Abstract
Background
Reports conflict as to whether Tourette Syndrome (TS) confers deficits in executive function. This study's aim was to evaluate executive function in youths with TS using oculomotor tasks while controlling for confounds of tic severity, age, medication and severity of comorbid disorders.
Method
Four saccade tasks requiring the executive functions of response generation, response inhibition, and working memory (prosaccade, antisaccade, 0-back and 1-back) were administered. Twenty youths with TS and low tic severity (TS-low), nineteen with TS and moderate tic severity (TS-moderate), and twenty-nine typically developing control subjects (Controls) completed the oculomotor tasks.
Results
There were small differences across groups in the prosaccade task. Controlling for any small sensorimotor differences, TS-moderate subjects had significantly higher error rates than Controls and TS-low subjects in the 0-back and 1-back tasks. In the 1-back task, these patients also took longer to respond than Controls or TS-low subjects.
Conclusions
In a highly controlled design, the findings demonstrate for the first time that increased tic severity in TS is associated with impaired response inhibition and impaired working memory and that these executive function deficits cannot be accounted for by differences in age, medication or comorbid symptom severity.
Keywords: attention deficit hyperactivity disorder, cognitive control, executive functionl, n-back, obsessive-compulsive disorder, saccades
INTRODUCTION
Tourette Syndrome (TS) is a neuropsychiatric disorder with onset in childhood that is characterized by stereotyped, repetitive actions and noises called tics. Over half of children with TS also suffer from comorbid attention deficit hyperactivity disorder (ADHD) and/or obsessive-compulsive disorder (OCD). Reports conflict as to whether children with TS suffer from deficits in executive function (Muller, Johannes, Wieringa, Weber, Muller-Vahl et al., 2003, Verte, Geurts, Roeyers, Oosterlaan & Sergeant, 2005, Wechsler, 1999) or not (Crawford, Channon & Robertson, 2005), and if executive function deficits are due to comorbid conditions (Eddy, Rizzo & Cavanna, 2009). Yet another view asserts that impaired executive function may not only be linked to comorbid conditions, but also severity of overall symptomology (Ozonoff, Strayer, McMahon & Filloux, 1998). Intact executive function encompasses a cognitive flexibility to devise, revise, and carry out a plan in fulfillment of a goal, and thus requires generation of desired behaviors (response generation), inhibition of inappropriate alternatives (response inhibition), and the memory to remain focused on the goal (working memory).
The exact pathophysiology of TS is unknown, but evidence points to involvement of the basal ganglia (Cheon, Ryu, Namkoong, Kim, Kim et al., 2004), the frontal cortex (Fredericksen, Cutting, Kates, Mostofsky, Singer et al., 2002, Peterson, Skudlarski, Anderson, Zhang, Gatenby et al., 1998, Peterson, Staib, Scahill, Zhang, Anderson et al., 2001), and distinct neural circuitry interconnecting the two. Of the putative five parallel frontostriatal circuits, the oculomotor and motor circuits control eye and skeletomotor movements, respectively, whereas the other three direct cognitive and emotional behavior (Alexander, DeLong & Strick, 1986). Functional imaging, lesion, and single-unit recording studies have provided intimate knowledge of the neural control of saccadic eye movements (Pierrot-Deseilligny, Milea & Muri, 2004). In clinical populations, measuring eye movements is a non-invasive means of investigating psychomotor and higher cognitive processes (Hutton, 2008). Thus, eye movement tasks are well suited to quickly test executive function in TS. They have been shown to have test–retest reliability (Gooding, Mohapatra & Shea, 2004) and to be more sensitive measures of executive function than neuropsychological tasks such as the Stroop, finger tapping, Trail Making, and Continuous Performance tasks alone (Broerse, Holthausen, van den Bosch & den Boer, 2001).
Several studies have employed oculomotor paradigms to investigate executive function in TS (Dursun, Burke & Reveley, 2000, Farber, Swerdlow & Clementz, 1999, Jackson, Mueller, Hambleton & Hollis, 2007, Mostofsky, Lasker, Singer, Denckla & Zee, 2001, Mueller, Jackson, Dhalla, Datsopoulos & Hollis, 2006, Munoz, Le Vasseur & Flanagan, 2002, Nomura, Fukuda, Terao, Hikosaka & Segawa, 2003, Straube, Mennicken, Riedel, Eggert & Muller, 1997). These studies, however, often did not carefully control for tic severity, age, medication, and comorbid symptom severity, possibly confounding results. Tics fluctuate in intensity and frequency on the order of weeks to months, and in many individuals peak in mid-adolescence (Bruun & Budman, 2005). Greater tic severity likely results from more pronounced disturbance of the oculomotor and motor frontostriatal circuits. Thus, accounting for tic severity evaluates the impact of corresponding pathophysiology on executive function performance. Controlling for age is important because TS severity changes drastically throughout development, and because executive function and oculomotor function develop during childhood (Klein & Foerster, 2001, Mostofsky et al., 2001). Despite the fact that psychiatric medications are known to alter executive function and eye movement performance (Reilly, Lencer, Bishop, Keedy & Sweeney, 2008), TS participants in past studies were commonly tested while actively taking pharmacological treatments (Dursun et al., 2000, Farber et al., 1999, Jackson et al., 2007, Mueller et al., 2006, Munoz et al., 2002, Straube et al., 1997). Finally, comorbidities such as ADHD or OCD may independently impact executive functions. Multiple eye movement studies in children with ADHD alone or OCD alone report increased time to respond (or increased coefficient of variation) and increased error rate compared to control subjects on tasks that require executive functions (Klein, Raschke & Brandenbusch, 2003, Rosenberg, Averbach, O'Hearn, Seymour, Birmaher et al., 1997).
Here, we examine executive function, specifically response generation, response inhibition, and working memory, in TS adolescents using oculomotor tasks while controlling for tic severity, age, medication, and comorbid symptom severity. We administered four oculomotor tasks: a reflexive and voluntary task and two novel spatial n-back tasks (Jeter, Patel & Sereno, 2011). The n-back task is a working memory task in which subjects must continually revise and update their mental set to respond to the location of the stimulus n items before the final stimulus. We hypothesized that TS subjects with increased tic severity would show oculomotor executive functioning deficits compared to TS subjects of lower tic severity and Controls.
METHOD
SUBJECTS
Both typically developing control subjects (Controls) and children with TS (TS-overall), ages 10–16, participated in the study. Based on tic severity, children with TS were classified with low tic severity (TS-low) or moderate tic severity (TS-moderate). Across groups, children were matched within six months of age. TS subjects were recruited from the Child and Adolescent Neurology Clinic at The University of Texas Medical School at Houston. Control children were recruited from the community by flyer and word of mouth. Before testing, each subject's parent or guardian gave informed consent and each subject gave assent. The study was approved by The University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects in accordance with the Declaration of Helsinki.
A qualified pediatric neurologist confirmed all patients met the DSM-IV diagnostic criteria for TS. Controls were excluded if they, or a sibling or parent, had an active neurological or psychiatric disorder. IQ was estimated using a two-subtest version (Vocabulary and Matrix Reasoning) of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). The Yale Global Tic Severity Scale (YGTSS) (Leckman, Riddle, Hardin, Ort, Swartz et al., 1989) was used to evaluate vocal and motor tics; the Attention Deficit Hyperactivity Disorder Rating Scale – IV (ADHD-IV) (DuPaul, Power, Anastopoulos & Reid, 1998) was used to assess inattention and hyperactivity; the Obsessive Compulsive Inventory – Child Version (OCI-CV) (Foa, Coles, Huppert, Pasupuleti, Franklin et al., 2010) was used to measure obsessive-compulsive characteristics. For each subject, the child (including Controls), his or her parent or guardian, and an investigator (CBJ) independently rated symptom severity. The child, parent/guardian, and investigator scores were averaged for each diagnostic rating scale because the intraclass correlation coefficients among the three raters suggested fair to excellent reproducibility (see Results section). None of the Controls met criteria for TS, ADHD, or OCD. Children with TS who were taking neurological and/or psychiatric medications were included only if they were willing and able to be weaned completely off these medications at least one week prior to testing (n=5 in TS-low and n=7 in TS-moderate groups).
EYE TRACKING
Apparatus
Subjects sat 72 cm from a monitor (LCD, 17 inch, 75 Hz refresh rate, 1024×768 pixels) connected to a Power G4 Macintosh computer running OS 9. A chin rest and forehead bar stabilized the head. An infrared eye-tracking camera system (ISCAN ETL-200, Burlington, Massachusetts), connected to the Macintosh via USB port, measured eye movement with 0.5° and 240 Hz spatial and temporal resolutions, respectively. A custom program developed using commercially available software (Vision Shell; Code Warrior) presented stimuli (0.2°×0.2° white squares) in landmark boxes (1.1°×1.1°) positioned 7° to the top, bottom, right and left of the central fixation point (0.2° gray circle) on a black screen.
For each trial in all experiments, the eye position data were analyzed online, with trials automatically canceled and re-presented later in random order when subjects broke fixation early or blinked. Percent of trials cancelled as a result of a blink for the three groups were as follows: Controls (5.60%), TS-low (6.85%) and TS-moderate (6.88%). Saccade onset was indicated by an eye velocity above 18°/s leaving the 5.8° fixation window and saccade termination by an eye velocity below 4.6°/s, with correct initial saccades defined as landing within 2° of the target.
Eye Movement Tasks
Eye movements of subjects were calibrated before each task by fixating nine different locations indicated on the screen. After standardized verbal instructions, subjects completed an 8-trial practice block and were encouraged to ask questions before proceeding. Tasks were administered in a semi-counterbalanced order, with the n-back tasks always presented first. Prosaccade and Antisaccade tasks were comprised of 48 trials, with equal target presentation in each of the four landmark positions, whereas the n-back tasks were comprised of 96 trials, with equal final and n – 1 stimulus presentations in each of the four landmarks.
Prosaccade
This task served as a control task measuring reflexive response generation and verifying sensorimotor function was intact. To initiate each trial, subjects fixated the central fixation point for 400 ms. A peripheral target appeared randomly in one of the four landmark boxes simultaneously with the offset of the fixation point, signaling subjects to make a speeded saccade to the target.
Antisaccade
This task tested the subject's ability to inhibit a reflexive saccade and generate a voluntary response. To initiate each trial, subjects fixated the central fixation point for 400 ms. A peripheral target appeared randomly in one of the four landmark boxes simultaneously with the offset of the fixation point, signaling subjects to make a speeded saccade to the target's mirror location on the opposite side of the screen.
0-back
This task was a test of working memory. To initiate each trial, subjects fixated the central fixation point for 750 ms. While subjects continued to fixate, two or three stimuli appeared in sequence, each in a unique landmark. Stimuli appeared for 80 ms with a 350 ms interstimulus interval. Subjects continued to fixate throughout a 500ms delay period until the central fixation point was extinguished, signaling them to make a saccade to the remembered location of the last, or 0-back, stimulus (be it the second of two or third of three stimuli in the sequence). The random presentation of two or three stimuli per trial prevented subjects from using predictive strategies to time the initiation and direction of the saccade. All subjects saw the same 96 trials of stimulus sequences.
1-back
This task was composed of the same set of trials as the 0-back task and was otherwise identical except that when the central fixation point was extinguished, this signaled the subject to make a saccade to the remembered location of the next-to-last, or 1-back, stimulus (be it the first of two or second of three stimuli in the sequence).
Working Memory Load
This assessment was not a task, but rather a difference measure of the two n-back tasks evaluating the added demand placed on working memory by the 1-back task compared to the 0-back task (calculated as 1-back minus 0-back).
Data Analysis
Clinical
An intraclass correlation coefficient (ICC) was used as a measure of agreement between the child, parent/guardian, and investigator ratings of symptom severities. An ICC ≤ 0.4 was defined as poor agreement, between 0.4 and 0.75 was defined as fair to good agreement, and ≥ 0.75 was defined as excellent agreement.
To assess the influence of tic severity on oculomotor performance while keeping comorbid symptom severity equated, we divided TS subjects into two subgroups: TS subjects of low tic severity (TS-low; n=20) and TS subjects of moderate tic severity (TS-moderate; n=19). Subjects with tic severities at or below the median tic severity score (YGTSS=31.33) comprised the TS-low subgroup, whereas subjects with tic severities greater than the median were classified as TS-moderate. Possible differences between TS-overall and Control groups due to differences in age, gender, and IQ were considered in three separate linear models. These linear models then were reanalyzed with Group now having three levels (TS-low, TS-moderate, and Controls) to assess the influence of age, gender, and IQ on tic severity.
Eye Movements
Saccade latency was defined as the time from target onset to saccade initiation. Trials with saccade latencies below 100 ms or above 900 ms were excluded (4.4% of all trials). For each task, remaining trials were used to calculate error rate, defined as the number of trials with direction errors (incorrect trials) divided by the total number of trials not excluded. Only correct trials with saccade latencies less than 2.5 standard deviations around the subject's mean were used to calculate mean saccade latency (82.3% of all trials). For both saccade latency and error rate, working memory load was defined as the difference between their values in the 1-back and 0-back tasks (1-back minus 0-back).
To determine the effect of age on eye movement performance, Pearson's correlations were used to estimate the correlation of the eye movement variables with age. For error rate, data were natural log transformed before conducting Pearson correlations to equalize variance across age. In order to relate our results to that in the literature, we first compared the eye movement performance of all children with TS and Controls. Saccade latencies were analyzed using mixed effect models for each task separately and error rate was analyzed using Poisson regression models for each task separately. The main factor in these models was Group (TS-overall and Controls) and were adjusted for covariates: age, gender, and IQ. In order to adjust for Prosaccade latency and error rate differences across groups (i.e., the sensorimotor control task), the other three executive function tasks as well as Working Memory Load were analyzed with the previously mentioned covariates, but also Prosaccade latency (for latency measures) and Prosaccade error rate (for error rate measures). We then reanalyzed the mixed effect and Poisson regression models detailed above, with group having three levels (TS-low, TS-moderate, Controls).
RESULTS
Inter-observer Reproducibility in Ratings of Symptom Severities
The ICCs among 3 raters (child, parent/guardian, and investigator) were: 0.90 for the Yale Global Tic Severity Scale, 0.74 for the Attention Deficit Hyperactivity Disorder Rating Scale and 0.53 for Obsessive Compulsive Inventory – Child Version.
Participant Demographics
Our population of 68 subjects included 47 males and 21 females, with an average age of 13.7±2.09 years. In our patient sample, tic severity did not significantly differ across age (see online supplementary Figure S1). When TS subjects (n=39) as a whole were compared to Controls (n=39), the two groups significantly differed on the three clinical measures (severity of tics, ADHD, and OCD symptoms), but not by age, gender, or IQ (Table 1). Across TS subgroups (TS-low, n=20; TS-moderate, n=19) and Controls, children were matched within 6 months of age. Critically, the TS subgroups and Controls did not significantly differ by age, gender, or IQ and the TS subgroups did not differ by severity of ADHD or OCD symptoms (Table 1).
Table 1.
Demographic and Diagnostic Rating Scale Data
| Controls (n=29) | TS-overall (n=39) | TS-low (n=20) | TS-moderate (n=19) | Controls vs. TS-overall P | TS-low vs. Controls TS-moderate vs. Controls TS-moderate vs. TS-low |
|
|---|---|---|---|---|---|---|
| Age (years) | 13.2±0.4 | 13.0±0.3 | 13.1±0.4 | 12.9±0.5 | 0.23 | ns |
| Gender (M:F) | 19:10 | 28:11 | 12:8 | 16:3 | 0.93 | ns |
| IQ | 97.3±2.0 | 98.4±2.4 | 95.2±3.4 | 101.5±3.3 | 0.73 | ns |
| YGTSS | 0.7±0.2 | 36.0±2.8 | 24.3±1.3 | 48.3±4.0 | <0.001* | TS-low>C, TS-moderate>C TS-moderate>TS-low |
| ADHD-IV | 43.3±4.5 | 78.3±3.3 | 79.6±4.6 | 76.9±4.8 | <0.001* | TS-low>C TS-moderate>C |
| OCI-CV | 3.9±0.5 | 10.8±0.8 | 11.3±1.0 | 10.3±1.3 | <0.001* | TS-low>C TS-moderate>C, |
Format for rating scales: mean± standard error. M, male; F, female; YGTSS, Yale Global Tic Severity Scale (score range 0–100); ADHD-IV, Attention Deficit Hyperactivity Disorder Rating Scale – IV (percentile rank 1–100); OCI-CV, Obsessive Compulsive Inventory – Child Version (score range 0–42). Planned comparisons: p<0.001; ns, not significant;
significant
Correlations between Oculomotor Variables and Age
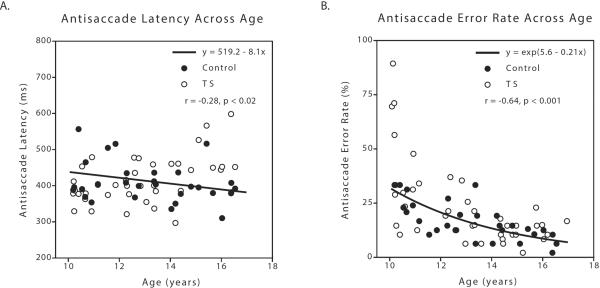
Regardless of disease status (i.e., group or subgroup), saccade latency and error rate each inversely correlated with age on most tasks, consistent with other studies (Klein & Foerster, 2001, Mostofsky et al., 2001). Specifically, Antisaccade latency (r(67)=−0.28; p=0.02; Figure 1A) and error rate (r(67)=−0.64, p <0.0001; Figure 1B) as well as 1-back saccade latency (r(67)=−0.29, p=0.02, data not shown) and error rate (r(67)=−0.38, p<0.005, data not shown) had significant inverse relationships with age. The Prosaccade and 0-back tasks did not. This supports the conclusion that comparisons of oculomotor parameters among groups of children, even in restricted age ranges such as the present study, require the groups to be carefully age-matched. To account for the systematic effect of age on eye movement variables, age was included as a covariate in all analyses of eye movement variables.
Figure 1.
Antisaccade latency and error rate each inversely correlate with age. A. Latency on the Antisaccade task decreased across age in all subjects. B. Error rate on the Antisaccade task decreased across age in all subjects. Trend line is calculated from the linear regression analysis between natural log-transformed error rate and age. TS, Tourette Syndrome
Oculomotor Variables: TS subjects vs. Controls
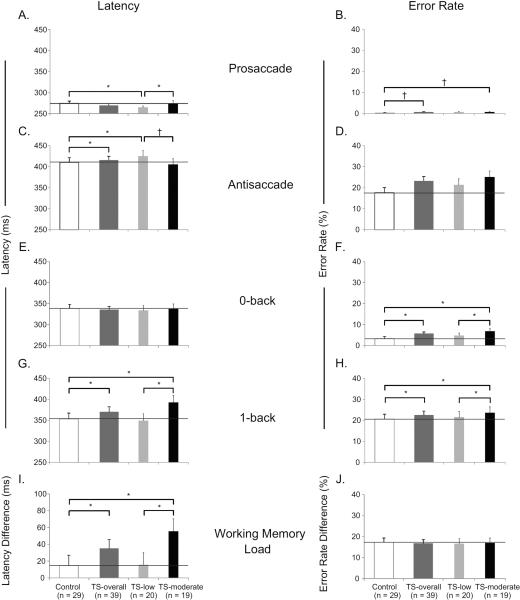
Results from each of the four tasks and working memory load are shown in Table 2. For the Prosaccade task, TS-overall subjects did not differ on latency (4.4±2.8; t(63)=1.58, p=0.12; Figure 2A) compared to Controls but had a marginally-significant tendency for slightly higher error rate (95% confidence interval (CI)=[0.85, 10.74]; Wald Chi-square(63)=2.94, p=0.09; Figure 2B). Thus, subsequent analyses in other tasks and conditions of latency and error rate included Prosaccade latency and Prosaccade error rate as covariates, respectively. For the Antisaccade task, when all TS subjects were compared to Controls, they had longer latency (−11.5±5.1; t(62)=−2.26, p=0.03; Figure 2C), but did not differ in errors (CI=[0.83, 1.15]; Wald Chi-square(62)=0.08, p=0.77; Figure 2D). In the 0-back task, TS-overall subjects showed no difference from Controls on latency (2.5±3.7; t(62)=0.68, p=0.50; Figure 2E), but displayed a greater error rate (CI=[1.41, 2.36]; Wald Chi-square(62)=20.70, p<0.0001; Figure 2F). For the 1-back task, TS-overall subjects had longer latency than Controls (−12.2±5.5; t(62)=−2.20, p=0.03; Figure 2G), as well as higher error rate (CI=[1.00, 1.25]; Wald Chi-square(62)=4.01, p=0.045; Figure 2H). Likewise, working memory demand (difference between the 1-back and 0-back tasks) differed between TS-overall and Controls on latency (−21.4±6.1; t(62)=−3.54, p<0.001; Figure 2I) but not error rate (CI=[0.82, 1.05]; Wald Chi-square(60)=1.34, p=0.25; Figure 2J).
Table 2.
Comparison of eye movement performance from TS patients and Controls in all the tasks
| Task | Variable | Controls (29) | TS-overall (39) | P value |
|---|---|---|---|---|
| Prosaccade | Latency (ms) | 274.8±5.3 | 269.7±4.6 | 0.12 |
| Error rate (%) | 0.22±0.19 | 0.66±0.16 | 0.09+ | |
|
| ||||
| Antisaccade | Latency (ms) | 410.4±10.9 | 415.4±9.4 | 0.03* |
| Error rate (%) | 17.6±2.4 | 23.2±2.1 | 0.77 | |
|
| ||||
| 0-back | Latency (ms) | 338.3±9.7 | 335.3±8.2 | 0.50 |
| Error rate (%) | 3.3±1.0 | 5.7±0.8 | <0.0001 | |
|
| ||||
| 1-back | Latency (ms) | 353.4±13.8 | 370.3±12.0 | 0.03* |
| Error rate (%) | 20.6±2.3 | 22.5±1.9 | 0.045* | |
|
| ||||
| Working Memory Load (1-back – 0-back) | Latency (ms) | 15.0±12.1 | 35.0±10.6 | <0.001* |
| Error rate (%) | 17.3±2.0 | 16.8±1.7 | 0.25 | |
Format for all values: mean+standard error. P values are from planned comparisons from mixed effect models for latency and Poisson regression models for error rate;
significant;
marginally-significant tendency
Figure 2.
Comparison of eye movement performance across Control group, TS group and TS subgroups.
Oculomotor Variables: Comparison across TS subgroups and Controls
The next analysis repeated the mixed effect and Poisson regression models described above on our eye movement measures, but divided TS subjects by tic severity to determine whether executive function differed by severity of tics. Results from each of the four tasks and the working memory load are shown in Table 3. Here, we only discuss the results of planned comparisons across TS subgroups and Controls. On the Prosaccade task, TS-low subjects were significantly faster to respond than Controls (−9.1±3.3; t(62)=−2.72, p<0.01; Figure 2A) and TS-moderate subjects (−9.4±3.8; t(62)=−2.51, p=0.01; Figure 2A). TS-moderate subjects had a marginally-significant tendency for more errors than Controls (CI=[0.98, 15.08]; Wald Chi-square(62)=3.73, p=0.054; Figure 2B) Thus, once again, subsequent analyses in other tasks and conditions of latency and error rate included Prosaccade latency and Prosaccade error rate as covariates, respectively. For the Antisaccade task, TS-low subjects had significantly longer latency than Controls (18.0±6.1; t(61)=2.95, p<0.005) and showed a marginally-significant tendency for longer latency than TS-moderate subjects (13.4±7.0; t(61)=1.92, p=0.06, effect size=13.64; Figure 2C). There were no significant differences across TS subgroups and Controls for Antisaccade error rate, 0-back latency, or Working Memory Load error rate difference. There were four findings (0-back error rate, 1-back latency, 1-back error rate, and Working Memory Load latency difference) where TS-moderate subjects were significantly different from both Controls and TS-low subjects. Specifically, TS-moderate subjects had a significantly higher 0-back error rate than both Controls (CI=[1.80, 3.20]; Wald Chi-square(61)=35.69, p<0.0001) and TS-low subjects (CI=[1.33, 2.45]; Wald Chi-square(61)=14.51, p=0.0002; Figure 2F). Similarly, on the 1-back task, TS-moderate subjects not only took longer to respond than Controls (28.4±6.7; t(61)=−4.25, p=<0.0001), but also than TS-low subjects (−32.1±7.5, t(61)=−4.25, p=<0.0001; Figure 2G). Likewise, TS-moderate subjects also committed significantly more 1-back errors than Controls (CI=[1.07, 1.34]; Wald Chi-square(61)=8.39, p=0.04) and TS-low subjects (CI=[1.02, 1.37]; Wald Chi-square(61)=4.69, p=0.03, Figure 2H). Finally, in response to increased memory load, not only did TS-moderate subjects take significantly longer to respond than Controls (−33.7±7.3, t(61)=−4.60, p<0.0001), but they also took longer to respond than TS-low subjects (−24.3±8.3, t(61)=−2.95, p=0.0045; Figure 2I).
Table 3.
Comparison of eye movement performance across TS subgroups and Controls in all the tasks
| Task | Variable | Comparison (Group 1 vs. Group 2) | Group 1 (N) | Group 2 (N) | P value |
|---|---|---|---|---|---|
| Prosaccade | Latency (ms) | TS-low vs. Control | 265.1±3.4(20) | 274.8±5.3 (29) | <0.01* |
| TS-moderate vs. Control | 274.5±6.5 (19) | 274.8±5.3 (29) | 0.92 | ||
| TS-moderate vs. TS-low | 274.5±6.5 (19) | 265.1±3.4 (20) | 0.01* | ||
| Error rate (%) | TS-low vs. Control | 0.64±0.23 | 0.22±0.19 | 0.25 | |
| TS-moderate vs. Control | 0.68±0.24 | 0.22±0.19 | 0.054+ | ||
| TS-moderate vs. TS-low | 0.68±0.24 | 0.64±0.23 | 0.41 | ||
|
| |||||
| Antisaccade | Latency (ms) | TS-low vs. Control | 425.0±13.2 | 410.4±10.9 | <0.005* |
| TS-moderate vs. Control | 405.4±13.5 | 410.4±10.9 | 0.46 | ||
| TS-moderate vs. TS-low | 405.4±13.5 | 425.0±13.2 | 0.06+ | ||
| Error rate (%) | TS-low vs. Control | 21.3±2.9 | 17.6±2.4 | 0.88 | |
| TS-moderate vs. Control | 25.1±2.9 | 17.6±2.4 | 0.82 | ||
| TS-moderate vs. TS-low | 25.1±2.9 | 21.3±2.9 | 0.94 | ||
|
| |||||
| 0-back | Latency (ms) | TS-low vs. Control | 333.5±11.6 | 338.3±9.7 | 0.45 |
| TS-moderate vs. Control | 337.2±12.0 | 338.3±9.7 | 0.72 | ||
| TS-moderate vs. TS-low | 337.2±12.0 | 333.5±11.6 | 0.73 | ||
| Error rate (%) | TS-low vs. Control | 4.8±1.2 | 3.3±1.0 | 0.10 | |
| TS-moderate vs. Control | 6.8±1.2 | 3.3±1.0 | <0.0001* | ||
| TS-moderate vs. TS-low | 6.8±1.2 | 4.8±1.2 | 0.0002 | ||
|
| |||||
| 1-back | Latency (ms) | TS-low vs. Control | 349.1±16.4 | 353.4±13.8 | 0.58 |
| TS-moderate vs. Control | 392.6±16.8 | 353.4±13.8 | <0.0001* | ||
| TS-moderate vs. TS-low | 392.6±16.8 | 349.1±16.4 | <0.0001* | ||
| Error rate (%) | TS-low vs. Control | 21.5±2.7 | 20.6±2.3 | 0.85 | |
| TS-moderate vs. Control | 23.6±2.8 | 20.6±2.3 | 0.04* | ||
| TS-moderate vs. TS-low | 23.6±2.8 | 21.5±2.7 | 0.03* | ||
|
| |||||
| Working Memory Load (1-back – 0-back) | Latency (ms) | TS-low vs. Control | 15.6±14.5 | 15.0±12.1 | 0.20 |
| TS-moderate vs. Control | 55.4±14.9 | 15.0±12.1 | <0.0001* | ||
| TS-moderate vs. TS-low | 55.4±14.9 | 15.6±14.5 | 0.0045* | ||
| Error rate (%) | TS-low vs. Control | 16.6±2.4 | 17.3±2.0 | 0.18 | |
| TS-moderate vs. Control | 16.9±2.4 | 17.3±2.0 | 0.23 | ||
| TS-moderate vs. TS-low | 16.9±2.4 | 16.6±2.4 | 0.93 | ||
Format for all values: mean±standard error. P values are from planned comparisons from mixed effect models for latency and Poisson regression models for error rate; Means and standard error values for Controls are the same as those in table 2;
significant,
marginally-significant tendency
DISCUSSION
To our knowledge, this is the first eye movement study investigating executive function in TS to control for age, medication, IQ, gender and comorbid symptom severity across groups, allowing attribution of executive function deficits to increasing tic severity itself. These results support our hypothesis that increased tic severity in TS adolescents results in impaired executive functions, including response generation, response inhibition, and working memory. Specifically, TS subjects with increased tic severity had significantly higher error rates on the 0-back task than either TS subjects with low tic severity or Control groups. In the 1-back task, these subjects not only took longer to respond than Controls, but also than TS subjects with low tic severity, demonstrating slowing in voluntary tasks that could not be accounted for by sensorimotor slowing in response generation. Finally, the added working memory demand (1-back task compared to the 0-back task) caused greater slowing for TS subjects with increased tic severity, as evidenced by longer working memory load latencies compared to Controls and TS subjects with low tic severity. These findings suggest that TS subjects with moderate tic severity have executive function deficits.
For the n-back tasks, significant differences between subgroups appeared in 0-back error rate, and appeared as latency and error rate differences in the harder 1-back task. The pattern of error differences in the 0-back task (see Fig 2F) is the same as the pattern of error rate differences in the 1-back task (see Fig 2H). This similarity across the two tasks is supported by the strikingly equal Working Memory Load calculation across the different groupings (1-back minus 0-back performance; see Fig 2J). Despite the similarity in pattern there was an increase in the overall errors across tasks (overall mean ~22% vs. ~5% for 1-back vs. 0-back). In addition to this overall increase across all groups in error rates in the more demanding 1-back task, we also saw an increase in latency (overall mean ~370 ms vs. ~335 ms). More importantly, by breaking the TS-overall group down, we were able to show that this increase in latency with increasing working memory load was dependent on tic severity. Hence, it appears that whereas the subjects with higher tic severity (TS-moderate) had similar increases in error rates (comparable to other groups, see Fig 2J) in the 1-back task, the increased working memory load made them take longer to inhibit, manipulate, and ultimately generate the correct eye movement (see Fig 2I). Consistent with these findings, prior work has shown that deficits in memory search in girls with Tourette Syndrome have been suggested to be responsible for longer latencies in completing a letter-word fluency test (Schuerholz, Singer & Denckla, 1998).
Past eye movement studies in TS provide mixed results on both reflexive and voluntary performance. On a prosaccade task, studies report TS individuals have faster latencies (Farber et al., 1999), slower latencies (Mostofsky et al., 2001, Munoz et al., 2002) or comparable latencies to controls (Nomura et al., 2003, Straube et al., 1997). On the antisaccade task, a collection of groups found TS individuals took longer to respond (Dursun et al., 2000, Farber et al., 1999, Mostofsky et al., 2001, Munoz et al., 2002, Straube et al., 1997). Several found no difference in antisaccade errors (Munoz et al., 2002, Straube et al., 1997), whereas others identified increased errors compared to controls (Dursun et al., 2000, Farber et al., 1999, Mostofsky et al., 2001). Similar incongruities are found in the literature with memory-guided saccade tasks. Comparable to our n-back tasks, memory-guided tasks require participants to look to the location of a single remembered target. Nomura and colleagues found TS individuals were slower to respond in a memory-guided task compared to controls, but do not report errors (Nomura et al., 2003). Conversely, another study found no difference in latency, but do report an increase in misguided sequences for individuals with TS (Straube et al., 1997). These disagreements likely extend from not considering potential confounds, which make it difficult to interpret the findings.
Previous eye movement investigations in TS did not closely control for tic severity. While we grouped subjects with Tourette Syndrome by tic severity, we also reported results for TS-overall vs. Controls for two important reasons. First, this allows comparison of our results to the prior literature. Second, this analysis shows the importance of considering tic severity. Without considering tic severity (i.e., TS-overall), we would report significant differences from Controls in 0-back and 1-back errors, and 1-back and Working Memory Load latencies, when TS-low subjects show no differences from Controls. We found that tic severity significantly impacts several oculomotor measures of executive function; TS-moderate subjects made more errors in the 0-back condition and took longer to respond in the 1-back condition and Working Memory load calculation compared to both Controls and TS-low patients, with the latter responding comparably to Controls. Neuropsychological studies corroborate our findings, with increasing performance deficits in tasks of response inhibition and sustained attention as symptom severity increases (Ozonoff et al., 1998). Unfortunately, these studies did not report or carefully control age, comorbidities, or medication status.
Saccade parameters differ across the lifespan (Klein & Foerster, 2001). Most previous TS studies enrolled adults or a broad range of ages rather than only children (Dursun et al., 2000, Farber et al., 1999, Munoz et al., 2002, Straube et al., 1997). Only two studies have considered oculomotor development (Mostofsky et al., 2001, Nomura et al., 2003), and one found increased reflexive response time in participants less than 10 years old. There are also important developmental changes in antisaccade error rates. Only one prior study with TS individuals considered an age effect on antisaccade errors and found more errors in those less than 10 years old than those older than 10 years (Mostofsky et al., 2001). We report significant age effects even within the very narrow age range of 10–16, regardless of disease status. This suggests small non-significant inequalities in age between groups involving adolescents, when not controlled, could artificially lead to differences. Our oculomotor study is the first in TS to control for age so carefully, with case-control matching within six months of age.
The majority of individuals with TS also have symptoms of other disorders or conditions, the two most common being ADHD and OCD. Previous work shows oculomotor changes in these comorbid conditions (Klein et al., 2003, Rosenberg et al., 1997). By comparing low and moderate tic severity groups with equivalent comorbid symptom severity, we were able to definitively attribute the eye movement deficits we report to tic severity and not comborbidity status.
In our study, all TS subjects were off medication at least a week prior to testing. Psychiatric medications are known to alter eye movement performance (Babin, Hood, Wassef, Williams, Patel et al., 2011, Reilly et al., 2008). Yet, TS participants of previous studies were commonly tested while actively taking pharmacological treatments for their symptoms. Risperidone (dopamine and 5HT2A antagonist) is a common pharmacotherapy in TS and has been shown to slow reflexive saccade response time (Sweeney, Bauer, Keshavan, Haas, Schooler et al., 1997). Hence, it is possible TS studies that do not control for medication may show saccadic response time increases due to medication differences. For example, one previous study examined performance in risperidone-treated TS individuals and found increased reflexive eye movement response times (Munoz et al., 2002). Interestingly, risperidone has been shown to decrease antisaccade errors (Burke & Reveley, 2002). Studies in risperidone-treated TS individuals that do not control for medication may show normal antisaccade error rate (Munoz et al., 2002) that may be due to medication normalizing an antisaccade deficit in TS. While some groups did attempt to probe for a drug effect and argued for its absence, such post hoc analyses are known to be underpowered (Reilly et al., 2008). Hence, differences among previous studies may be engendered by medication differences.
Recent functional neuroimaging work in TS has focused on the cortical origins of the multiple frontalstriatal circuits. In a seminal paper, Sowell and colleagues reported thinning of gray matter in the motor and sensory cortices of children with pure TS, providing express evidence for involvement of the motor loop in TS (Sowell, Kan, Yoshii, Thompson, Bansal et al., 2008). Not only was thinning more pronounced in teens than children, but also was coupled with more severe tics, paralleling the progression of tic severity through adolescence.
Many imaging studies in TS have found significant changes in the gray and white matter of the dorsolateral prefrontal cortex (DLPFC), a cortical area that enables spatial memory, executive function, and attention. The DLPFC also allows proper response inhibition of prosaccades in an antisaccade task (Pierrot-Deseilligny et al., 2004). An early anatomical magnetic resonance imaging (MRI) study reported larger DLPFC volume in children with TS compared to controls (Peterson et al., 2001). Further, bilateral DLPFC cortical thickness correlated inversely with worst-ever tic severity (Sowell et al., 2008). So, too, in the orbitofrontal cortex (OFC), a frontal lobe area involved in inhibitory control, cortical volume negatively correlated with tic severity (Peterson et al., 2001), that is, more volume with decreasing tic severity. More white matter is found under these frontal lobe regions in individuals with TS compared to controls, implying greater connectivity with deep brain structures (Fredericksen et al., 2002).
Owing to known frontostriatal dysfunction, individuals with TS are expected to have neuropsychological deficits in addition to their tics (Eddy et al., 2009). Yet, TS-low subjects did not demonstrate executive function deficits in our oculomotor study. Because increased DLPFC and OFC brain matter is associated with less tic severity, several authors have interpreted their similar results as evidence of an adaptive, compensatory mechanism (Baym, Corbett, Wright & Bunge, 2008, Peterson et al., 2001, Spessot, Plessen & Peterson, 2004). This view is further supported by a functional MRI study in which effortful tic suppression activated vast areas of the prefrontal cortex (Peterson et al., 1998). Moreover, TS individuals had increased electroencephalogram coherence among frontal regions not only during voluntary tic suppression, but also during a Go-No Go task (Serrien, Orth, Evans, Lees & Brown, 2005). Critically, TS individuals had equivalent performance on the task as controls, suggesting the increased coherence was behaviorally compensatory.
Some have argued that because increased tic severity in early adolescence is coupled with the rigid expectations of school and social settings, youth with TS continually tap these prefrontal regions to suppress tics. Over time, activity-dependent enlargement of prefrontal cortices builds the capacity for inhibitory functions (Spessot et al., 2004). The prefrontal cortex has long been connected with this type of self-regulatory control, arbitrating working memory and inhibition. The same frontal regions also moderate the cognitive control of voluntary eye movements (Pierrot-Deseilligny et al., 2004). Thus, we theorize that in TS individuals with low tic severity, while abnormalities of motor and sensory cortices underpin the presence of tics, the enlarged prefrontal regions of DLPFC and OFC not only adaptively protect against worse symptoms, but also attenuate neuropsychological and eye movement deficits. Given that tic severity waxes and wanes, it is possible that executive function deficits with increased tic severity may accelerate progression. If thinning of motor and sensory cortices progresses, tics worsen and executive function deficits outstrip prefrontal compensation (as in our TS-moderate patients). This model predicts that marked motor and sensory thinning evokes severe tics, and emaciation of the prefrontal compensatory mechanism produces profound neuropsychological and eye movement deficits.
CONCLUSION
Our study is the first to investigate the impact of tic severity on oculomotor performance in TS while controlling for confounds of age, medication, and comorbid symptom severity. As demonstrated here, significant changes on oculomotor measures with even small changes in age in adolescents underscores the importance of controlling for age. We found that individuals with moderate levels of tic severity demonstrate significant deficits in voluntary response generation, response inhibition, and working memory whereas individuals with low levels of tic severity largely did not differ from Controls. These executive function deficits with increased tic severity were not due to increased comorbid symptom severities. We suggest that TS individuals with low levels of tic severity may recruit executive functions to manage their tics, and thus are successfully compensating, showing little evidence of executive function deficits on oculomotor tasks. Critically, that TS subjects with increased tic severity displayed significant executive function deficits underscores the importance of assessing tic severity when considering treatment selection, education accommodation, and academic performance.
Supplementary Material
KEY POINTS.
Controlling for medication, age, and comorbid symptom severities, we found that increasing tic severity was associated with executive function deficits.
Specifically, individuals with moderate levels of tic severity demonstrated deficits in voluntary response generation, response inhibition, and working memory whereas individuals with low levels did not differ from Controls, and may be successfully compensating.
These executive function deficits were not due to increased comorbid symptom severities.
Given that tic severity waxes and wanes, executive function deficits with increased tic severity underscores the importance of time and state of assessment when considering treatment selection and affects on academic performance.
We also demonstrate executive function changes with even small age differences in adolescents, underscoring the importance of controlling for age.
ACKNOWLEDGEMENTS
This research was supported by grants from the NIH [(P30-EY010608), The University of Texas Health Science Center at Houston Clinical and Translational Science Award (TL1RR024147)], NSF (0924636), and the Philanthropic Educational Organization National Scholar Award.
Footnotes
Supporting Information Additional Supporting Information is provided along with the online version of this article.
All authors have declared that they have no competing or potential conflicts of interest.
References
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Babin SL, Hood AJ, Wassef AA, Williams NG, Patel SS, Sereno AB. Effects of haloperidol on cognition in schizophrenia patients depend on baseline performance: a saccadic eye movement study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1753–1764. doi: 10.1016/j.pnpbp.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baym CL, Corbett BA, Wright SB, Bunge SA. Neural correlates of tic severity and cognitive control in children with Tourette syndrome. Brain. 2008;131:165–179. doi: 10.1093/brain/awm278. [DOI] [PubMed] [Google Scholar]
- Broerse A, Holthausen EA, Van Den Bosch RJ, Den Boer JA. Does frontal normality exist in schizophrenia? A saccadic eye movement study. Psychiatry Res. 2001;103:167–178. doi: 10.1016/s0165-1781(01)00275-x. [DOI] [PubMed] [Google Scholar]
- Bruun DB, Budman CL. The natural history of Gilles de la Tourette Syndrome. In: Kurlan R, editor. Handbook of Tourette's Syndrome and Related Tic and Behavioral Disorders. Marcel Dekker; New York: 2005. pp. 23–38. [Google Scholar]
- Burke JG, Reveley MA. Improved antisaccade performance with risperidone in schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72:449–454. doi: 10.1136/jnnp.72.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Namkoong K, Kim CH, Kim JJ, Lee JD. Dopamine transporter density of the basal ganglia assessed with [123I]IPT SPECT in drug-naive children with Tourette's disorder. Psychiatry Res. 2004;130:85–95. doi: 10.1016/j.pscychresns.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Crawford S, Channon S, Robertson MM. Tourette's syndrome: performance on tests of behavioural inhibition, working memory and gambling. J Child Psychol Psychiatry. 2005;46:1327–1336. doi: 10.1111/j.1469-7610.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- Dupaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. The Guilford Press; 1998. [Google Scholar]
- Dursun SM, Burke JG, Reveley MA. Antisaccade eye movement abnormalities in Tourette syndrome: evidence for cortico-striatal network dysfunction? J Psychopharmacol. 2000;14:37–39. doi: 10.1177/026988110001400104. [DOI] [PubMed] [Google Scholar]
- Eddy CM, Rizzo R, Cavanna AE. Neuropsychological aspects of Tourette syndrome: a review. J Psychosom Res. 2009;67:503–513. doi: 10.1016/j.jpsychores.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Farber RH, Swerdlow NR, Clementz BA. Saccadic performance characteristics and the behavioural neurology of Tourette's syndrome. J Neurol Neurosurg Psychiatry. 1999;66:305–312. doi: 10.1136/jnnp.66.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Coles M, Huppert JD, Pasupuleti RV, Franklin ME, March J. Development and validation of a child version of the obsessive compulsive inventory. Behav Ther. 2010;41:121–132. doi: 10.1016/j.beth.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Fredericksen KA, Cutting LE, Kates WR, Mostofsky SH, Singer HS, Cooper KL, Lanham DC, Denckla MB, Kaufmann WE. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58:85–89. doi: 10.1212/wnl.58.1.85. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Mohapatra L, Shea HB. Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychol Med. 2004;34:921–932. doi: 10.1017/s003329170300165x. [DOI] [PubMed] [Google Scholar]
- Hutton SB. Cognitive control of saccadic eye movements. Brain Cogn. 2008;68:327–340. doi: 10.1016/j.bandc.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Mueller SC, Hambleton K, Hollis CP. Enhanced cognitive control in Tourette Syndrome during task uncertainty. Exp Brain Res. 2007;182:357–364. doi: 10.1007/s00221-007-0999-8. [DOI] [PubMed] [Google Scholar]
- Jeter CB, Patel SS, Sereno AB. Novel n-back spatial working memory task using eye movement response. Behav Res Methods. 2011;43:879–887. doi: 10.3758/s13428-011-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- Klein CH, Raschke A, Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40:17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Lasker AG, Singer HS, Denckla MB, Zee DS. Oculomotor abnormalities in boys with Tourette syndrome with and without ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:1464–1472. doi: 10.1097/00004583-200112000-00018. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Jackson GM, Dhalla R, Datsopoulos S, Hollis CP. Enhanced cognitive control in young people with Tourette's syndrome. Curr Biol. 2006;16:570–573. doi: 10.1016/j.cub.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Muller SV, Johannes S, Wieringa B, Weber A, Muller-Vahl K, Matzke M, Kolbe H, Dengler R, Munte TF. Disturbed monitoring and response inhibition in patients with Gilles de la Tourette syndrome and co-morbid obsessive compulsive disorder. Behav Neurol. 2003;14:29–37. doi: 10.1155/2003/832906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Le Vasseur AL, Flanagan JR. Control of volitional and reflexive saccades in Tourette's syndrome. Prog Brain Res. 2002;140:467–481. doi: 10.1016/s0079-6123(02)40069-6. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Fukuda H, Terao Y, Hikosaka O, Segawa M. Abnormalities of voluntary saccades in Gilles de la Tourette's syndrome: pathophysiological consideration. Brain Dev. 2003;25(Suppl 1):S48–54. doi: 10.1016/s0387-7604(03)90009-x. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL, Mcmahon WM, Filloux F. Inhibitory deficits in Tourette syndrome: a function of comorbidity and symptom severity. J Child Psychol Psychiatry. 1998;39:1109–1118. [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain Cogn. 2008;68:415–435. doi: 10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Averbach DH, O'hearn KM, Seymour AB, Birmaher B, Sweeney JA. Oculomotor response inhibition abnormalities in pediatric obsessive-compulsive disorder. Arch Gen Psychiatry. 1997;54:831–838. doi: 10.1001/archpsyc.1997.01830210075008. [DOI] [PubMed] [Google Scholar]
- Schuerholz LJ, Singer HS, Denckla MB. Gender study of neuropsychological and neuromotor function in children with Tourette syndrome with and without attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:277–282. doi: 10.1177/088307389801300607. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Orth M, Evans AH, Lees AJ, Brown P. Motor inhibition in patients with Gilles de la Tourette syndrome: functional activation patterns as revealed by EEG coherence. Brain. 2005;128:116–125. doi: 10.1093/brain/awh318. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, Toga AW, Peterson BS. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11:637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spessot AL, Plessen KJ, Peterson BS. Neuroimaging of developmental psychopathologies: the importance of self-regulatory and neuroplastic processes in adolescence. Ann N Y Acad Sci. 2004;1021:86–104. doi: 10.1196/annals.1308.010. [DOI] [PubMed] [Google Scholar]
- Straube A, Mennicken JB, Riedel M, Eggert T, Muller N. Saccades in Gilles de la Tourette's syndrome. Mov Disord. 1997;12:536–546. doi: 10.1002/mds.870120410. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Bauer KS, Keshavan MS, Haas GL, Schooler NR, Kroboth PD. Adverse effects of risperidone on eye movement activity: a comparison of risperidone and haloperidol in antipsychotic-naive schizophrenic patients. Neuropsychopharmacology. 1997;16:217–228. doi: 10.1016/S0893-133X(96)00195-9. [DOI] [PubMed] [Google Scholar]
- Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with autism and Tourette syndrome. Dev Psychopathol. 2005;17:415–445. doi: 10.1017/s0954579405050200. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Harcourt Assessment, Inc; San Antonio, TX: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.