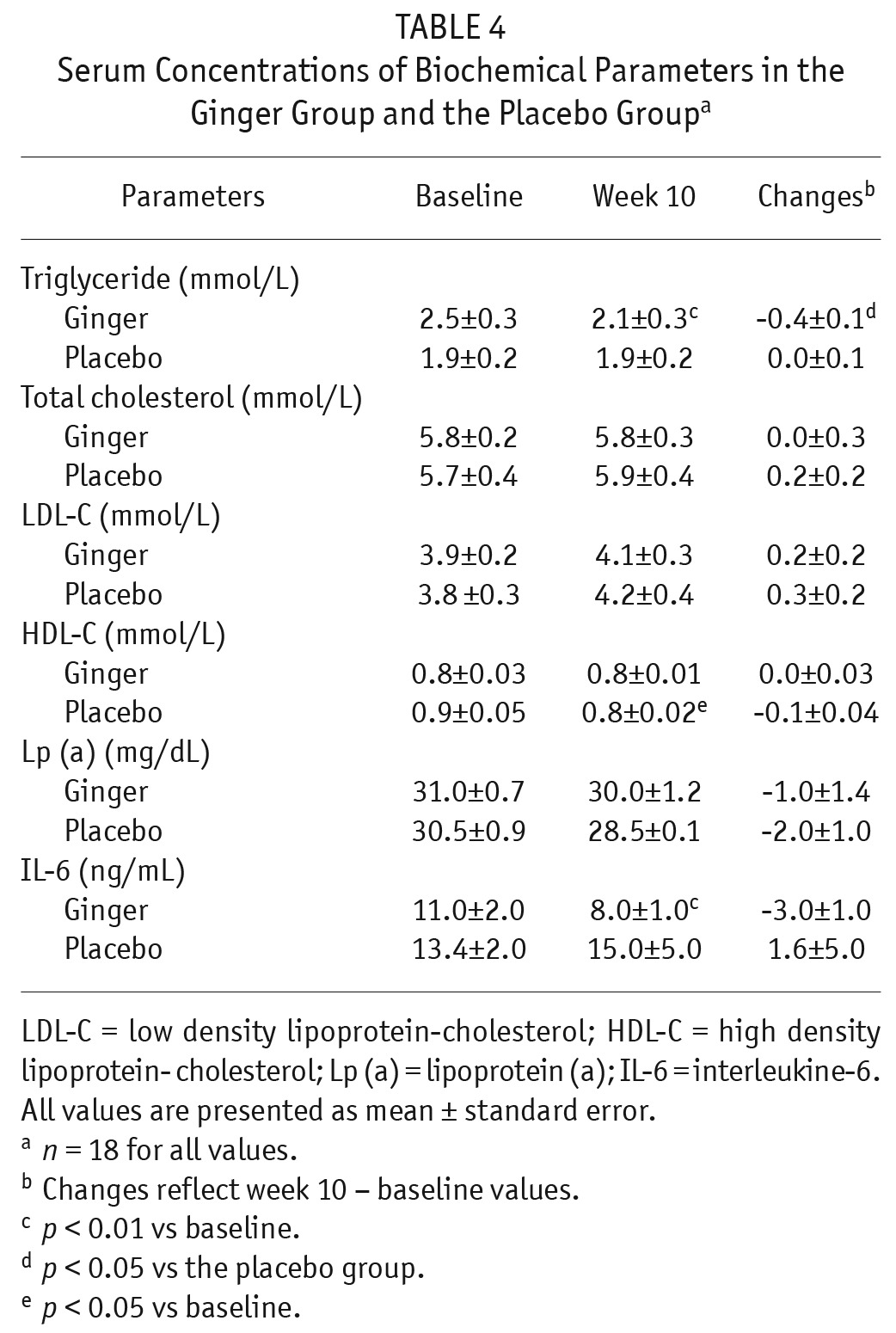
No significant changes were observed in serum total cholesterol, LDL-C, and Lp (a) (Table 4). Serum HDL-C concentration was significantly reduced in the placebo group at the end of week 10 compared with baseline (p < 0.05), whereas no statistically significant change was observed in the ginger group.
Serum IL-6 concentration was significantly decreased in the ginger group (p < 0.01) at the end of week 10 compared with baseline, whereas no significant change was observed in the placebo group. The reduction of serum IL-6 in the ginger group was not statistically significant in comparison with the placebo group (Table 4).
Discussion
Hypertriglyceridemia is among the most common lipid abnormalities in PD patients (3,22). In our study, approximately 58% of PD patients had a serum triglyceride level higher than normal (≥ 150 mg/dL according to National cholesterol Education Program criteria) (23). In PD patients, up to 80% of glucose from glucose-based PD solutions is absorbed (3,24), accounting for a daily glucose load of 100 – 300 g (25). The absorption of glucose from PD solutions and high serum glucose concentration lead to hyperinsulinemia (25), and increased hepatic synthesis of fatty acids and triglycerides (3). This results in an increase of very low density lipoprotein (VLDL) formation in the liver and consequently a rise in serum triglyceride concentration (3). Another cause for hypertriglyceridemia in PD patients is inflammation (26,27). Inflammatory cytokines such as IL-6 increase lipolysis in adipose tissue and cause a rise in serum-free fatty acids. This results in an increased synthesis of triglycerides and VLDL in the liver and a consequent rise in serum triglyceride concentration (26). In addition, inflammatory cytokines decrease the activity of lipoprotein lipase and cause an increase in serum concentration of triglyceride-rich lipoproteins such as VLDL (26,27).
In our study, daily administration of 1,000 mg ginger significantly decreased serum triglyceride concentrations up to 15% in PD patients. To date, no studies have examined the effects of ginger consumption on serum triglyceride concentration in PD patients. However, in agreement with our study, some animal studies have shown that ginger reduced serum triglyceride concentrations (28–30). In addition, few studies have investigated the effects of ginger in diabetic patients. Andallu et al. (17) indicated that daily administration of 3 g of ginger to diabetic patients with hypercholesterolemia for 3 months significantly decreased serum triglyceride concentrations. Mahluji et al. (16) showed that serum triglyceride concentration reduced significantly after a 2-month supplementation of diabetic patients with 2 g/day ginger. Arablou et al. (15) indicated that daily administration of 1,600 mg ginger to patients with diabetes type 2 for 12 weeks significantly decreased serum triglyceride concentration. However, Bordia et al. (31) reported that daily administration of ginger to patients with coronary artery disease in doses of 4 or 10 g for 3 months did not affect serum triglyceride concentration.
In our study, the effect of ginger consumption on the reduction of serum triglyceride concentration may be due to significant reductions in serum concentrations of glucose and IL-6, as 2 causes of hypertriglyceridemia in PD patients. In this study (32), serum fasting glucose reduced up to 20% in the ginger group and this reduction was significant in comparison with the placebo group. Some in vitro studies indicated that 2 active constituents of ginger (6-gingerol and 8-gingerol) enhanced cell glucose uptake by increasing gene expression of glucose transporter type 4 (33,34). In the present study, serum IL-6 concentration decreased significantly in the ginger group, but this reduction was not statistically significant in comparison with the placebo group. In agreement with our study, Mahluji et al. (35) showed that daily administration of 2 g of ginger to diabetic patients for 2 months significantly decreased serum IL-6, but this reduction was not significant in comparison with the placebo group.
Serum total cholesterol and LDL-C have been reported to be higher than normal in PD patients (3,24). The absorption of glucose from PD solutions and high serum glucose concentration lead to hyperinsulinemia and increased hepatic synthesis of cholesterol and LDL-C (3,22). In addition, high serum levels of total cholesterol and LDL-C in PD patients may be due to increased hepatic synthesis of apo B100, and consequently LDL, following loss of amino acids and proteins through PD (3,8).
In our study, ginger consumption had no significant effects on serum total cholesterol and LDL-C. In agreement with these findings, Bordia et al. showed that ginger consumption caused no changes in serum total cholesterol and LDL-C (31). In contrast, the majority of previous studies have indicated that ginger reduces serum concentrations of total cholesterol and/or LDL-C (15–17,28–30,36). This disparity may be due to the administration of a higher dose of ginger than was used in our study. In addition, in our study, 56% of PD patients in the ginger group received atorvastatin, as a cholesterol-lowering drug.
In PD patients, serum HDL-C levels are lower than the normal range (3,22,24). This may be due to decreased activities of lipoprotein lipase and hepatic lipase (22). In our study, the administration of ginger did not affect serum HDL-C. In agreement with this finding, previous studies on diabetic patients have shown that ginger consumption causes no change in serum HDL-C (15–17,31). In contrast, 2 animal studies have indicated that ginger increases serum HDL-C (28,30).
High serum Lp (a) concentration is a common lipid disorder in PD patients (3,5,22,37) and hyper Lp (a) constitutes a major risk factor for CVD (38–42). High serum levels of Lp (a) in PD patients may be due to increased hepatic synthesis of Lp (a) following loss of amino acids and proteins through PD (5,22,37,43,44). In our study, ginger consumption had no significant effect on serum Lp (a). No research was found in the available literature about the effect of ginger on serum Lp (a) for comparison with the results of the present study.
In conclusion, this study indicates that daily administration of 1,000 mg ginger reduces serum triglyceride concentration, which is a risk factor for CVD, in PD patients.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
This study was supported by the National Nutrition and Food Technology Research Institute of Iran.
The authors thank the staff of the Peritoneal Dialysis Unit in Shafa Clinic for their invaluable assistance, and the staff of the research laboratory of Research Institute for Endocrine Sciences and the Nutrition research laboratory of the Faculty of Nutrition and Food Technology for their technical assistance.
REFERENCES
- 1. Singh AK, Brenner BM. Dialysis in the treatment of renal failure. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005:1663–7. [Google Scholar]
- 2. Jungers P, Khoa TN, Massy ZA, Zingraff J, Labrunie M, Descamps-Latscha B, et al. Incidence of atherosclerotic arterial occlusive accidents in predialysis and dialysis patient: a multicentric study in the IIe de France district. Nephrol Dial Transplant 1999; 14(4):898–902. [DOI] [PubMed] [Google Scholar]
- 3. Wanner C. Altered lipid metabolism and serum lipids in renal disease and renal failure. In: Kopple JD, Massary SG, eds. Kopple and Massary's Nutritional Management of Renal Disease. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:42–55. [Google Scholar]
- 4. O'Neal D, Lee P, Murphy B, Best J. Low-density lipoprotein particle size distribution in end-stage renal disease treated with hemodialysis or peritoneal dialysis. Am J Kidney Dis 1996; 27(1):84–91. [DOI] [PubMed] [Google Scholar]
- 5. Shoji T, Nishizawa Y, Nishitani H, Yamakawa M, Morii H. High serum lipo-protein (a) concentrations in uremic patients treated with continuous ambulatory peritoneal dialysis. Clin Nephrol 1992; 38(5):271–6. [PubMed] [Google Scholar]
- 6. Fytili CI, Progia EG, Panagoutsos SA, Thodis ED, Passadakis PS, Sombolos KI, et al. Lipoprotein abnormalities in hemodialysis and continuous ambulatory peritoneal dialysis patients. Ren Fail 2002; 24(5):623–30. [DOI] [PubMed] [Google Scholar]
- 7. Kimak E, Berger B, Solski J, Janicka L, Ksiazek A. Comparison of lipid and lipoprotein profiles in long-term chronic ambulatory peritoneal dialysis (CAPD) in elderly patients with chronic renal failure (CRF). Int Urol Nephrol 2002; 33(1):203–4. [DOI] [PubMed] [Google Scholar]
- 8. Ong-Ajyooth L, Sirisalee K, Shayakul C, Vareesangthip K, Vasuvattakul S, Vanichakarn S, et al. Comparison of lipid abnormalities in continuous ambulatory peritoneal dialysis and hemodialysis patients. Transplant Proc 1994; 26(4):2077–9. [PubMed] [Google Scholar]
- 9. Siamopoulos KC, Elisaf MS, Bairaktari HT, Pappas MB, Sferopoulos GD, Nikolakakis NG. Lipid parameters including lipoprotein (a) in patients undergoing CAPD and hemodialysis. Perit Dial Int 1995; 15(8):342–7. [PubMed] [Google Scholar]
- 10. Garcia-Lopez E, Carrero JJ, Suliman ME, Lindholm B, Stenvinkel P. Risk factors for cardiovascular disease in patients undergoing peritoneal dialysis. Perit Dial Int 2007; 27(Suppl 2):S205–9. [PubMed] [Google Scholar]
- 11. Prichard S. Major and minor risk factors for cardiovascular disease in peritoneal dialysis. Perit Dial Int 2000; 20(Suppl 2):S154–9. [PubMed] [Google Scholar]
- 12. Shurraw S, Tonelli M. Statins for treatment of dyslipidemia in chronic kidney disease. Perit Dial Int 2006; 26(5):523–39. [PubMed] [Google Scholar]
- 13. Leong DJ, Choudhury M, Hirsh DM, Hardin JA, Cobelli NJ, Sun HB. Nutraceuticals: potential for chondroprotection and molecular targeting of osteoarthritis. Int J Mol Sci 2013; 14(11):23063–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int J Cardiol 2009; 131(3):408–9. [DOI] [PubMed] [Google Scholar]
- 15. Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr 2014; 65(4):515–20. [DOI] [PubMed] [Google Scholar]
- 16. Mahluji S, Attari VE, Mobasseri M, Payahoo L, Ostadrahimi A, Golzari SE. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int J Food Sci Nutr 2013; 64(6):682–6. [DOI] [PubMed] [Google Scholar]
- 17. Andallu B, Radhika B, Suryakantham V. Effect of aswagandha, ginger and mulberry on hyperglycemia and hyperlipidemia. Plant Food Hum Nutr 2003; 58(3):1–7. [Google Scholar]
- 18. Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparation ultracentrifuge. Clin Chem 1972; 18(6):499–502. [PubMed] [Google Scholar]
- 19. Burkart JM, Bargman JM. Adequacy of peritoneal dialysis, including fluid balance. In: Khanna R, Krediet RT, eds. Nolph and Gokal's Textbook of Peritoneal Dialysis. 3rd ed. New York: Springer; 2009:469–503. [Google Scholar]
- 20. Blake PG, Daugirdas JT. Physiology of peritoneal dialysis. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001:281–96. [Google Scholar]
- 21. Oreopoulos DG, Rao PS. Assessing peritoneal ultrafiltration, solute transport, and volume status. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001:361–72. [Google Scholar]
- 22. Lacour B, Massy Z, Drueke TB. Lipid metabolism. In: Massry SG, Glassock RJ, editors. Massry & Glassock's Textbook of Nephrology. 4th ed. Philadelphia: Williams & Wilkins; 2001:1346–56. [Google Scholar]
- 23. Raymond JL, Couch SC. Medical nutrition therapy for cardiovascular disease. In: Mahan LK, Escott-Stump S, Raymond JL, eds. Krause's Food and the Nutrition Care Process. 13th ed. Missouri: Elsevier/Saunders; 2012:746. [Google Scholar]
- 24. Prichard SS. Metabolic complications of peritoneal dialysis. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001:405–10. [Google Scholar]
- 25. Ritz E, Adamczak M, Wiecek A. Carbohydrate metabolism in kidney disease and kidney failure. In: Kopple JD, Massry SG, Kalantar-Zadeh K, eds. Nutritional Management of Renal Disease. 3rd ed. Amsterdam: Academic Press; 2013:21. [Google Scholar]
- 26. Cannon JG. Cytokines and eicosanoids. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:663. [Google Scholar]
- 27. Winter BK, Fiskum G, Gallo LL. Effect of L-carnitine on serum triglyceride and cytokine levels in rat models of cachexia and septic shock. Br J Cancer 1995; 72(5): 1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J Ethnopharmacol 2005; 97(2):227–30. [DOI] [PubMed] [Google Scholar]
- 29. Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr 2006; 96(4):660–6. [DOI] [PubMed] [Google Scholar]
- 30. ElRokh el-SM, Yassin NA, El-Shenawy SM, Ibrahim BM. Antihypercholesterolaemic effect of ginger rhizome (Zingiber officinale) in rats. Inflammopharmacology 2010; 18(6):309–15. [DOI] [PubMed] [Google Scholar]
- 31. Bordia A, Verma SK, Srivastava KC. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids 1997; 56(5):379–84. [DOI] [PubMed] [Google Scholar]
- 32. Imani H, Tabibi H, Najafi I, Atabak S, Hedayati M, Rahmani L. Effects of ginger on serum glucose, advanced glycation end products and inflammation in peritoneal dialysis patients. Nutrition 2015; [In press]. [DOI] [PubMed] [Google Scholar]
- 33. Son MJ, Miura Y, Yagasaki K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology 2015; [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Tran VH, Duke CC, Roufogalis BD. Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes. Planta Med 2012; 78(14):1549–55. [DOI] [PubMed] [Google Scholar]
- 35. Mahluji S, Ostadrahimi A, Mobasseri M, Ebrahimzade Attari V, Payahoo L. Anti-inflammatory effects of Zingiber officinale in type 2 diabetic patients. Adv Pharm Bull 2013; 3(2):273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Noory AS, Amreen AN, Hymoor S. Antihyperlipidemic effects of ginger extracts in alloxan-induced diabetes and propylthiouracil-induced hypothyroidism in rats. Pharmacognosy Res 2013; 5(3):157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heimburger O, Stenvinkel P, Berglund L, Tranaus A, Lindholm B. Increased plasma lipoprotein (a) in continuous ambulatory peritoneal dialysis is related to peritoneal transport of proteins and glucose. Nephron 1996; 72(2):135–44. [DOI] [PubMed] [Google Scholar]
- 38. Superko HR. Did grandma give you heart disease? The new battle against coronary artery disease. Am J Cardiol 1998; 82(9A):34Q–46Q. [DOI] [PubMed] [Google Scholar]
- 39. Spinler SA, Cziraky MJ. Lipoprotein (a): physiologic function, association with atherosclerosis, and effects of lipid-lowering drug therapy. Ann Pharmacother 1994; 28(3):343–51. [DOI] [PubMed] [Google Scholar]
- 40. Djurovic S, Berg K. Epidemiology of Lp (a) lipoprotein: its role in atherosclerotic/thrombotic disease. Clin Genet 1997; 52(5):281–92. [DOI] [PubMed] [Google Scholar]
- 41. Dahlen GH, Stenlund H. Lp (a) lipoprotein is a major risk factor for cardiovascular disease: pathogenic mechanisms and clinical significance. Clin Genet 1997; 52(5):272–80. [DOI] [PubMed] [Google Scholar]
- 42. Boonmark NW, Lawn RM. The lysine-binding function of Lp (a). Clin Genet 1997; 52(5):355–60. [DOI] [PubMed] [Google Scholar]
- 43. Ross EA, Shah GM, Kashyap ML. Elevated plasma lipoprotein (a) levels and hypoalbuminemia in peritoneal dialysis patients. Int J Artif Organs 1995; 18(12):751–6. [PubMed] [Google Scholar]
- 44. Anwar N, Bhatnagar D, Short CD, Mackness MI, Durrington PN, Prais H, et al. Serum lipoprotein (a) concentrations in patients undergoing continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 1993; 8(1):71–4. [DOI] [PubMed] [Google Scholar]


