Abstract
Fifteen years ago, our group reported the case of a 67-year-old man on peritoneal dialysis for 11 years, in whom ultrafiltration failure and impaired sodium sieving were associated with an apparently normal expression of aquaporin-1 (AQP1) water channels in peritoneal capillaries. At that time, AQP1 dysfunction was suggested as the cause of impaired free-water transport. However, recent data from computer simulations, and structural and functional analysis of the peritoneal membrane of patients with encapsulating peritoneal sclerosis, demonstrated that changes in the peritoneal interstitium directly alter osmotic water transport. In light of these insights, we challenge the initial hypothesis and provide several lines of evidence supporting the diagnosis of encapsulating peritoneal sclerosis in this patient and suggesting that severe peritoneal fibrosis accounted for the loss of osmotic conductance developed during the course of peritoneal dialysis.
Keywords: Water transport, osmosis, fibrosis, encapsulating peritoneal sclerosis
Prolonged exposure of the peritoneal membrane to non-physiologic dialysis solutions leads to vascular proliferation, fibrosis, and hyalinizing vasculopathy (1–4). A subgroup of patients ultimately develops encapsulating peritoneal sclerosis (EPS), the most severe form of peritoneal fibrosis, leading to bowel encapsulation and obstruction, malnutrition, and a high risk of death (5).
Long-term peritoneal dialysis (PD) is also associated with a progressive loss of ultrafiltration (UF) capacity, rise in peritoneal solute transport rate (PSTR), and decreased sodium sieving (6,7). Fifteen years ago, we reported the case of a long-term PD patient who had developed UF failure and loss of sodium sieving, in whom preserved aquaporin-1 (AQP1) expression in the peritoneal membrane suggested impaired function of water channels (8). Previous work indeed established that AQP1 mediates solute-free water transport, thereby diluting dialysate sodium concentration during the first part of a dwell with hypertonic glucose (9–12). However, recent data from computer simulations and analysis of the peritoneal membrane of patients with EPS demonstrated that peritoneal fibrosis per se contributes to the loss of osmotic conductance during prolonged exposure to PD (13–16).
We therefore hypothesized that peritoneal fibrosis, rather than altered AQP1 function, could have contributed to UF failure and abolition of sodium sieving in the case we reported 15 years ago. From a retrospective analysis of the medical file and complementary assessment of the peritoneal membrane, we provide several lines of evidence supporting the diagnosis of EPS and pointing toward the fibrotic interstitium as the cause of impaired osmotic water transport in this patient.
Material and Methods
Peritoneal function was assessed using a conventional 2.27% glucose-based (17) or a modified, 3.86% glucose-based peritoneal equilibration test (PET) (18), as previously described (16). The PET was part of the normal procedure of care for PD patients in our center.
Median, para-umbilical samples of the parietal peritoneum of the index case were obtained at autopsy. Samples from the same peritoneal area were obtained from control patients at catheter insertion or removal, as part of a routine protocol in our center (16). The use of human biopsy samples has been approved by the Ethical Review Board of the Cliniques universitaires Saint-Luc. Adequacy of each peritoneal specimen, peritoneal fibrosis, collagen volume fraction, and collagen structure were assessed as previously described (7,16). Immunofluorescence was performed using purified rabbit anti-von Willebrand factor (DAKO, Glostrup, Denmark) and polyclonal rabbit anti-human AQP1 (Chemicon International, Temecula, CA, USA) antibodies (16). Images were analyzed using ImageJ (1.47v).
Summary of the Initial Case Description, 1999
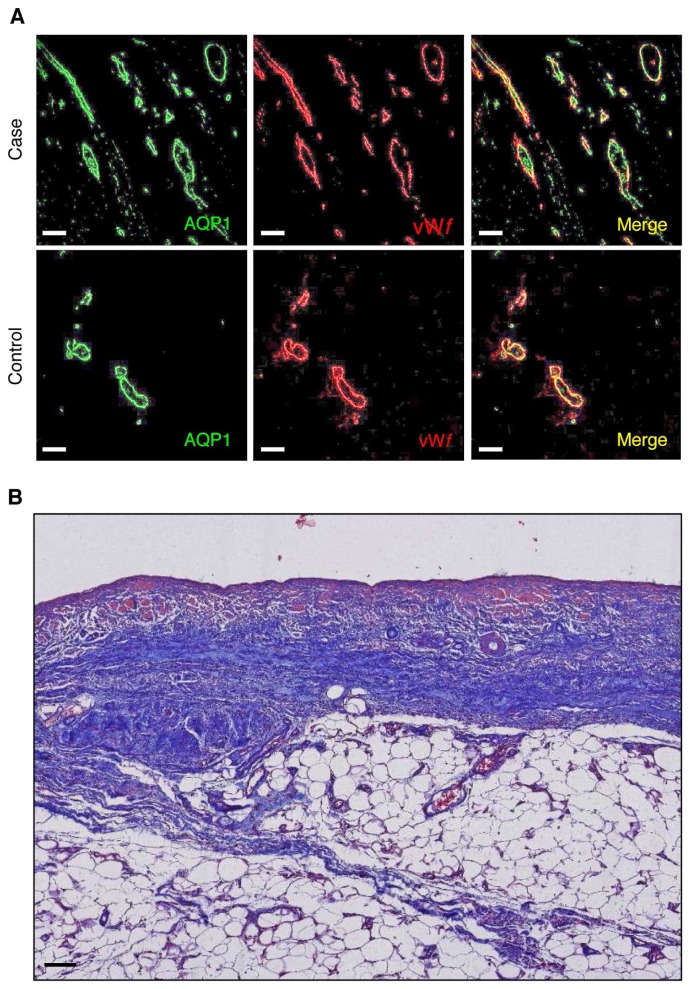
A 67-year-old man with end-stage renal disease due to AA amyloidosis secondary to pulmonary tuberculosis was started on continuous ambulatory PD in 1986. During the course of PD, the patient became anuric in 1992, and presented a unique episode of peritonitis due to Staphylococcus epidermidis in 1994, cured with systemic administration of vancomycin. From November 1995 onward, daily UF volume decreased progressively, requiring the systematic use of hypertonic 3.86% glucose to maintain euvolemia. Peritoneal transport testing using 3.86% glucose-based dialysate performed in March 1996 confirmed the diagnosis of UF failure (net UF < 400 mL), with a fast PSTR, and complete abolition of sodium sieving (Table 1). In March 1996, a switch to continuous cycling PD failed to improve daily UF, and the patient was transferred to hemodialysis. After 4 weeks of peritoneal membrane rest, and since the patient refused to continue hemodialysis, he was restarted on continuous cycling PD without improvement of the UF capacity. Daily UF at that time varied between 200 and 500 mL. In April 1997, the patient developed severe pulmonary infection due to Pseudomonas aeruginosa and died from septic shock. Post-mortem examination showed preserved AQP1 expression in the peritoneal membrane of the patient despite the loss of UF capacity and impaired free-water transport (Figure 1A), suggesting altered structure and/or function of water channels in the peritoneal capillaries (8).
TABLE 1.
Changes in Parameters of Peritoneal Water and Solute Transport Over Time
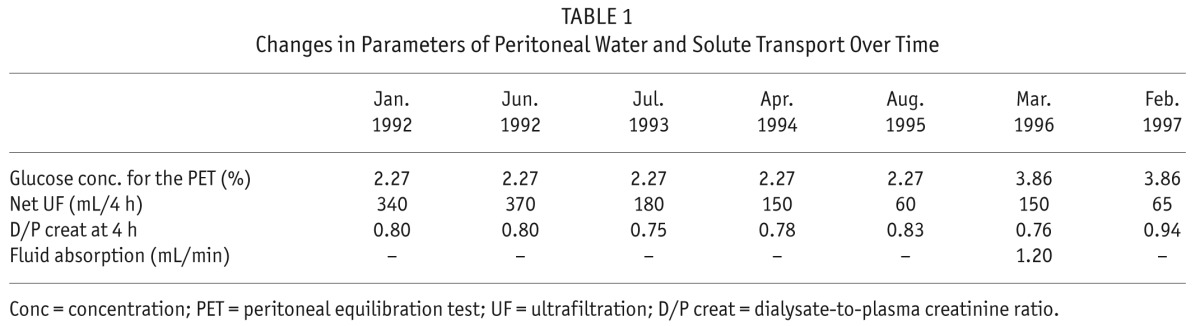
Figure 1 —
A. Peritoneal aquaporin-1 expression. Representative pictures of double immunostaining with anti-AQP1 (green channel) and anti-von Willebrand factor (vWf) (red channel) antibodies viewed under confocal fluorescence microscopy in peritoneal sections from the case vs a control PD patient. Aquaporin-1 is expressed in endothelial cells lining the capillaries and small vessels in the peritoneal membrane, both in the patient with impaired water transport (net UF = 65 mL, and sodium sieving = 0.01, during a 4-h modified 3.86% glucose-based PET) and in the PD control (net UF 940 mL, sodium sieving 0.06). Bar, 50 μm. B. Masson's trichrome staining. Structural analysis of the peritoneal membrane showed loss of mesothelial cells, accumulation of extracellular matrix in the submesothelial area, and signs of vasculopathy. Original magnification, 4x; bar, 100 μm. AQP1 = aquaporin-1; vWf = anti-von Willebrand factor; PD = peritoneal dialysis; UF = ultrafiltration; PET = peritoneal equilibration test.
Complementary Data from the Retrospective Review of the Medical File, 2015
Since recent data documented impaired osmotic water transport directly linked to extreme peritoneal fibrosis in patients with EPS, we reviewed the medical file of the patient in search of additional signs suggestive of EPS, a condition that was probably frequently overlooked at that time. We noticed that the patient presented 2 episodes of intestinal subocclusion, in April and May 1996, without any identified cause. Computed tomography scanner of the abdomen showed an extensive thickening of the bowel walls, peritoneal thickening, calcifications of the parietal and visceral peritoneal sheets, and bowel dilatation. The patient then progressively lost weight and developed a biological inflammatory syndrome (C-reactive protein levels between 2.6 and 13.6 mg/dL, normal range < 1.0, in the absence of any superimposed infection), along with food intolerance requiring total parenteral nutrition. At autopsy, the peritoneum appeared tanned, thickened, and brown, with a leathery appearance. These clinical findings definitely confirmed the retrospective diagnosis of EPS, defined by international guidelines as a clinical syndrome with signs of intermittent and persistent or recurrent complaints of gastrointestinal obstruction, with macroscopic or radiological confirmation of sclerosis, calcification, peritoneal thickening, or encapsulation of the intestines (5,19).
Additional structural evaluation of the peritoneal membrane demonstrated severe changes in the peritoneal interstitium of the patient, including vascular proliferation (vascular density 88.5 vessels/mm2), excessive accumulation of extracellular matrix in the submesothelial area (mean submesothelial thickness 512 μm) (Figure 1B), and increased density of collagen fibers (collagen volume fraction of 43%), comparable to those seen in EPS patients (16,20).
Discussion
Revisiting a case our group reported 15 years ago (8) suggested that severe fibrotic changes in the peritoneal interstitium accounted for UF failure and abolition of sodium sieving in a long-term PD patient with intact AQP1 expression.
The clinical features of recurrent bowel obstruction, food intolerance, and inflammatory syndrome suggested the patient could have developed EPS, a diagnosis confirmed by radiological and macroscopic findings. In addition, examination of the patient's peritoneal membrane showed excessive vascular proliferation, submesothelial thickening, and collagen density, comparable to those previously reported in patients with EPS and much higher than PD and uremic controls (vascular density: 97.5 ± 10.5, 52.5 ± 6.0, and 25.0 ± 2.0 vessels/mm2; submesothelial thickness: 549 ± 56, 286 ± 34, and 116 ± 17 μm; and collagen volume fraction: 41 ± 3% vs 22 ± 2 and 15 ± 1% in patients with EPS, PD, and uremic controls, respectively [16,20]). According to computer simulations based on the 3-pore membrane/fiber matrix (13) and the distributed (14,15) models, excessive deposition of extracellular matrix in the peritoneal interstitium is predicted to impair osmotic conductance and to reduce sodium sieving, through reduction of the mean hydraulic radius between collagen fibers and decreased concentration of glucose in contact with peritoneal capillaries (13–15,21). These predictions were recently confirmed in a cohort of long-term PD patients with EPS, in which impaired water transport was directly related to the degree of peritoneal fibrosis and density of collagen fibers in the peritoneal interstitium (16). These data highlighted the progressive reduction of sodium sieving when interstitial fibrosis increased, despite the fact that the relative capillary density of AQPs was kept unchanged, as in the present case. In addition, interstitial fibrosis was paralleled by an intense vascular proliferation and a fast PSTR, further reducing sodium sieving through early dissipation of the osmotic gradient.
Admittedly, we cannot completely rule out acquired dysfunction or biochemical modification of AQP1. To date, known post-translational modifications of AQP1 are limited to glycosylation, which has not been shown to affect either channel folding or oligomerization (22). However, phosphorylation and protonation of plant AQPs have been recently shown to influence the gating of water channels to counteract environmental stresses, through interaction with the intracytoplasmic D loop domain (for review, see 23). Similarly, some synthetic compounds are able to interact with the D loop of AQP1, and to modulate AQP1-mediated osmotic water transport, in vitro and in a mouse model of PD (24). Future research will need to test the possibility of post-translational changes of AQP1, i.e. during prolonged exposure to PD solutions or after an episode of peritonitis, and their potential impact on water transport.
In summary, sodium sieving is an indirect indicator of free-water transport and osmotic conductance to glucose that can not only be affected by hypothetical, acquired, dysfunction of water channels, but also by structural changes in the peritoneal membrane. Sodium sieving should therefore not be regarded as synonymous with AQP function, but rather as a functional evaluation of the whole pathway of water movement across the peritoneal membrane, from the patient's blood towards the dialysate. Revisiting the significance of UF failure and loss of sodium sieving provided clues to the post-mortem diagnosis of EPS and pointed toward the fibrotic interstitium as a major restriction barrier to water transport in this long-term PD patient.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
This work was supported in part by the Saint-Luc Foundation (to JM), the Horlait-Dapsens Foundation (to JM), the French Speaking Society of Dialysis (to JM), Baxter Healthcare (Extramural Grant to JM and OD), and the National Fund for Scientific Research (to JM and OD).
REFERENCES
- 1. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9. [DOI] [PubMed] [Google Scholar]
- 2. Williams JD, Craig KJ, von Ruhland C, Topley N, Williams GT, Biopsy Registry Study Group The natural course of peritoneal membrane biology during peritoneal dialysis. Kidney Int 2003; 88:S43–9. [DOI] [PubMed] [Google Scholar]
- 3. Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, et al. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 2008; 3:720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Devuyst O, Margetts PJ, Topley N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol 2010; 21:1077–85. [DOI] [PubMed] [Google Scholar]
- 5. Korte MR, Sampimon DE, Betjes MG, Krediet RT. Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol 2011; 7:528–38. [DOI] [PubMed] [Google Scholar]
- 6. Davies SJ. Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int 2004; 66:2437–45. [DOI] [PubMed] [Google Scholar]
- 7. Smit W, Schouten N, van den Berg N, Langedijk MJ, Struijk DG, Krediet RT, Netherlands Ultrafiltration Failure Study Group Analysis of the prevalence and causes of ultrafiltration failure during long-term peritoneal dialysis: a cross-sectional study. Perit Dial Int 2004; 24:562–70. [PubMed] [Google Scholar]
- 8. Goffin E, Combet S, Jamar F, Cosyns JP, Devuyst O. Expression of aquaporin-1 in a long-term peritoneal dialysis patient with impaired transcellular water transport. Am J Kidney Dis 1999; 33:383–8. [DOI] [PubMed] [Google Scholar]
- 9. Devuyst O, Rippe B. Water transport across the peritoneal membrane. Kidney Int 2015; 85:750–8. [DOI] [PubMed] [Google Scholar]
- 10. Rippe B, Stelin G, Haraldsson B. Computer simulations of peritoneal fluid transport in CAPD. Kidney Int 1991; 40:315–25. [DOI] [PubMed] [Google Scholar]
- 11. Ni J, Verbavatz JM, Rippe A, Boisdé I, Moulin P, Rippe B, et al. Aquaporin-1 plays an essential role in water permeability and ultrafiltration during peritoneal dialysis. Kidney Int 2006; 69:1518–25. [DOI] [PubMed] [Google Scholar]
- 12. Morelle J, Sow A, Vertommen D, Jamar F, Rippe B, Devuyst O. Quantification of osmotic water transport in vivo using fluorescent albumin. Am J Physiol Renal Physiol 2014; 307:F981–9. [DOI] [PubMed] [Google Scholar]
- 13. Rippe B, Venturoli D. Simulations of osmotic ultrafiltration failure in CAPD using a serial three-pore membrane/fiber matrix model. Am J Physiol Renal Physiol 2007; 292:F1035–43. [DOI] [PubMed] [Google Scholar]
- 14. Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal-plasma transport: theoretical considerations. Am J Physiol 1984; 246:R597–607. [DOI] [PubMed] [Google Scholar]
- 15. Flessner MF. Peritoneal transport physiology: insights from basic research. J Am Soc Nephrol 1999; 2:122–35. [DOI] [PubMed] [Google Scholar]
- 16. Morelle J, Sow A, Hautem N, Bouzin C, Crott R, Devuyst O, et al. Interstitial fibrosis restricts osmotic water transport in encapsulating peritoneal sclerosis. J Am Soc Nephrol 2015; 26(10):2521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Twardowski ZJ, Nolph KD, Khanna R, Prowant BF, Ryan LP, Moore HL, et al. Peritoneal equilibration test. Perit Dial Bull 1987; 7:138–47. [Google Scholar]
- 18. La Milia V, Pozzoni P, Virga G, Crepaldi M, Del Vecchio L, Andrulli S, et al. Peritoneal transport assessment by peritoneal equilibration test with 3.86% glucose: a long-term prospective evaluation. Kidney Int 2006; 69:927–33. [DOI] [PubMed] [Google Scholar]
- 19. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int 2000; 20:S43–55. [PubMed] [Google Scholar]
- 20. Mateijsen MA, van der Wal AC, Hendriks PM, Zweers MM, Mulder J, Struijk DG, et al. Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 1999; 19:517–25. [PubMed] [Google Scholar]
- 21. Rippe B, de Arteaga J, Venturoli D. Aquaporins are unlikely to be affected in marked ultrafiltration failure: results from a computer simulation. Perit Dial Int 2001; 21:S30–4. [PubMed] [Google Scholar]
- 22. Magni F, Sarto C, Ticozzi D, Soldi M, Bosso N, Mocarelli P, et al. Proteomic knowledge of human aquaporins. Proteomics 2006; 6:5637–49. [DOI] [PubMed] [Google Scholar]
- 23. Chaumont F, Tyerman SD. Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 2014; 164:1600–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yool AJ, Morelle J, Cnops Y, Verbavatz JM, Campbell EM, Beckett EA, et al. AqF026 is a pharmacologic agonist of the water channel aquaporin-1. J Am Soc Nephrol 2013; 24:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]