Abstract
Sleep is not a single state, but a complex set of brain processes that supports a number of physiological needs. Sleep deprivation is known to affect attention in many animals, suggesting that a key function of sleep is to regulate attention. Conversely, tasks that require more attention drive sleep need and sleep intensity. Attention involves the ability to filter incoming stimuli based on their relative salience, and this is likely to require coordinated synaptic activity across the brain. This capacity may have only become possible with the evolution of related neural mechanisms that support two key sleep functions: stimulus suppression and synaptic plasticity. We argue here that sleep and attention may have co-evolved as brain states that regulate each other.
Keywords: sleep, attention, brain, evolution
Sleep and attention
Daily experience is continuously shaped by how we perceive our surroundings. Selective attention heightens our awareness of some things, while dampening our awareness of others. In contrast, our perception fades more broadly during sleep, leaving us mostly unaware of the outside world. Although these two different perceptual states – sleep and selective attention – may at first seem like polar opposites, they both appear to regulate our awareness by suppressing incoming information from the environment. Understanding how and why the brain gives rise to these seemingly different perceptual states could reveal a deep connection between sleep and attention.
In recent years, sleep and attention have been investigated in smaller animal models that have provided insights into the molecular and physiological nature of these states, as well as their functions. These studies extend to invertebrate animals such as the fruit fly, Drosophila melanogaster, which despite having vastly different neuroanatomy from that of humans, has similar capacities for sleep and selective attention [1-4]. Even more surprisingly, the nematode Caenorhabditis elegans (C. elegans), which consists of a mere 302 neurons, appears to have sleep-like behaviour characterized by quiescence, reduced arousal and homeostasic regulation [5]. However, a problem that arises here is how to define ‘sleep’ and ‘attention’, and whether these are similar in all animals. C. elegans research suggests that sleep may have begun as an energy-conserving ‘down-state’ in order to cope with an animal's developmental needs (e.g., molting) and the impact of environmental stress (e.g., heat) [5, 6]. However in animals with more complex nervous systems, such as insects and mammals, sleep is not simply tied to development or stress but is an everyday occurrence that is needed to support cognitive functions. Selective attention is one such cognitive function, describing an animal's capacity to actively ‘ignore’ irrelevant stimuli in order to act on selected stimuli. As brains became more complex, sleep may have evolved to support the extensive daily plasticity required for curating selective attention mechanisms, while simultaneously maintaining its more primitive functions.
In this review, we focus on the role of sleep in regulating attention and how these two processes may have co-evolved as interconnected and complementary states, much like the indivisible “yin” and “yang” of Chinese philosophy. Firstly, it could be argued that the daily need for sleep arose at the same time as animals became capable of operant learning (see Box 1) and selective attention, with the emergence of centralized nervous systems. Secondly, although sleep and attention are different behavioural states, they share the fundamental property of blocking information flow, allowing animals to suppress the outside world on either a global or a selective level. Thirdly, sleep and attention regulate each other; sleep is crucial for maintaining optimal levels of attention, and recent evidence suggests that attentional demands can also regulate sleep need. We propose that sleep and attention are complementary brain states that co-evolved as brains became more complex.
Highlights.
-
1)
sleep and selective attention are both perceptual suppression mechanisms
-
2)
sleep functions may have co-evolved with selective attention
-
3)
sleep and selective attention regulate each other
The evolution of sleep functions
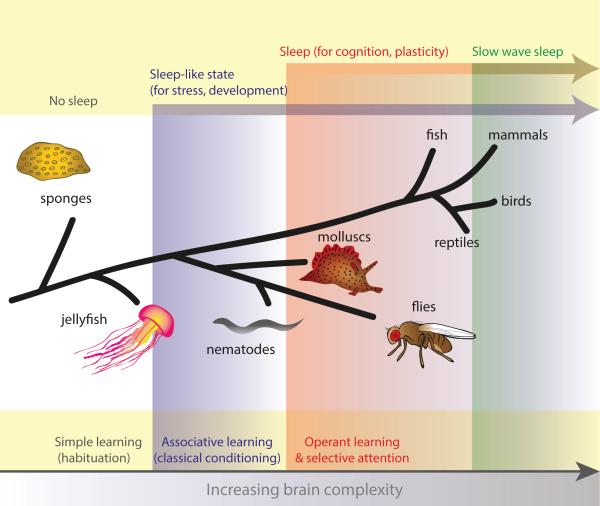
Sleep is a reversible state of reduced activity and lowered sensory awareness, that has been found in most animal species studied so far [7]. However, it is becoming clear that sleep need and sleep functions can vary widely among animals. To understand how sleep functions evolved, it may help to begin with the simplest of animals. Firstly, it seems that sleep requires a nervous system with a certain degree of complexity, as sleep does not appear to be found in unicellular animals (e.g., paramecia), animals without neurons (e.g., adult sponges), or in animals without a central nervous system (e.g., jellyfish) (Figure 1), although this has not been well studied. The simplest animal in which a sleep-like state has been identified is the nematode, C. elegans (Figure 1). The sleep-like state in these nematodes is different from sleep in other animals in that it seems specifically tied to development, occurring just prior to each molting period (‘lethargus’), but unlike other animals they do not sleep on a regular basis throughout adult life [5]. This raises the possibility that an ancient function of being in a restive, unresponsive state may have been to allow an animal to direct its energy into crucial developmental pathways. Support for this idea comes from the fruit fly, Drosophila, in which it has been shown that depriving a juvenile fly of sleep, when its brain is still developing, leads to long lasting cognitive and behavioural deficits [8, 9]. We also know that human infants sleep substantially more than adults, sleeping even in the womb, and that this is thought to be important for brain development [10].
Figure 1. Evolution of sleep and cognitive processes.
As brains became more complex during evolution, animals gained the capacity for more sophisticated forms of cognition such as operant learning and selective attention (bottom yellow panel). At the same time, sleep evolved as a behavioural state that supported not only primitive functions such as stress and development, but also the plasticity needed for learning and attention (top yellow panel). Slow-wave sleep may be one mechanism to achieve synaptic plasticity and support cognition in mammals and birds, whereas this may be achieved in other animals by prolonged down-states in neural activity that occur during sleep. Importantly, sleep can be divided into two types: a primitive sleep-like state for stress responses and key developmental pathways which originated in animals with simpler nervous systems (e.g., certain nematodes, blue arrow) and a sleep state needed for the daily plasticity demands of cognition in animals with brains (e.g., from flies and molluscs to vertebrates, red arrow at the top of figure).
Another function of sleep that must have arisen early in evolution is the ability to cope with stress from the external environment (Figure 1). Again, this is evident in C. elegans, which shows stress-induced quiescence. C. elegans enters a quiescent sleep-like state in response to environmental stressors such as heat, cold, osmotic stress and tissue damage, and failure to become quiescent in response to these stressors affects survival [6, 11]. Perhaps similarly, flies require stress-response genes to cope with sleep deprivation [12] and need to sleep more to recover from bacterial infection [11]. It is not clear why being in a quiescent, unresponsive state should aid stress recovery, although recent evidence in C. elegans suggests that it helps avoid further cellular stress and protein aggregation that may occur when cells remain active [6]. Alternatively, sleep could play a more active role in harnessing the immune system to combat infection. For example, humans sleep more following infection, possibly because sleep facilitates an adaptive immune system response [13]. In any case, given that responding to environmental stress is a fundamental requirement for any animal and appears to influence sleep need across a broad range of species, it is likely to be a more ancient and primitive function of sleep. Importantly, these sleep functions appear to be acute rather than chronic: sleep is in response to a sudden stress or (e.g., heat), or is linked to a developmental transition (e.g., molting). It is quite unlike the daily homeostatic ‘correction’ that requires hours of downtime in all animals endowed with a brain.
As brains became more complex, animals developed adaptive behaviours such as learning, memory and selective attention. It seems plausible that primitive sleep functions co-evolved together with novel sleep functions that also benefitted from behavioural quiescence, such as synaptic plasticity mechanisms to support cognitive behaviours. Although extensive evidence suggests that sleep facilitates learning and memory consolidation in mammals [13-15], there is relatively less evidence for this in simple organisms, perhaps because it depends on the type of learning. The simplest form of learning, habituation (desensitization to a single stimulus following repeated presentation), is present in jellyfish [16] (Figure 1) and even certain plants [17]. However, with the possible exception of some jellyfish that call for a closer look [18], the fact that life-forms without a central nervous system do not need to sleep is telling because it suggests that this simple form of learning does not require sleep-dependent plasticity. Likewise, C. elegans shows habituation as well as simple classical conditioning, (in which an animal learns to elicit a reflexive response to a conditioned stimulus through repeated stimulus pairings) [19]. However, sleep has not been shown to be important for maintaining optimal behavioural functions in adult C. elegans.
Sleep appears to be particularly important for cognitive processes in animals capable of operant learning and selective attention (Figure 1). Operant (or instrumental) learning is a process whereby an animal learns the consequences of its own actions so that it can modify its behaviour accordingly (Box 1). Animals do this by selecting some motor programs while actively suppressing others. Although operant behaviour has not been demonstrated in nematodes, it is present in invertebrates with more complex nervous systems, such as Drosophila, as well as in some molluscs such as the sea-slug, Aplysia [20, 21]. It is possible that some animals, such as some nematodes for example, may be found to be incapable of operant learning because they might lack the capacity for selective attention. Operant learning appears to require the ability to actively suppress irrelevant information while enhancing processing of relevant information. This is in contrast to classical conditioning, in which an animal does not require selective attention because it is only faced with a single option – to involuntarily learn an association between two stimuli. Interestingly, the capacity for operant learning and selective attention appears to have coincided with new sleep functions to support cognition and brain plasticity (Figure 1). These functions of sleep in cognition probably arose already in insects; in flies, sleep deprivation is detrimental to operant visual learning and courtship conditioning [9, 22, 23], whereas inducing sleep can improve these memory readouts, and can even restore behavioural plasticity to Drosophila mutants with operant learning deficits [24] [25]. In contrast, sleep-dependent processes supporting cognition have not been demonstrated for C. elegans or animals with simpler nervous systems (Figure 1), although it is not clear whether this is because it does not exist or because it has not been properly tested. Sleep therefore may have evolved into a brain state that achieved a multitude of functions including development, stress recovery, and brain plasticity. However, the evolutionary novelty in flies and most other bilaterians is that sleep became a rapidly reversible brain state that responds to ongoing plasticity demands, rather than as part of a developmental pathway or an acute stress response.
Sleep and attention are suppression states
Although sleep and attention may outwardly appear to be very different behavioural states, they are similar in that they both involve filtering out information from our awareness. For example, when we are asleep, we are generally unaware of outside noises unless they are very intrusive. Similarly, when we are awake we are able to tune into a conversation in a noisy environment by ignoring other noise - the ‘cocktail party effect’. But how does the brain achieve this?
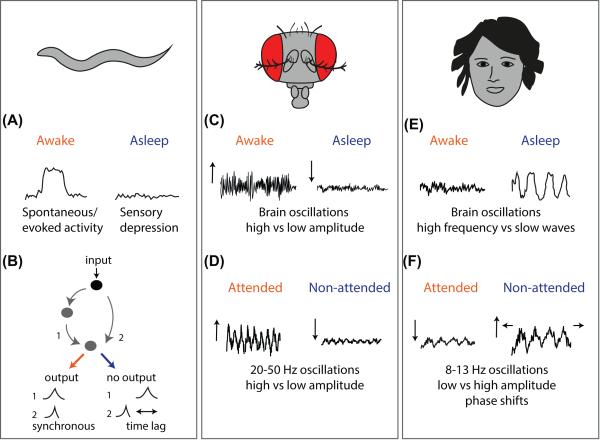
Information processing in neural networks can be suppressed in different ways. One way is to reduce the intrinsic excitability of individual neurons. This is already evident in simple animals, such as C. elegans, in which two different types of sensory neurons, ALM and ASH, show reduced excitability during the worm's sleep-like state [26-28] (Figure 2). A similar reduction in intrinsic or evoked excitability has also recently been observed in the Kenyon neurons during Drosophila sleep [29], suggesting that suppression at the individual neuron level is a key characteristic of the sleep state. However, behaviours are not simply produced from individual neurons; they also rely on the coordinated communication between neurons. Interestingly, it was recently discovered that the coordination of a particular neural circuit was disrupted during nematode sleep-like behaviour, due to a lag in neural response in one of the interneurons within the circuit, ultimately leading to a loss of behavioural output [26] (Figure 2). These studies are important because they suggest that two basic ways to block information flow is by reducing neural activity or by disrupting the timing and coordination of neural responses - two suppression mechanisms that could represent the building blocks to sleep and attention mechanisms in animals with more complex nervous systems. Any increase in brain complexity would therefore be intimately linked to an increased capacity to block information flow.
Figure 2. Sleep and attention are suppression states.
A) In the nematode, C. elegans, sensory neurons display less spontaneous activity and reduced responses to external stimuli during sleep-like states, and B) the timing of neural responses within a circuit becomes less coordinated [26, 28]; during wake, stimulation of a sensory neuron (black circle) leads to synchronous firing of two different interneurons (grey circles), resulting in a motor response, whereas the output is suppressed during sleep-like behaviour due to a time-lag in one of the interneurons [26]. C) Sleep in the fruit fly Drosophila melanogaster is characterized by an overall suppression of neural activity recorded in the local field potential oscillations in the brain [56, 57]. D) In Drosophila, attention towards a visual stimulus coincides with an increase in 20-50 Hz oscillations in the brain, whereas the same oscillations are suppressed when the stimulus is ignored [36, 37]. E) In humans, wake is characterized by high frequency activity, whereas during slow-wave sleep, large amplitude slow-wave oscillations indicate prolonged up-states (neural activity) and down-states (suppression of neural activity). F) Alpha oscillations (8-13) Hz are thought to provide a gating mechanism for visual attention in humans; brain regions with low amplitude alpha oscillations correspond with attention to a stimulus, whereas high amplitude or phase shifted alpha oscillations suppress the processing of unattended stimuli [40, 42-45].
Suppression of information processing at the single cell level has been observed in insects during selective attention and voluntary behaviour. In dragonflies, selection and suppression dynamics are observed in object-detection neurons when presented with competing visual cues [30], analogous to selective attention in primates [31]. Active behaviour also involves suppression mechanisms: a recent study in Drosophila found that responses of motion-detection neurons are inhibited when flies make voluntary turning movements [32]. In other words, flies can produce an ‘efference copy’ (a copy of the fly's motor command) to transiently suppress perception of self-generated motion, similar to primates during eye movements [33]. Interestingly, rapidly reversible suppression of the same types of motion-sensitive neurons has also been found to occur during sleep in honeybees [34]. Thus, at the level of a single neuron, it might be impossible to tell whether an insect is asleep or just paying attention to something else.
In animals with brains, suppression during sleep and selective attention is also evident at the level of neural oscillations. Oscillations are produced from the synchronous firing of many neurons, and these oscillations can then feed back to influence neuronal firing patterns, thereby creating complex and emergent network activity [35]. This interplay between neural firing and oscillations allows for a wider range of neural activity that is probably necessary for selective attention, operant learning and plasticity. Oscillatory activity associated with selective attention has been found even in the fruit fly (Figure 2), where oscillations in the 20-50 Hz frequency range have been associated with selection and suppression of novel or salient stimuli [36-38]. In the mammalian brain, gamma oscillations (30-90 Hz) are associated with sensory processing, through coordinated excitation and inhibition [39], while lower frequency alpha oscillations (8-13 Hz) may be more important for prioritising or organising gamma activity [40, 41] (discussed below). While it is notable that oscillatory activity is a fundamental property of larger and smaller brains, the exact frequency range involved is probably less important than its capacity to organize or suppress information flow.
During selective attention in humans, oscillatory activity in the alpha frequency range is thought to suppress unattended stimuli through pulsed inhibition, such that the brain can efficiently process a selected stimulus at a given time [40, 42]. Alpha oscillations are stronger in unattended brain regions and weaker in brain regions processing an attended object [43-45] (Figure 2). Suppression can occur because network oscillations influence the subthreshold oscillations of individual neurons, placing them in a hyperpolarized state or a depolarized state, in which the neuron is either less or more likely to fire, respectively; when alpha oscillation amplitudes are greater, the suppression is also stronger. This also means that neurons may fire at different phases of an oscillation, depending on their threshold for inhibition, creating a ‘temporal phase code’ that regulates attention [40]. This kind of phase coding is not unique to alpha frequencies but also occurs with slightly slower theta frequencies (4-7hz) in rats, and also with slow wave oscillations (1-4hz) in macaque monkeys [46-49]. Oscillatory neural activity therefore allows information flow to be shaped by rhythmic pulses of inhibition and release from inhibition. Furthermore, the patterns of neural activity that occur during selective attention could also determine which circuits are strengthened (ones that are active during attention) and which are weakened (ones that are inactive while stimuli are being ignored). Thus, the emergence of oscillatory activity in the brain is likely to have produced new modes of suppression, giving rise to more diverse patterns of network activity and plasticity in the brain.
During sleep in mammals and birds, information flow can be disrupted by slow-wave oscillations. Rhythmic firing across many neurons can lead to the loss of information, as it inhibits the more varied patterns of neural activity that occur during wakefulness. Why would slow wave oscillations be present as a mode of suppression during sleep, but not attention? One possibility is that because slow waves spread much greater distances than shorter frequency waves [50-52], they can engulf the whole brain (especially larger mammalian brains) into a suppression state, rather than just some neuronal networks. This may allow for the greater levels of suppression (blocking out most stimuli) seen during sleep compared to selective attention. During slow-wave sleep, signals are therefore less able to propagate long distances. This has been demonstrated by transcranial magnetic stimulation in humans: when a stimulus is delivered during wakefulness, oscillations are induced and spread through the cortex, whereas during slow-wave sleep they are initiated but do not propagate [53].
By creating a suppression state that blocks out the external world, slow wave sleep also provides a unique environment for synaptic plasticity processes that may support learning, attention and memory [54, 55]. However, slow-wave sleep appears to be the exception among animals, being observed only in mammals and birds. In flies, a global decrease in activity instead is observed across all frequencies, in both spontaneous and pharmacologically-induced sleep [25, 56, 57] (Figure 2). Since slow wave sleep essentially involves rhythmic periods of hyperpolarisation, one possibility is that neurons simply require prolonged ‘down states’ in which neuronal assemblies are inactive. In mammals and birds ‘down states’ may occur optimally during slow-wave sleep, whereas in other sleeping animals, including flies, they may involve an overall depression in neural activity across a broader frequency range. Both modes of suppression could essentially block information flow and allow synaptic plasticity functions to occur at the same time (Figure 2). Suppression states that regulate plasticity can also be triggered by changes in the neuromodulatory environment. For example, a recent study in Drosophila found that during sleep there is a suppression of dopaminergic signalling, which promotes memory retention by preventing forgetting [58]. Interestingly, dopamine levels are also associated with suppression mechanisms that occur during selective attention in Drosophila [59]. In other words, like sleep, attention may use similar neuromodulatory mechanisms to block information flow, albeit in a more localised or variegated fashion. Whether these neuromodulatory effects are conserved across animals, and whether they correlate with sleep functions, remains an important area for future research.
It is important to note that while sleep is a state of general sensory suppression, it does not block all information processing. Like selective attention, the ability to block out most sensory information during sleep may help to process information more selectively. For example, sleep promotes reactivation and consolidation of recently encoded memories [14], making these memories more easily retrieved during subsequent wakefulness [60]. Memories consolidated during sleep can also be influenced to some degree by exposure to external sensory information during sleep such as odors [61-63], which can even alter the content of our dreams [64]. Yet unlike wakefulness, which creates a brain state that is optimal for encoding new information through sensory experience, sleep causes the brain to be largely shut off from the sensory world.
Sleep and attention regulate each other
An increasingly popular view suggests that sleep is inextricably linked with synaptic plasticity [14, 15, 54]. If sleep is the price we pay for plasticity [54], what is the consequence of losing sleep? One obvious effect of having a bad night's sleep is the inability to pay attention effectively the following day. When we are sleep deprived, our reaction times are slower and our behaviour becomes unstable and unpredictable [65, 66]. However, although our attention is affected, basic sensory responses such as vision or auditory processing remain largely intact [67-70]. Attention allows us to focus on a single percept while suppressing others, and may therefore be particularly vulnerable to sleep loss because it is highly dependent on precisely timed suppression mechanisms across the brain, to dynamically select and suppress competing stimuli. This is evident even in the fruit fly, where attention-like behaviour is associated with increased temporal coordination between brain regions [71]. It follows that any systemic imbalance in synaptic function could disrupt this level of brain-wide coordination. In addition, sleep deprivation affects our ability to deal with high perceptual load (large amounts of information), and disrupts functional connectivity between different brain regions [72-74]. Sleep could be the price we pay for our capacity to pay attention – to maintain precisely timed communication across multiple circuits in the brain for selection and suppression of stimuli
Learning processes that require selective attention are also likely to be modulated by sleep, compared to more simple forms of learning such as classical conditioning or habituation. As mentioned earlier, operant learning involves attention processes as it requires an animal to learn to selectively associate an action with a consequence, from many possible associations, whereas classical conditioning is a much simpler form of learning that does not necessarily require selective attention (e.g. it can even occur during sleep [75] and minimally conscious states [76]). In Drosophila, sleep deprivation has been shown to affect learning and memory in operant learning tasks such as in courtship conditioning or aversive phototaxis suppression [9, 22, 23], whereas effects on classical conditioning are more subtle, depending on the time of day and the time tested following training [77, 78]. Furthermore, a closer examination of sleep effects on classical conditioning found that there was no effect of sleep on acquisition of memories via classical conditioning; only memory retention or “forgetting” was affected [58]. Similarly, studies in honeybees and rodents found that sleep deprivation did not affect classical olfactory reward conditioning; however, extinction of the memory was impaired by sleep deprivation [79, 80]. Compared to classical conditioning, extinction learning may be more similar to operant learning in that it requires a more complex network of brain structures [55] and also requires selective attention; unlike classical conditioning which involves spoon-feeding associations to the animal, extinguishing a memory relies on an animal's ability suppress pre-potent responses and to pay attention on its own to important cues in its environment, creating new memories to overwrite the old. Altogether, these experiments suggest that learning processes that require more attention and more coordinated activity across the brain have a greater dependency on sleep.
While it is clear that sleep regulates attention, the reverse may also be true: that attention drives sleep need. This is because attention is crucial for many forms of learning, and the more an animal learns during the day, the more it might require sleep-dependent plasticity to regulate and respond to the synaptic changes accrued during wake. But how can we know if attentional load influences sleep? There are already a number of clues from studies in humans, rodents and flies that sleep need increases following tasks or experiences that specifically engage selective attention. In humans, training in visual learning, perception and working memory tasks leads to increases in slow-wave activity, a marker of sleep pressure, in brain regions that are important for visual processing and attention [50, 81-83]. Slow wave activity also increases in rats following operant learning or when they explore a novel environment [84, 85]. Although none of these studies has looked specifically at how much attention drives sleep need, it is likely that attention is a key factor because it is intricately linked with operant learning and is also engaged by novel exploration through exposure to salient events. Similarly, flies that are reared in more social environments or exposed to visual and tactile stimuli need to sleep more, as well as more deeply [57, 86-88], possibly because they are exposed to greater novelty. Interestingly, human attention disorders such as autism, schizophrenia and attention-deficit/hyperactivity disorder (ADHD) have also been associated with reduced or disrupted sleep [89-91], whereas periods of intense learning such as childhood and adolescence are associated with a greater need for sleep and a greater amount of slow-wave activity after learning [10, 92]. In summary, a number of studies suggest that sleep may be influenced by learning and attention in different species, supporting the view that sleep and attention may regulate each other bi-directionally.
Conclusions & future directions
Although sleep appears to be widely conserved among species, recent advances in studying potential sleep functions in animal models have highlighted the need for a better definition of sleep; one way to do this is to consider how it may have evolved. Early in evolution, sleep may have simply been a ‘down state’ to conserve energy required for development and to cope with environmental stress. However, as nervous systems became more complex, this down state came under the control of the brain to primarily regulate the plasticity processes required for attention and learning – which we now know are crucial functions of sleep. Should the more primitive forms of sleep that are tied to development or stress processes (seen in C. elegans, for example) really be called ‘sleep’ or ‘sleep-like’? Certainly, there may be many insights to be gained in relation to sleep's role in stress and development; metabolite clearance during sleep [93] could fall under stress-related sleep functions, and any physical changes in neuronal wiring that occur during sleep may involve conserved developmental pathways. However, the link between behavioural quiescence and these primitive sleep functions in animals does not tell the full story about the importance of sleep for cognition. It is important to emphasize that, however we define sleep, there may be key differences in sleep function when comparing a worm to a fly to a human, and one general term (sleep) may not be completely appropriate as it potentially confuses the field.
Although sleep-like states may serve various functions in different species, one common property of these states is that they decrease responsiveness to the outside world. Likewise, selective attention is also fundamentally a decrease in responsiveness to most external stimuli, except for the one being momentarily attended to. The capacity to suppress information flow may have begun in simple nervous systems by reducing neural activity and coordinated circuit communication, later evolving into more complex modes of suppression that involve interplay between neural spiking and global oscillations. Blocking information flow globally may produce a loss of behavioural responsiveness that could simultaneously accommodate a variety of sleep functions, such as synaptic plasticity relating to memory and attention processes, but also more ancient stress responses. For example, slow-wave sleep has been hypothesised to provide an optimal brain state for memory consolidation as it suppresses encoding of new memories [94]. Alternatively, the synaptic homeostasis hypothesis for sleep (SHY), proposed by Tononi and Cirelli, [54], suggests that slow-wave sleep evolved as a mechanism to proportionally downscale synapses. However, slow-wave sleep is also associated with basic cellular processes such as metabolite clearance [93], suggesting that ancient sleep functions may have been repackaged with more recent sleep functions associated with learning.
If there is indeed proportional synaptic renormalization that takes place during sleep (most likely involving strengthening as well as downscaling of synapses [95, 96]), then it is probably to counterbalance the necessarily non-proportional synaptic changes across the brain that result from selective attention during wakefulness. Sleep and attention may therefore share a deeper relationship than previously thought, using similar suppression mechanisms to ignore the outside world while simultaneously influencing each other by regulating synaptic plasticity in a complementary way. This view raises several questions that could be addressed in future research, by examining the mechanisms of sleep and attention in different animals and how they are related at a behavioural, electrophysiological and molecular level (Box 2). More broadly, it may be productive to study sleep and wakefulness as complementary states rather than as fundamentally different brain phenomena.
Box 1: Operant learning.
Operant learning is a process by which an animal learns to adapt actions to achieve certain consequences in the environment. It is different from classical conditioning (making associations between paired stimuli) in that the animal must select the appropriate motor sequence to earn rewards or avoid punishment, and therefore has some degree of control over its learning. Although classical conditioning may be affected by internal drive (e.g. hunger or thirst, to receive a reward) it does not necessarily require selective attention as it can occur during sleep or minimally conscious states [75, 76]. In contrast, operant learning requires selective attention because the animal learns to select the correct action and suppress maladaptive motor programs. A number of behavioural paradigms have been used to study operant learning in different model organisms, with some examples outlined below.
Rodents: Operant learning in rodents is often studied by associating a reward (e.g. food) or punishment (e.g. electric shock) with a lever press.
Fruit flies: A commonly used operant learning task used in Drosophila studies is courtship conditioning, whereby a male fly exposed to a mated female will learn to suppress courtship behaviour as the male is persistently rejected by the mated female during training.
Molluscs: In the sea slug, Aplysia, operant learning can occur by appetitive learning, whereby biting and swallowing behaviour increases in the presence of food [20]. Another study has shown that operant learning can occur for gill withdrawal behaviour, which can be suppressed by association with electric shocks [21].
Does operant learning exist in worms? Operant learning has not been demonstrated for C. elegans, and it is therefore not entirely clear whether worms are incapable of operant learning or whether it has not been found because this question has not yet been addressed. The closest thing to operant behaviour observed in C. elegans is the ability to cross an aversive barrier to reach an attractive odor [97, 98], as this involves suppression of avoidance of the aversive stimulus. However, chemotaxis (which even bacteria are capable of) may not really require having selective control over movements, and is therefore arguably not an operant behaviour. To know whether worms have operant learning, one could examine mating behaviour, the most complex motor sequence of a worm. For example, do male worms suppress their mating behaviour if they are only exposed to other male worms (similar to the courtship conditioning assay in Drosophila)?
Box 2. Outstanding Questions.
How do attention processes increase sleep drive? Although sleep need appears to depend on waking experiences that involve attention, whether attention regulates sleep has not been directly tested. Future studies could investigate whether increasing attentional load or activating attention networks in the brain can influence sleep need. This then raises the question of how these processes tell the brain how much it needs to sleep. How would driving attention processes in the brain lead to electrical signatures of sleep, such as enhanced slow-wave activity in mammals or reduced neural activity in flies? Sleep onset could be triggered by an accumulation of certain molecular factors within neurons, or could be signalled by the recognition of poorly tuned neural networks. One interesting avenue could be to investigate the role of glia, as they have been found to modulate sleep pressure, sleep rebound and cognitive consequences of sleep loss [99]
How do the different electrical activity patterns observed during sleep, such as slow-wave activity in mammals or attenuated activity in flies, achieve sleep functions that optimize attention? In mammals, REM sleep promotes consolidation of procedural memories (e.g., learning to ride a bike), whereas slow-wave sleep primarily supports declarative memories (e.g. learning names) [14]. However, whether specific types of neural activity optimize selective attention processes has not been investigated, e.g., can oscillatory activity during sleep (either spontaneous, or induced) influence subsequent patterns of brain activity that occur during attention tasks, and does this correlate with performance on these tasks?
What are the molecular processes harnessed by these different patterns of neural activity? Molecules associated with sleep function have begun to be identified, for example Fragile-X mental retardation protein (FMRP) in synaptic pruning during sleep [86]. However, a key question is how neural activity during sleep harnesses molecular processes that achieve sleep functions. Future work could examine how stimulating neurons in a way that resembles sleep triggers changes in the molecular states of those neurons, by performing expression profiling or by examining more closely the behaviour of certain candidate molecules within neurons or glia following sleep-like activity.
Do sleep and attention use common neural mechanisms to suppress information processing? Although different patterns of electrical activity in the brain have been identified during sleep (e.g., slow wave oscillations) and have also been associated with attention processes (e.g., alpha oscillations), it is not clear whether they use similar or different mechanisms to orchestrate information flow. For example, do slow wave oscillations create a temporal phase code that effectively inhibits processing of stimuli, like alpha oscillations? Or do slow wave oscillations simply disrupt temporal phase codes altogether, by causing prolonged ‘down states’ of neural inactivity? Furthermore, considering that slow-wave sleep has not been identified in all animals, one might ask to what degree functional roles for oscillatory activity translate across different organisms. For example, can animals that appear to lack slow wave sleep (e.g., most animals other than birds and mammals) achieve the same functions with oscillatory activity in a different frequency domain, or perhaps even by simply shutting down altogether?
Did sleep functions co-evolve with selective attention? This is a broad question that reflects the central hypothesis of this article. In terms of ‘co-evolution’, one approach to understanding this question is to examine sleep and attention in different species, to see whether they always co-exist, or whether they can be separated. For example one prediction may be that animals lacking selective attention will not require sleep for daily plasticity processes, only for more primitive functions such as stress and development. Another approach could be to create virtual nervous systems that are either capable or incapable of selective attention, and see whether these networks also need to be regularly fine-tuned by sleep-like functions such as synaptic homeostasis.
Trends Box.
Emerging evidence suggests that sleep serves a number of distinct functions, even though these might all have a common need for behavioral quiescence.
In animals with simple nervous systems, sleep-like states are associated with development and environmental stress.
In animals with more complex nervous systems, sleep also has a major role in supporting cognitive processes such as learning and attention.
Sleep need is increased by behaviors that require selective attention and operant (instrumental) learning.
Sleep and attention have been viewed as fundamentally different states, but they may involve similar mechanisms for suppressing external stimuli.
Sleep and selective attention may have complementary effects on synaptic plasticity
Acknowledgements
We thank Sara Mednick, Jason Mattingley, Martyna Grabowska, Aoife Larkin, Rowan Tweedale, Ben De Bivort, and Nick Strausfeld for helpful comments on the manuscript and figures. This work was supported by an Australian Research Council Future Fellowship (FT100100725) to BvS and an Australian Postgraduate Award to LK.
Glossary
- Selective attention
the ability to focus on one stimulus while actively ignoring others, whereby processing of a salient stimulus is enhanced in the brain while irrelevant information is suppressed.
- Attentional load
or ‘perceptual load’ [100] - the amount of information processing required during an attention task, with high load tasks requiring large amounts of information processing and low load tasks requiring lower amounts of processing capacity.
- Bilatarians
animals with bilateral symmetry (front and back, left and right), as opposed to a radial symmetry.
- Classical conditioning
learning that results in a reflexive behaviour being elicited in response to a neutral stimulus, by repeated pairing of the neutral stimulus with the stimulus that triggers the reflex (also known as Pavlovian conditioning).
- Oscillations
rhythmic electrical activity in the brain, that is often classified according to frequency range: e.g., alpha oscillations (8 – 13 Hz) and gamma oscillations (30 – 90 Hz)
- Operant learning
learning that involves association of one's own actions with consequences in the environment (e.g. reward/punishment), such that an animal adjusts its motor patterns to achieve a goal.
- Sleep pressure
the internal drive to sleep
- Slow-wave sleep
oscillatory brain activity during sleep occurring in the 0.5-4 Hz frequency range
- Synaptic plasticity
strengthening or weakening of synapses in the nervous system as a result of experience
- Synaptic homeostasis / synaptic renormalization
a type of plasticity thought to occur during sleep, proposed by the synaptic homeostasis hypothesis (SHY) [54], whereby changes in synaptic strength during wakefulness (usually increases in strength as a result of learning) are modified during sleep (‘downscaled’, or proportionally weakened on a global level) to maintain a balance in synaptic weight in the brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 2.Sareen P, et al. Attracting the attention of a fly. Proc Natl Acad Sci U S A. 2011;108:7230–7235. doi: 10.1073/pnas.1102522108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw PJ, et al. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 4.van Swinderen B. Attention in Drosophila. Int Rev Neurobiol. 2011;99:51–85. doi: 10.1016/B978-0-12-387003-2.00003-3. [DOI] [PubMed] [Google Scholar]
- 5.Raizen DM, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 6.Nelson MD, et al. FMRFamide-like FLP-13 Neuropeptides Promote Quiescence following Heat Stress in Caenorhabditis elegans. Current Biology. 2014;24:2406–2410. doi: 10.1016/j.cub.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 8.Kayser MS, et al. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science. 2014;344:269–274. doi: 10.1126/science.1250553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seugnet L, et al. Sleep deprivation during early-adult development results in long-lasting learning deficits in adult Drosophila. Sleep. 2011;34:137–146. doi: 10.1093/sleep/34.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roffwarg HP, et al. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 11.Lenz O, et al. FMRFamide signaling promotes stress-induced sleep in Drosophila. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw PJ, et al. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 13.Rasch B, Born J. About sleep's role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diekelmann S, Born J. SLEEP The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 15.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annual Review of Psychology. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MC, Wuensch KL. An Investigation of Habituation in the Jellyfish Aurelia-Aurita. Behavioral and Neural Biology. 1994;61:54–59. doi: 10.1016/s0163-1047(05)80044-5. [DOI] [PubMed] [Google Scholar]
- 17.Gagliano M, et al. Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia. 2014;175:63–72. doi: 10.1007/s00442-013-2873-7. [DOI] [PubMed] [Google Scholar]
- 18.Seymour JE, et al. Do box jellyfish sleep at night? Medical Journal of Australia. 2004;181:707–707. doi: 10.5694/j.1326-5377.2004.tb06529.x. [DOI] [PubMed] [Google Scholar]
- 19.Ardiel EL, Rankin CH. An elegant mind: Learning and memory in Caenorhabditis elegans. Learn Memory. 2010;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- 20.Brembs B, et al. Operant reward learning in Aplysia: Neuronal correlates and mechanisms. Science. 2002;296:1706–1709. doi: 10.1126/science.1069434. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins RD, et al. Operant conditioning of gill withdrawal in Aplysia. J Neurosci. 2006;26:2443–2448. doi: 10.1523/JNEUROSCI.3294-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seugnet L, et al. Persistent Short-Term Memory Defects Following Sleep Deprivation in a Drosophila Model of Parkinson Disease. Sleep. 2009;32:984–992. doi: 10.1093/sleep/32.8.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seugnet L, et al. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–1117. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donlea JM, et al. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dissel S, et al. Sleep Restores Behavioral Plasticity to Drosophila Mutants. Curr Biol. 2015 doi: 10.1016/j.cub.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JY, Sternberg PW. Multilevel Modulation of a Sensory Motor Circuit during C. elegans Sleep and Arousal. Cell. 2014;156:249–260. doi: 10.1016/j.cell.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi S, et al. Analysis of NPR-1 reveals a circuit mechanism for behavioral quiescence in C. elegans. Neuron. 2013;78:869–880. doi: 10.1016/j.neuron.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz J, et al. Reduced activity of a sensory neuron during a sleep-like state in Caenorhabditis elegans. Current Biology. 2011;21:R983–R984. doi: 10.1016/j.cub.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Bushey D, et al. Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc Natl Acad Sci U S A. 2015;112:4785–4790. doi: 10.1073/pnas.1419603112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiederman SD, O'Carroll DC. Selective attention in an insect visual neuron. Curr Biol. 2013;23:156–161. doi: 10.1016/j.cub.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 31.Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Kim AJ, et al. Cellular evidence for efference copy in Drosophila visuomotor processing. Nat Neurosci. 2015;18:1247–1255. doi: 10.1038/nn.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser W, Steiner-Kaiser J. Neuronal correlates of sleep, wakefulness and arousal in a diurnal insect. Nature. 1983;301:707–709. doi: 10.1038/301707a0. [DOI] [PubMed] [Google Scholar]
- 35.Buzsaki G, et al. The origin of extracellular fields and currents - EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang S, Juusola M. Intrinsic activity in the fly brain gates visual information during behavioral choices. PLoS One. 2010;5:e14455. doi: 10.1371/journal.pone.0014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Swinderen B. Attention-like processes in Drosophila require short-term memory genes. Science. 2007;315:1590–1593. doi: 10.1126/science.1137931. [DOI] [PubMed] [Google Scholar]
- 38.van Swinderen B, Greenspan RJ. Salience modulates 20-30 Hz brain activity in Drosophila. Nat Neurosci. 2003;6:579–586. doi: 10.1038/nn1054. [DOI] [PubMed] [Google Scholar]
- 39.Buzsaki G, Wang XJ. Mechanisms of Gamma Oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen O, et al. An oscillatory mechanism for prioritizing salient unattended stimuli. Trends Cogn Sci. 2012;16:200–206. doi: 10.1016/j.tics.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Frontiers in human neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handel BF, et al. Alpha Oscillations Correlate with the Successful Inhibition of Unattended Stimuli. J Cognitive Neurosci. 2011;23:2494–U2552. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- 44.Rihs TA, et al. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- 45.Worden MS, et al. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-06-j0002.2000. art. no.-RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen O, Lisman JE. Hippocampal CA3 region predicts memory sequences: Accounting for the phase precession of place cells. Learn Memory. 1996;3:279–287. doi: 10.1101/lm.3.2-3.279. [DOI] [PubMed] [Google Scholar]
- 47.Lisman JE, Idiart MAP. Storage of 7+/−2 Short-Term Memories in Oscillatory Subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 48.Mehta MR, et al. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- 49.Montemurro MA, et al. Phase-of-firing visual stimuli in coding of natural primary visual cortex. Current Biology. 2008;18:375–380. doi: 10.1016/j.cub.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Huber R, et al. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 51.Stroh A, et al. Making Waves: Initiation and Propagation of Corticothalamic Ca2+ Waves In Vivo. Neuron. 2013;77:1136–1150. doi: 10.1016/j.neuron.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Volgushev M, et al. Long-range correlation of the membrane potential in neocortical neurons during slow oscillation. Slow Brain Oscillations of Sleep, Resting State and Vigilance. 2011;193:181–199. doi: 10.1016/B978-0-444-53839-0.00012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massimini M, et al. Triggering sleep slow waves by transcranial magnetic stimulation. P Natl Acad Sci USA. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tononi G, Cirelli C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vorster AP, Born J. Sleep and memory in mammals, birds and invertebrates. Neuroscience and biobehavioral reviews. 2015;50:103–119. doi: 10.1016/j.neubiorev.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Nitz DA, et al. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 57.van Alphen B, et al. A Dynamic Deep Sleep Stage in Drosophila. J Neurosci. 2013;33:6917–6927. doi: 10.1523/JNEUROSCI.0061-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berry JA, et al. Sleep Facilitates Memory by Blocking Dopamine Neuron-Mediated Forgetting. Cell. 2015 doi: 10.1016/j.cell.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Swinderen B, Andretic R. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc Biol Sci. 2011;278:906–913. doi: 10.1098/rspb.2010.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dumay N. Sleep not just protects memories against forgetting, it also makes them more accessible. Cortex. 2015 doi: 10.1016/j.cortex.2015.06.007. online July 27, 2015. [DOI] [PubMed] [Google Scholar]
- 61.Diekelmann S, et al. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 62.Rasch B, et al. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 63.Rihm JS, et al. Reactivating memories during sleep by odors: odor specificity and associated changes in sleep oscillations. J Cogn Neurosci. 2014;26:1806–1818. doi: 10.1162/jocn_a_00579. [DOI] [PubMed] [Google Scholar]
- 64.Schredl M, et al. Information processing during sleep: the effect of olfactory stimuli on dream content and dream emotions. Journal of sleep research. 2009;18:285–290. doi: 10.1111/j.1365-2869.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 65.Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- 66.Lim J, Dinges DF. A Meta-Analysis of the Impact of Short-Term Sleep Deprivation on Cognitive Variables. Psychological Bulletin. 2010;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casagrande M, et al. Orienting and alerting: effect of. 24 h of prolonged wakefulness. Experimental Brain Research. 2006;171:184–193. doi: 10.1007/s00221-005-0269-6. [DOI] [PubMed] [Google Scholar]
- 68.Horne JA, et al. Effects of Sleep-Deprivation on Signal-Detection Measures of Vigilance - Implications for Sleep Function. Sleep. 1983;6:347–358. doi: 10.1093/sleep/6.4.347. [DOI] [PubMed] [Google Scholar]
- 69.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 70.Killgore WD, et al. Lack of degradation in visuospatial perception of line orientation after one night of sleep loss. Percept Mot Skills. 2007;105:276–286. doi: 10.2466/pms.105.1.276-286. [DOI] [PubMed] [Google Scholar]
- 71.Paulk ACP, Kirszenblat L, Zhou Y, van Swinderen B. Closed-loop behavioral control increases coherence in the fly brain. The Journal of Neuroscience. 2015 doi: 10.1523/JNEUROSCI.0691-15.2015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chee MWL, Chuah YML. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. P Natl Acad Sci USA. 2007;104:9487–9492. doi: 10.1073/pnas.0610712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chee MWL, Tan JC. Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. Neuroimage. 2010;51:835–843. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 74.Kong DY, et al. Reduced visual processing capacity in sleep deprived persons. Neuroimage. 2011;55:629–634. doi: 10.1016/j.neuroimage.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 75.Arzi A, et al. Humans can learn new information during sleep. Nat Neurosci. 2012;15:1460–1465. doi: 10.1038/nn.3193. [DOI] [PubMed] [Google Scholar]
- 76.Bekinschtein TA, et al. Classical conditioning in the vegetative and minimally conscious state. Nat Neurosci. 2009;12:1343–U1176. doi: 10.1038/nn.2391. [DOI] [PubMed] [Google Scholar]
- 77.Le Glou E, et al. Circadian Modulation of Consolidated Memory Retrieval Following Sleep Deprivation in Drosophila. Sleep. 2012;35:1377–1384. doi: 10.5665/sleep.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, et al. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep. 2009;32:1417–1424. doi: 10.1093/sleep/32.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hussaini SA, et al. Sleep deprivation affects extinction but not acquisition memory in honeybees. Learn Memory. 2009;16:698–705. doi: 10.1101/lm.1578409. [DOI] [PubMed] [Google Scholar]
- 80.Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav. 2005;84:343–349. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 81.Landsness EC, et al. Sleep-Dependent Improvement in Visuomotor Learning: A Causal Role for Slow Waves. Sleep. 2009;32:1273–1284. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mascetti L, et al. The Impact of Visual Perceptual Learning on Sleep and Local Slow-Wave Initiation. J Neurosci. 2013;33:3323–U3487. doi: 10.1523/JNEUROSCI.0763-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pugin F, et al. Local Increase of Sleep Slow Wave Activity after Three Weeks of Working Memory Training in Children and Adolescents. Sleep. 2015;38:607–614. doi: 10.5665/sleep.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanlon EC, et al. Effects of Skilled Training on Sleep Slow Wave Activity and Cortical Gene Expression in the Rat. Sleep. 2009;32:719–729. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huber R, et al. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 86.Bushey D, et al. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donlea JM, et al. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ganguly-Fitzgerald I, et al. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 89.Cortesi F, et al. Sleep in children with autistic spectrum disorder. Sleep Med. 2010;11:659–664. doi: 10.1016/j.sleep.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Monti JM, et al. Sleep and circadian rhythm dysregulation in schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;43:209–216. doi: 10.1016/j.pnpbp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 91.Silvestri R, et al. Sleep disorders in children with Attention-Deficit/Hyperactivity Disorder (ADHD) recorded overnight by video-polysomnography. Sleep Med. 2009;10:1132–1138. doi: 10.1016/j.sleep.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 92.Wilhelm I, et al. Sleep slow-wave activity reveals developmental changes in experience-dependent plasticity. J Neurosci. 2014;34:12568–12575. doi: 10.1523/JNEUROSCI.0962-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie LL, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mednick SC, et al. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34:504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frank MG. Erasing Synapses in Sleep: Is It Time to Be SHY? Neural Plast. 2012;2012:264378. doi: 10.1155/2012/264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tononi G, Cirelli C. Time to Be SHY? Some Comments on Sleep and Synaptic Homeostasis. Neural Plast. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishihara T, et al. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell. 2002;109:639–649. doi: 10.1016/s0092-8674(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 98.Shinkai Y, et al. Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J Neurosci. 2011;31:3007–3015. doi: 10.1523/JNEUROSCI.4691-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fellin T, et al. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. P Natl Acad Sci USA. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lavie N. Perceptual load as a necessary condition for selective attention. Journal of experimental psychology. Human perception and performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]