Abstract
♦ Background:
Reverse epidemiology of blood pressure (BP) in end-stage kidney disease (ESKD) is manifested as higher mortality at lower blood pressure. We hypothesize that this phenomenon is partially mediated by deterioration of cardiac structure and function.
♦ Methods:
Seventy-seven prevalent ESKD patients starting renal replacement therapy on peritoneal dialysis (PD) from 2007 to 2012 were evaluated for the primary outcome of all-cause mortality. Longitudinal data were obtained from 1,930 patient-encounters including monthly clinic BP and serial echocardiograms. Generalized linear mixed models using data from the last observation moving backward, and time-to-event analysis using time-varying Cox-survival models to estimate mortality risk at different blood pressure categories were applied.
♦ Results:
There were 39 males (50.6%). Mean age was 51 years (standard deviation [SD] = 15). During follow-up, 20 patients (25%) died. As compared to systolic blood pressure (SBP) of 140 – 159 mmHg, unadjusted risk of mortality was 7.3 (95% confidence interval [CI]: 1.5 – 35.7, p = 0.008) at level < 120 mmHg. Systolic BP trended down to an average of 117 mmHg prior to death in non-survivors as compared to 141 mmHg in survivors (p < 0.05). In non-survivors, percentage with concentric left ventricular hypertrophy (LVH) decreased by 20% at the expense of a 20% reciprocal increase in eccentric hypertrophy associated with a 30% increase in percentage with low ejection fraction (EF) (< 50%). After adjusting for EF, risk of mortality at SBP < 120 mmHg attenuated to 3.4 (95% CI: 0.7 – 17.7, p = 0.14).
♦ Conclusion:
We conclude that higher mortality associated with lower BP may be mediated in part by worsening heart function in ESKD patients receiving PD.
Keywords: Peritoneal dialysis, end-stage kidney disease, blood pressure, mortality, heart failure
Hypertension is the second most common cause of kidney failure responsible for over 150,000 patients with end-stage kidney disease (ESKD) in the United States (1). Hypertension is highly prevalent in ESKD, such that up to 90% of patients are reported to have hypertension (2–4). Over 50% of deaths in ESKD are attributed to cardiovascular disorders (4) of which hypertension is the most prevalent disorder. Unlike the general population, where there is a linear increase in mortality with an increase in systolic blood pressure (BP), there is a “U” shape relationship between risk of mortality and systolic BP in ESKD referred to as reverse epidemiology (5–11). This phenomenon is a matter of intense debate and has generated uncertainty about the optimal range of BP control in ESKD. While the 2005 National Kidney Foundation Kidney Disease Outcomes Quality Initiatives clinical practice guidelines recommend a pre-dialysis BP < 140/90 and a postdialysis BP < 130/80 mmHg in patients on hemodialysis (HD), targeting such a level has been challenged in several reports (5,6). Presumptive etiologies including age-comorbidity index, survival bias, competing risk factors, chronic inflammation/malnutrition, the uremic milieu, and heart failure have all been proposed to explain this reverse epidemiology (5,6,12–14). However, to our knowledge no longitudinal study has examined the echocardiographic changes of left ventricular (LV) geometry and function in ESKD patients and its association with BP. As peritoneal dialysis (PD) imposes less severe hemodynamic fluctuations from ultrafiltration than HD (15–17), echocardiographic studies in PD patients may allow a more robust evaluation of the relationship between BP and LV function than HD. Therefore, echocardiographic studies in PD patients may provide a unique opportunity to capture the dynamic changes of LV geometry in ESKD. In this study, we aim to compare changes of LV geometry and function over time in a well characterized group of ESKD patients on PD by survival status, and to examine the impact of different categories of BP on risk of mortality. We hypothesize that reverse epidemiology of BP is partially mediated by deterioration of cardiac structure and function.
Methods
Study Subjects
This study is a single-center longitudinal observation. The study population is defined as prevalent patients with ESKD on PD from January 2007 to July 2012. Inclusion criteria were: 18 years of age or older, initiation of renal replacement therapy with PD as the modality in patients with ESKD from any etiology, at least 3 months of follow-up available after the PD start, and having routinely performed serial echocardiography examinations during follow-up. Exclusion criteria were recovery from kidney failure leading to termination of PD, transitioning to HD or kidney transplantation, as well as transferring of care to another outpatient unit within 3 months after dialysis initiation. All consecutive eligible patients who passed the enrollment criteria were included. They consisted of 77 patients with ESKD from different causes including 24 patients (31.2%) from diabetes, 24 (31.2%) from glomerulopathy, 5 (6.5%) from polycystic kidney disease, 4 (5.2%) from hypertension, 4 (5.2%) from genitourinary reflux, and 16 patients (20.8%) from other etiologies as cause of their kidney failure.
Data Collection
Data were collected by review of 1,930 patient-visits from the medical records of the included patients from their date of the first dialysis treatment until the date of death or the end of the study, which ever happened first. All patients were typically seen for monthly clinic visits after initiation of PD, except during a prolonged hospitalization. Clinical characteristics including demographics (age, gender, and race), physical examination findings (height, weight, systolic and diastolic BP), medications, and lab values were obtained at baseline and subsequent monthly outpatient clinic visits. The longitudinal data included sitting BPs, heart rate, weight, hemoglobin, serum albumin, PD prescription, Kt/V (delivered and residual kidney function), history of interval hospitalization or development of PD complications, and sequential echocardiograms. Urea kinetic modeling was used to calculate the weekly delivered Kt/V from PD and this was added to the Kt/V of residual kidney function if present to determine the total Kt/V for each patient. Loss of residual kidney function was defined as anuria or a renal Kt/V of 0. Left ventricular echocardiography results were obtained from 285 interpretable routinely performed serial echo studies. The baseline echocardiogram was selected as the study done nearest to the date of initiation of PD (median = 1 month). The median interval between subsequent echocardiograms was 11 months. Body surface area (BSA) at the time of echocardiography was estimated using the formula: [height (cm) × weight (kg)/3,600]0.5 (18). Standard M-mode echocardiography with echo probe at or just below the tips of the mitral valve leaflets obtained from the corresponding parasternal long- and short-axis cuts (19) provided the LV measurements. Left ventricular mass was calculated from measurements of other LV dimensions using the formula: LV mass = [1.04 × (IVST + PWT + LVEDD)3 – LVEDD3], where IVST, PWT, and LVEDD stand for interventricular septal thickness, posterior wall thickness, and LV end-diastolic dimension, respectively (20). Left ventricular mass index (LVMI) is defined as LV mass standardized by BSA. Left ventricular hypertrophy (LVH) is defined as LVMI greater than 110 g/m2 in females and greater than 130 g/m2 in males (21–23). Relative wall thickness (RWT) was calculated as (2 × PWT)/LVEDD. Concentric LVH (cLVH) is defined as LVH with an RWT of 0.44 or greater. Eccentric LVH (eLVH) is defined as LVH with an RWT of less than 0.44. Patients were followed until July 2012, until the primary endpoint of death from any cause, or until they were censured for change to HD as modality, transfer to another center, or received a kidney transplant. Approval of institutional review board was obtained. Tests of intraobserver variability of the echocardiograms revealed excellent reproducibility with intraclass correlation coefficient of 0.98 for LVEDD, 0.94 for LVESD, 0.94 for IVST, 0.97 for PWT, and 1.0 for EF < 50%, and coefficients of variation of 1.8% for LVEDD, 5.8% for LVESD, 4.8% for IVST, and 5.2% for PWT.
Statistical Analysis
The descriptive statistics were mean and standard deviation (SD) for continuous variables, and frequency and percentages for categorical variables. Median (interquartile range [IQR]) was used for description of variables with skewed distribution. Chi-square was used to test the categorical variables by different subgroups. Analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons was applied to compare means across different categories. Linear regression analysis was applied to explore the significance of linear trends among the subgroups. We used 2 complementary analytic approaches to study the longitudinal variables. First, to compare means by survival status at different time points, the averaged values of several observations within 3-month time blocks were compared in the 2 groups of deceased and survivors starting from the last observation moving backward. The rationale for averaging variables into 3-month time blocks is to minimize the impact of isolated fluctuations and providing robust values representative of the 3-month blocks to be used for the downstream analyses. When a patient missed a clinic visit for reasons such as hospitalization, values from the other 2 clinic visits were averaged to represent the corresponding time block. Random effect generalized linear mixed models were used to explore the interaction of the trends of longitudinal variables obtained from successive visits over time by survival status. Similar mixed linear models were used to assess the trend of change in LVMI and the LV configurations over time. In the second approach, and to explore the impact of different categories of BP on outcome, we applied time-to-event analyses from the starting date of PD moving forward using various Cox survival models. These models included unadjusted to fully adjusted models to estimate relative risk of death at different categories of BP: A) at the time of initiation of PD, and B) throughout the duration of study by using BP, and other covariates as time-varying variables. The reference category for systolic BP was the 140 – 159 mmHg group, and for diastolic BP was the 70 – 79 mmHg group. For incremental level of adjustments, we only kept the covariates which were independently associated with mortality for the next model after adjusting for the variables with significant imbalance at baseline. The analysis was conducted using SPSS version 21 (Armonk, NY, USA) and SAS version 9.3 (Cary, NC, USA).
Results
There were 39 males (50.6%) and 38 females (49.4%). Mean age was 51 years (SD = 15). There were 60 white patients (77.9%), 15 black patients (19.5%), and 2 patients (2.6%) from other races. The median follow-up time was 35 months (IQR: 21 – 58 months). During this observation 20 patients (25%) died. All 20 expired patients were known to have 1 or more forms of longstanding cardiopulmonary disorders prior to death including diastolic dysfunction in 7 patients, systolic heart failure in 6, pulmonary hypertension in 6, coronary artery disease in 3, and chronic arrhythmia in 1 patient. However, the immediate cause of death was non-cardiac in 8 patients and included sepsis in 3, accident in 1, systemic lupus erythematosus in 1, intra-cranial bleeding in 1, mesenteric ischemia in 1, and unknown in 1 patient. The cardiac causes of death in the remaining 12 patients were deterioration of heart failure in 10 (with 8 patients transitioning to hospice), and cardiac arrhythmia in 2 patients. Table 1 shows the distribution of baseline variables by category of baseline systolic BP. Accordingly, the distribution of age, sex, race, weight, height, BSA, medications, and modality of PD was not significantly different by category of baseline systolic BP. Although there is a trend toward higher utilization of antihypertensive medications in association with higher BP groups, the trend did not reach statistical significance. Total Kt/V was found to decline with progressively higher categories of BP, and this was largely driven by lower values of residual renal function in the higher BP categories (p < 0.05). The only notable change in laboratory parameters was the increasing trend in serum phosphorous with increased levels of BP, despite similar use of phosphate binders. There were no other significant differences in other lab values among the BP subgroups.
TABLE 1.
Distribution of Baseline Characteristics by Category of Systolic Blood Pressure at Initiation of Peritoneal Dialysis
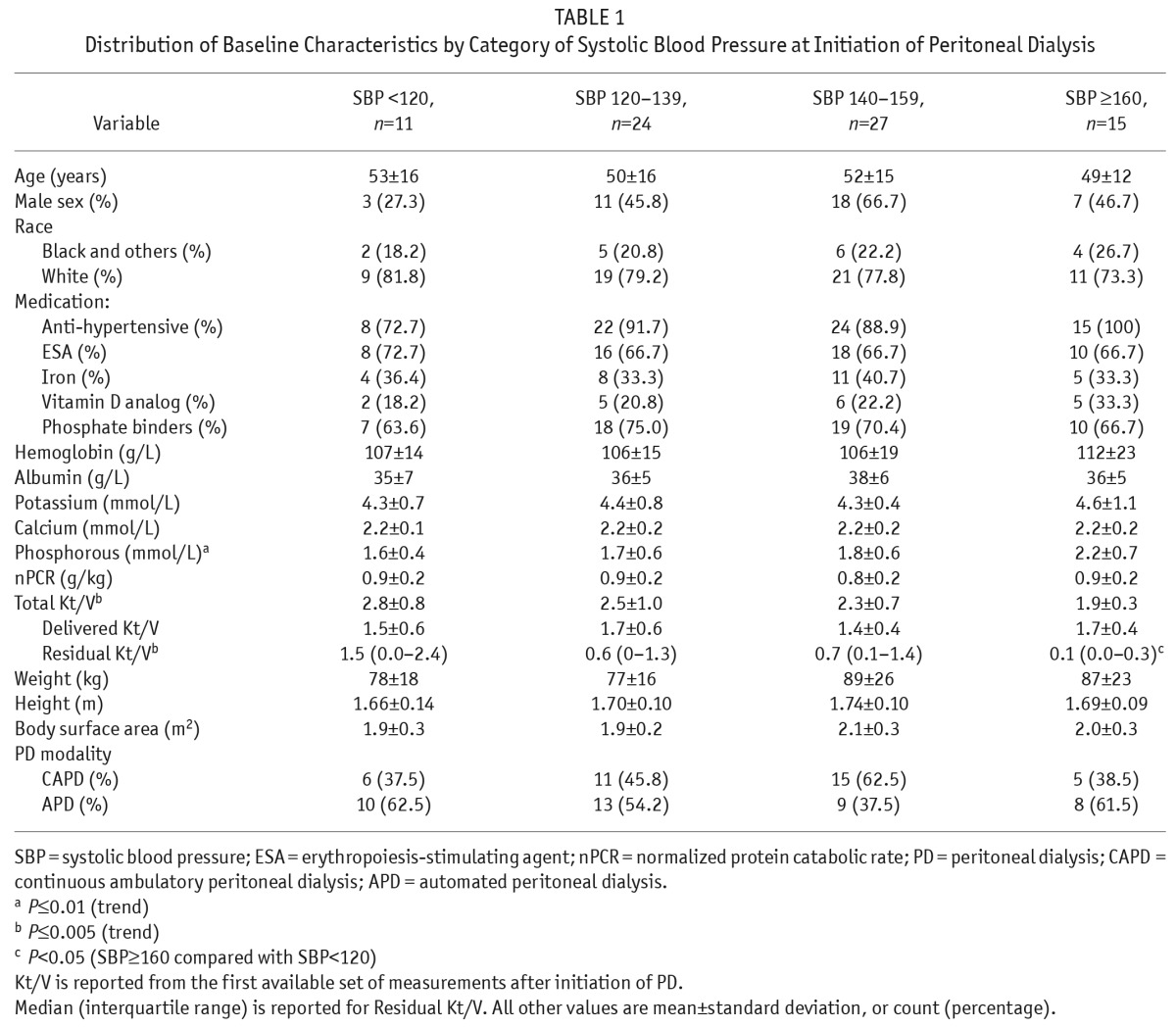
Table 2 shows the distribution of parameters of the baseline echocardiograms. An increase in IVST (p = 0.01) and PWT (p < 0.05) was noted with successive categories of higher systolic BP. Based on the increase in these components with higher BP categories, LV mass was also seen to increase with successive categories of systolic BP, so that patients with systolic BP of 160 mmHg or more had a significantly higher LV mass than those with systolic BP < 120 mmHg (p < 0.01). Similarly, there was a significant increase in the trend of mean LVMI with each increase in systolic BP category (p < 0.05).
TABLE 2.
Distribution of the First Echocardiographic Parameters after Initiation of Peritoneal Dialysis by Categories of Systolic Blood Pressure
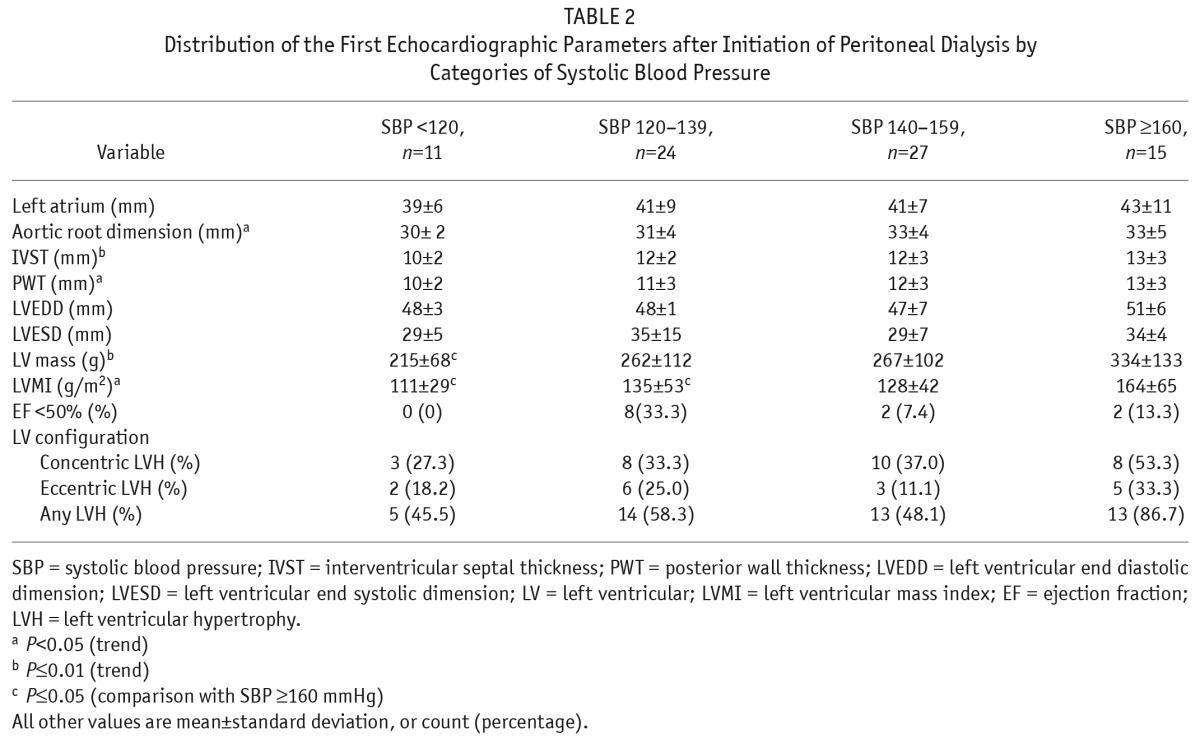
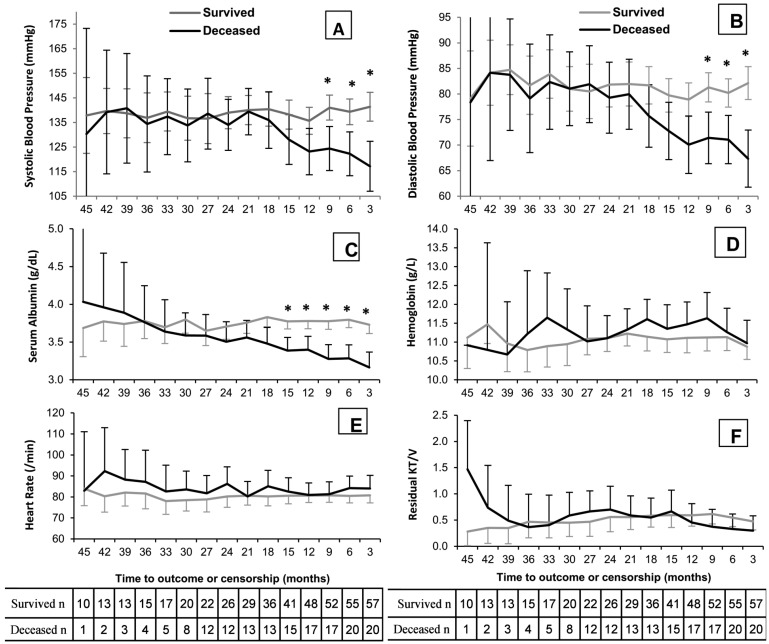
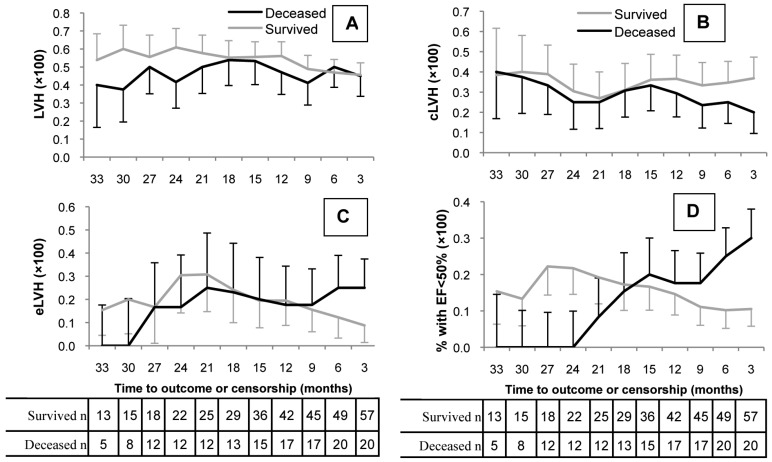
Figure 1 shows the trend of BP and other clinical and laboratory values over time by survival status. Systolic BP remained relatively unchanged in survivors over the 45 months of follow-up, but it started to decline around 21 months prior to death in the group of patients who died during the study period (Figure 1A). The difference in mean SBP became statistically significant at about 9 months prior to death as compared to survivors (p < 0.05). The interaction of systolic BP trend by survival status appeared highly significant (p = 0.004). Similarly, the trend of diastolic BP remained relatively unchanged over time in survivors but it started to decline at about 21 months prior to death in non-survivors and was statistically significant at 9 months prior to death (p < 0.05, Figure 1B). The interaction of diastolic BP trend by survival status is significant (p = 0.019). Mean systolic BP (± SD) between 90 and 180 days prior to death was 126 (± 24) mmHg and 119 (± 19) mmHg in patients with cardiac and non-cardiac causes of death (p = 0.49), respectively. Similarly, the mean diastolic BP at the same interval was 71 (± 12) mmHg and 69 (± 11) mmHg, respectively (p = 0.67). The rationale for choosing one 90-day lag time prior to death for the above comparison is to eliminate the dramatic effects of acute events such as sepsis or volume overload on blood pressure at the time of death. Serum albumin began to decline in non-survivors 2 years prior to death (p = 0.005), with a mean difference that reached statistical significance at 15 months prior to death as compared to survivors (Figure 1C). No difference was observed between survivors and non-survivors in hemoglobin, heart rate, or residual Kt/V over the study period. Figure 2 reveals the distribution of different LV configurations over time by survival status. The percentage of patients with LVH was similar in each group over the study duration (Fig 2A). However, cLVH declined from 40% over 30 months prior to death to 20% at the last observation in non-survivors (Figure 2B), while a reciprocal increase in eLVH from 0% over 30 months prior to death to 25% at the last observation was observed in this group (Figure 2C). Among survivors, no significant change in percentage of patients with cLVH or eLVH was seen over time (Figures 2B and 2C). Lastly, among the non-survivors only, there was a progressive rise in the proportion with an ejection fraction (EF) of less than 50%, from 0% over 24 months prior to death to 30% by the time of the last echocardiogram (trend-survival interaction p value = 0.044, Figure 2D).
Figure 1 —
Comparison of distributions and trends of time-dependent variables by survival status: A) *Systolic BP (p<0.05). Trend-survival interaction: p=0.004. B) *Diastolic BP (p<0.05). Trend-survival interaction p=0.019. C) *Serum albumin (p<0.05). Trend-survival interaction: p=0.005. D) Hemoglobin: p=0.113. E) Heart rate: p=0.291. E) KT/V of residual renal function: p<0.001. Estimations are based on generalized mixed linear models with time as random effect. Values are mean and 95% confidence interval. BP = blood pressure.
Figure 2 —
Percentage with LVH, concentric LVH (cLVH), eccentric LVH (eLVH), and low EF (EF<50%) by survival status at intervals prior to death or the end of study: A) LVH: p=NS. B) cLVH Trend-survival interaction p=0.001. C) eLVH trend-survival interaction has not reached statistical significance (p=0.634). D) low EF (<50%) Trend-survival interaction p=0.044. Estimations are based on generalized mixed linear models with time as random effect. Values are percentage ±SE. LVH = left ventricular hypertrophy; EF = ejection fraction; NS = not significant; SE = standard error.
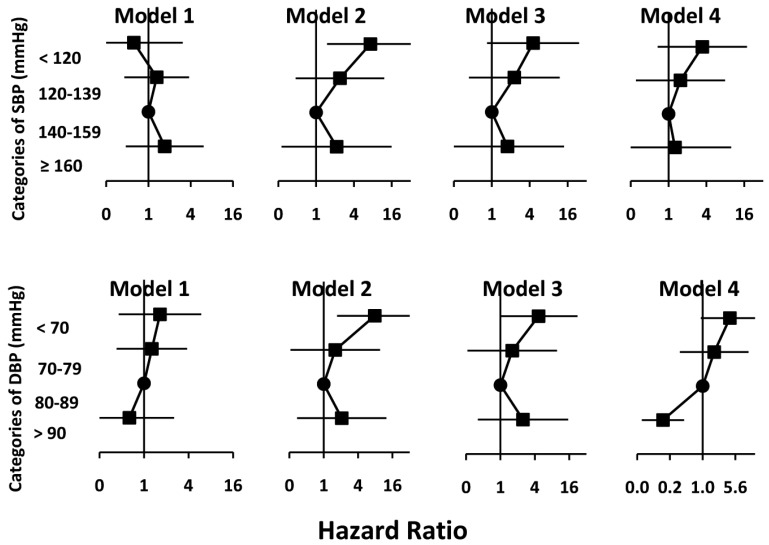
Cox survival models describing the relative risk of mortality at different categories of systolic BP are presented in Figure 3. Model 1 shows that the risk of mortality across different categories of baseline systolic and diastolic BP obtained from early visits after initiation of PD has not been significantly different from the reference categories using an unadjusted model. Model 2 illustrates that if the BP was used as a time-varying variable taking into account the risk attributed to change in BP over time, systolic BP < 120 mmHg was associated with a 7.3-fold (95% confidence interval [CI] 1.5 – 35.7, p = 0.014) and diastolic BP of < 70 mmHg was associated with a 7.8-fold (95% CI 1.7 – 35.7, p = 0.008) higher risk of all-cause mortality as compared to the reference categories in unadjusted models. Model 3 is model 2 additionally adjusted for serum albumin as a time-varying variable. The result is that the relative risks (RRs) observed in model 2 declined to 4.5 (95% CI 0.8 – 24.3, p = 0.078) for systolic BP < 120 mmHg, and to 4.7 (95% CI 1.0 – 22.3, p = 0.05) for diastolic BP < 70 mmHg. Further adjusting by low EF (<50%) resulted in attenuation of the observed RRs to 3.4 (95% CI 0.7 – 17.7, p = 0.14) for systolic BP < 120 mmHg, and to 4.2 (95% CI 0.9 – 19.6, p = 0.066) for diastolic BP < 70 mmHg (Model 4). Given differences in outcome observed in a few studies by modality of PD (24,25), we looked at the impact of modality of PD and PD prescription (including total volume and fill volume) as time-varying covariates throughout the duration of observation and have tested their independence on outcome. However, because modality and components of prescription of PD did not stand as independent predictors of mortality, we did not include them in the fully adjusted model (Model 4).
Figure 3 —
Cox survival models showing hazard ratio of death at different categories of BP compared to reference category of “140–159 mmHg” for SBP and “80–89 mmHg” for DBP, according to different models with increasing level of adjustment. Model 1: Unadjusted with BP at initiation of peritoneal dialysis. Model 2: Unadjusted with BP as a time-varying variable. Model 3: Model 2 + adjusted for albumin as a time-varying variable. Model 4: Model 3 + adjusted for low EF as a time-varying variable. BP = blood pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure; EF = ejection fraction.
Discussion
In this study we noticed a similar mean systolic and diastolic BP at baseline shortly after initiation of PD in both survivors and non-survivors. However, up to 24 months prior to death in the non-survivor group, systolic and diastolic BP started to decline when compared to those patients who survived to the study endpoint. Aligned with these observations, we found a lack of prognostic value for different categories of BP shortly after initiation of PD. However, when we used BP as a time-dependent variable, systolic BP of less than 120 mmHg and diastolic BP of less than 70 mmHg appeared to be significant predictors of all-cause mortality. We also found a linear increase in the proportion of patients with a low EF (< 50%) in non-survivors as compared to survivors. Prognostic values of low systolic and diastolic BP attenuated toward null after adjusting for EF and albumin.
Most of the epidemiologic studies of BP in ESRD patients have mainly been done with patients on HD (6–11,13,26,27). Although these studies are highly powered owing to their large sample size, they are limited by lack of granularity, absence of echocardiographic studies (6–11,13,26,27), and/or having used a single BP value per patient for analysis despite their prospective designs (6,8,10). There are fewer similar studies in PD patients. In an analysis of 2,770 PD patients from the UK renal registry (UKRR) (28), greater systolic and diastolic BP were shown to be associated with decreased mortality in the first year after the start of PD, but such an association was not observed in a subgroup of patients who were listed for transplant in the first 6 months after initiation of PD. This was explained by better health status and cardiovascular function of patients listed for transplant as compared to others (28). In an analysis of 1,219 randomly selected PD patients from the Centers for Medicare and Medicaid Services (CMS) dataset, diastolic BP < 72 mmHg was associated with an over 3-fold higher risk of 1-year mortality (29). In another observation from the UK, analysis of 168 patients on home HD and CAPD revealed a U-shaped risk of all-cause mortality with the lowest risk at mean arterial pressure of about 100 mmHg (30). In an analysis of 1,053 PD patients from USRDS, Goldfarb et al. showed a similarly higher mortality in association with systolic BP of 111 mmHg (31). Overall, our findings are in agreement with these studies that show a relatively higher mortality with lower BP. Several different proposed explanations include, but are not limited to, survival bias, competing risk factors, chronic inflammation-malnutrition complex, autonomic neuropathy, detrimental effect of uremic milieu, and heart failure (5,6,12,28). A major limitation of these previous studies is lack of echocardiography and parameters of heart function.
There are several interesting findings in our study. First, we did not find a significant association between different categories of BP at baseline and mortality. However, when BP was used as a time-dependent covariate, low BP was significantly associated with higher mortality. This observation is similar with Li and Stidley's reports (26,27), and suggests that while the assumption of proportionality by categories of baseline BP is violated due to a change in the relationship between baseline BP with mortality over time, other ongoing mechanisms might have been responsible for the association of low BP with mortality. To that end, we evaluated whether worsening cardiac function might have been a legitimate mechanism by which low BP is associated with increased mortality. Although a graded worsening of cardiac remodeling by progressive stages of non-dialysis CKD is described in other reports (32–35), dynamic changes of heart configuration over time in dialysis-dependent patients is less studied. To our knowledge, this study is the first illustration of dynamic changes of LV configuration over time in ESRD patients. Accordingly, a decline in percentage with cLVH over time at the expense of an increase in the percentage with eLVH, was associated with a rise in percentage of patients with low EF (< 50%) in the non-surviving cohort. When we adjusted the association of low BP with mortality by low EF, significant relative risk of low BP was attenuated toward the null. Lack of a statistically significant difference in mean BP by cause of death in 1 lag time prior to death is also a reflection of similar cardiovascular status irrespective of cause of death. We also noticed a progressive decline in serum albumin even earlier than the decline in BP as an independent predictor of mortality. No other parameters including weight, hemoglobin, heart rate, modality or prescription of PD was associated with the primary outcome, and the association of lower residual kidney function with mortality was not independent from serum albumin level. Altogether these observations support the hypothesis that reverse epidemiology of BP in ESKD is partially mediated by heart failure and malnutrition-inflammation complex, and that BP in ESKD is a marker of cardiovascular function and stability, and as the heart function worsens BP also declines. These observations also suggest that sustained systolic BP < 120 mmHg as opposed to a transient drop of BP may predict mortality, as the former often represents underlying heart failure while the later may reflect transient fluctuations from a variety of reasons such as the effect of antihypertensive medications or volume status.
This observation has important clinical implications. Low BP is a phenotype which does not have a universally identical meaning. While it may represent a relatively benign transient condition such as volume depletion or overmedication, a sustained low BP may represent more serious underlying cardiovascular dysfunction, particularly in patients with a history of longstanding hypertension. While the lowest category of BP may represent heart failure, the highest category is not necessarily protective. The optimal target of BP remains unclear, as is the need for use of cardio-protective medications in patients with heart failure in ESKD.
This study has several strengths. First, it has a high level of granularity with details on clinical course and outcome of the PD patients with ESKD, which allowed exploration of the dynamic changes of time-dependent covariates over time. To our knowledge, this is the first study to illustrate the dynamic changes of LV configuration in ESKD and its association with all-cause mortality. Blood pressure was obtained consistently from regular monthly clinic visits. Echocardiograms were performed periodically with equal median number of studies in both survivors and non-survivors (n = 3, p = 0.97) eliminating the possibility of ascertainment bias. The potential for selection bias due to our exclusion criteria is very low, as patients who were transitioned to HD or other units were not different compared to others, were too few, and had too short of a follow-up, so that neither was their exclusion a source of bias nor their inclusion a meaningful observation. The study also has important limitations. First, this is a single-center observation with a relatively low sample size and therefore the generalizability of the findings is limited. For this reason, we are unable to comment on the prognostic value of a systolic BP above 160 mmHg as this category is underpowered. We did not have ambulatory home BP measures from our patients. The generalized linear mixed model is a conservative approach assessing the longitudinal changes of LV configurations, as its basic assumption is constancy between observations. Therefore, the trend of change in deceased patients is likely underestimated. Although we have measures of change in LV geometry the structural changes do not necessarily represent the full spectrum of cardiac function and its decline over time, and therefore other cardiac function tests may shed more light on the association between BP and mortality in ESKD. Body weight and its longitudinal change were assessed, which did not seem to significantly modify the observed relationships. However, change in body weight did not represent the possible change in body composition that might have happened between the study groups.
Conclusion
We conclude that higher mortality associated with lower BP may be mediated in part by worsening heart function in ESKD patients receiving PD.
Disclosures
FA received support from 5T32DK7378-34 grant. The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. USRDS Reference tables: End Stage Renal Disease. http://www.usrds.org/2013/pdf/v2_condensed_rt__13.pdf 2013 USRDS Annual Data Report.
- 2. Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 2003; 115(4):291-2–7. [DOI] [PubMed] [Google Scholar]
- 3. Del Vecchio L, Lusenti T, Del Rosso G, Malandra R, Balducci A, Losito A, Group for Arterial Hypertension of the Italian Society of Nephrology Prevalence of hypertension in a large cohort of Italian hemodialysis patients: results of a cross-sectional study. J Nephrol 2013; 26(4):745–54. [DOI] [PubMed] [Google Scholar]
- 4. KDOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005; 45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 5. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003; 63(3):793–808. [DOI] [PubMed] [Google Scholar]
- 6. Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension 2005; 45(4):811–17. [DOI] [PubMed] [Google Scholar]
- 7. Myers OB, Adams C, Rohrscheib MR, Servilla KS, Miskulin D, Bedrick EJ, et al. Age, race, diabetes, blood pressure, and mortality among hemodialysis patients. J Am Soc Nephrol 2010; 21(11):1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 1999; 33(3):507–17. [DOI] [PubMed] [Google Scholar]
- 9. Raimann JG, Usvyat LA, Thijssen S, Kotanko P, Rogus J, Lacson E, Jr., et al. Blood pressure stability in hemodialysis patients confers a survival advantage: results from a large retrospective cohort study. Kidney Int 2012; 81(6):548–58. [DOI] [PubMed] [Google Scholar]
- 10. Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2012; 82(5):570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Usvyat LA, Barth C, Bayh I, Etter M, von Gersdorff GD, Grassmann A, et al. Interdialytic weight gain, systolic blood pressure, serum albumin, and C-reactive protein levels change in chronic dialysis patients prior to death. Kidney Int 2013; 84(1):149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 2003; 42(5):864–81. [DOI] [PubMed] [Google Scholar]
- 13. Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 1998; 54(2):561–9. [DOI] [PubMed] [Google Scholar]
- 14. Vrtovsnik F, Porcher R, Michel C, Hufnagel G, Queffeulou G, Mentre F, et al. Survival of elderly patients on peritoneal dialysis: retrospective study of 292 patients, from 1982 to 1999. Perit Dial Int 2002; 22(1):73–81. [PubMed] [Google Scholar]
- 15. Kwan BC, Szeto CC. Is peritoneal dialysis kinder for the heart? Perit Dial Int 2011; 31(2):135–7. [DOI] [PubMed] [Google Scholar]
- 16. McIntyre CW. Hemodynamic effects of peritoneal dialysis. Perit Dial Int 2011; 31(Suppl 2):S73–6. [DOI] [PubMed] [Google Scholar]
- 17. Selby NM, McIntyre CW. Peritoneal dialysis is not associated with myocardial stunning. Perit Dial Int 2011; 31(1):27–33. [DOI] [PubMed] [Google Scholar]
- 18. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987; 317(17):1098. [DOI] [PubMed] [Google Scholar]
- 19. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2(5):358–67. [DOI] [PubMed] [Google Scholar]
- 20. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57(6):450–8. [DOI] [PubMed] [Google Scholar]
- 21. Casale PN, Devereux RB, Milner M, Zullo G, Harshfield GA, Pickering TG, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 1986; 105(2):173–8. [DOI] [PubMed] [Google Scholar]
- 22. Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol 1984; 4(6):1222–30. [DOI] [PubMed] [Google Scholar]
- 23. Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol 1987; 59(9):956–60. [DOI] [PubMed] [Google Scholar]
- 24. Sanchez AR, Madonia C, Rascon-Pacheco RA. Improved patient/technique survival and peritonitis rates in patients treated with automated peritoneal dialysis when compared to continuous ambulatory peritoneal dialysis in a Mexican PD center. Kidney Int Suppl 2008; (108):S76–80. [DOI] [PubMed] [Google Scholar]
- 25. Sun CY, Lee CC, Lin YY, Wu MS. In younger dialysis patients, automated peritoneal dialysis is associated with better long-term patient and technique survival than is continuous ambulatory peritoneal dialysis. Perit Dial Int 2011; 31(3):301–7. [DOI] [PubMed] [Google Scholar]
- 26. Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 2006; 17(2):513–20. [DOI] [PubMed] [Google Scholar]
- 27. Li Z, Lacson E, Jr., Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 2006; 48(4):606–15. [DOI] [PubMed] [Google Scholar]
- 28. Udayaraj UP, Steenkamp R, Caskey FJ, Rogers C, Nitsch D, Ansell D, et al. Blood pressure and mortality risk on peritoneal dialysis. Am J Kidney Dis 2009; 53(1):70–8. [DOI] [PubMed] [Google Scholar]
- 29. Rocco MV, Frankenfield DL, Prowant B, Frederick P, Flanigan MJ. Risk factors for early mortality in U.S. peritoneal dialysis patients: impact of residual renal function. Perit Dial Int 2002; 22(3):371–9. [PubMed] [Google Scholar]
- 30. Lynn KL, McGregor DO, Moesbergen T, Buttimore AL, Inkster JA, Wells JE. Hypertension as a determinant of survival for patients treated with home dialysis. Kidney Int 2002; 62(6):2281–7. [DOI] [PubMed] [Google Scholar]
- 31. Goldfarb-Rumyantzev AS, Baird BC, Leypoldt JK, Cheung AK. The association between BP and mortality in patients on chronic peritoneal dialysis. Nephrol Dial Transplant 2005; 20(8):1693–1701. [DOI] [PubMed] [Google Scholar]
- 32. Afshinnia F, Spitalewitz S, Chou SY, Gunsburg DZ, Chadow HL. Left ventricular geometry and renal function in hypertensive patients with diastolic heart failure. Am J Kidney Dis 2007; 49(2):227–36. [DOI] [PubMed] [Google Scholar]
- 33. Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 2012; 23(10):1725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nardi E, Palermo A, Mule G, Cusimano P, Cottone S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens 2009; 27(3):633–41. [DOI] [PubMed] [Google Scholar]
- 35. Moran A, Katz R, Jenny NS, Astor B, Bluemke DA, Lima JA, et al. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: the multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis 2008; 52(5):839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]