Abstract
Palladium-catalyzed allylic substitution reactions are among the most efficient methods to construct C–C bonds between sp3-hybridized carbons. In contrast, much less work has been done with nickel catalysts. This may be due, in part, to the different mechanisms of allylic substitution reactions. Palladium catalysts generally undergo substitution via a “soft” nucleophile pathway, wherein the nucleophile attacks the allyl group externally. Nickel catalysts are usually paired with “hard” nucleophiles, which attack the metal before C–C bond-formation. Herein is introduced a rare nickel-based catalyst that promotes substitution with diarylmethane pronucleophiles via the “soft” nucleophile pathway. Preliminary studies on the asymmetric allylic alkylation are promising.
Keywords: Cross-Coupling, Asymmetric Catalysis
Graphical Abstract
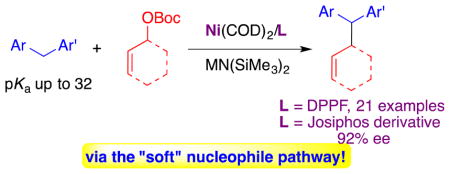
Just a softy: Contrary to what would be predicted, organosodium nucleophiles derived from diarylmethane pronucleophiles are shown to behave as soft nucleophiles in Ni-catalyzed allylic substitution reactions. This general reaction (21 examples) is demonstrated to proceed by the double inversion pathway. A promising asymmetric version (92% ee) is demonstrated.
Metal-catalyzed allylic substitution reactions remain one of the most efficient approaches to construct C(sp3)–C(sp3) bonds. Among transition metal catalysts used in allylic substitutions, palladium has met with the greatest success. Many enantioselective palladium catalysts have been developed and elegantly applied to the synthesis of natural products.[1–6]
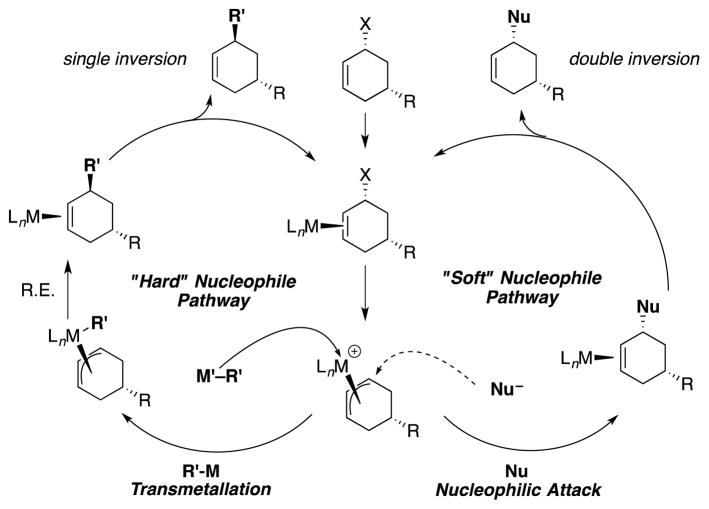
The mechanisms of allylic substitution reactions promoted by a variety of catalysts with different nucleophiles have been investigated.[1,2] From these studies, trends in reaction pathways have emerged and are now well accepted.[1] The reaction pathway has been found to depend on the nature of the nucleophile.[1] In general, anionic nucleophiles (Nu–) are divided into two classes based on the pKa of the pronucleophile, Nu–H: carbon nucleophiles derived from pronucleophiles with pKa’s < 25 are considered stabilized or “soft” nucleophiles while those from pronucleophiles with pKa’s > 25 are categorized as unstabilized or “hard” nucleophiles. The difference between these two classes is that soft nucleophiles attack the π-allyl moiety externally while hard nucleophiles bind directly to the metal center (via transmetallation) before C–C bond-formation with the allyl group (Scheme 1). Importantly, it has proven easier to control enantioselectivity with soft nucleophiles in Pd catalyzed asymmetric allylic alkylations (AAA) than with hard nucleophiles.[1,3,7–10] Thus, expanding the scope of soft nucleophiles in Pd-catalyzed AAA has attracted attention.[11–14]
Scheme 1.
Mechanism of Transition Metal Catalyzed Allylic Substitution
In contrast to Pd-catalyzed allylic substitutions, which have been extensively used with soft nucleophiles, Ni catalysts have generally been paired with hard nucleophiles, such as Grignard reagents and other main group organometallics.[6,15–29] An advantage of nickel catalysts over palladium is their lower cost.
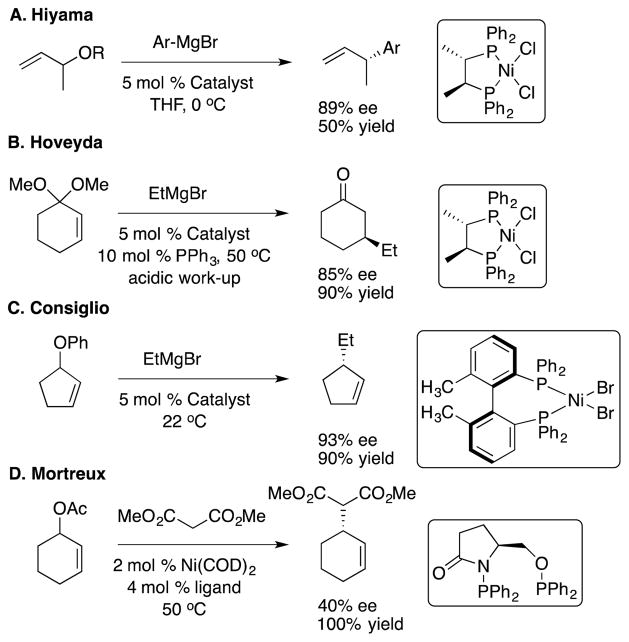
Early examples of nickel catalyzed allylic substitution reactions include Hiyama and coworkers use of (S,S)-chiraphos (Scheme 2A).[15] In a clever application of achiral ligands to optimize enantioselectivity,[30] Hoveyda and coworkers used the [(S,S)-chiraphos]Ni catalyst in the presence of PR3 and Grignard reagents to develop a synthesis of enol ethers and ketones with high ee (Scheme 2B).[16] Consiglio and coworkers determined that EtMgBr attacked the nickel center (transmetallation) first followed by reductive elimination to form the product.[6,19] They found the excellent enantioselectivity with EtMgBr, but MeMgBr and (n-Pr)MgBr exhibited significantly lower enantioselectivities (Scheme 2C).[18] Unlike hard nucleophiles, soft nucleophiles in Ni-catalyzed AAA exhibit poor enantioselection (Scheme 2D). [31]
Scheme 2.
Previous Ni-Catalyzed Asymmetric Allylic Alkylation Reactions.
Our interest in the Tsuji-Trost reaction has been to expand the scope of soft nucleophiles. We recently demonstrated that diarylmethane pronucleophiles behave as soft nucleophiles in Pd-catalyzed allylic substitutions under basic conditions, raising the pKa limit of soft nucleophiles from 25 to at least 32.[13] In the current study, we asked 1) if diarylmethane pronucleophiles were suitable substrates for Ni catalyzed allylic substitutions, 2) if they would react via the hard or soft nucleophile pathway, and 3) if highly enantioselective versions would be possible. Herein, we communicate that these basic nucleophiles react via the soft nucleophile pathway and disclose a promising preliminary Ni catalyzed AAA.
We initiated our study of the Ni-catalyzed allylic substitution by examining 24 of the most common mono and bidentate phosphine ligands with Ni(COD)2, KN(SiMe3)2 and allylOBoc (see Supporting Information for details). DPPF was the most promising ligand [72% 1H NMR assay yield (AY), Table 1, entry 1], outperforming van Leeuwen’s Xantphos, which was the ligand of choice in our Pd-catalyzed version of this reaction.[13,14] We then examined the nickel to ligand ratio, however, attempts to reduce the ligand loading led to lower yields (entries 2 and 3). DME proved to be a better solvent than THF, CPME (cyclopentyl methyl ether), dioxane and 2-MeTHF (entry 1 vs. 4–7). Nickel sources NiCl2 and NiBr2 resulted in decreased yields (entries 8 and 9 vs. 1). Finally, 88% isolated yield was obtained with 7.5 mol % Ni loading (entry 10).
Table 1.
Optimization of Allylic Alkylation with Diphenylmethane 1a.[a]
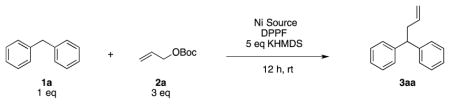
| ||||
|---|---|---|---|---|
| Entry | Ni Source | Ni/DPPF (mol %) | Solvent | Yield[b] (%) |
| 1 | Ni(COD)2 | 5/10 | DME | 72 |
| 2 | Ni(COD)2 | 5/5 | DME | 39 |
| 3 | Ni(COD)2 | 5/7.5 | DME | 46 |
| 4 | Ni(COD)2 | 5/10 | THF | 46 |
| 5 | Ni(COD)2 | 5/10 | CPME | <5 |
| 6 | Ni(COD)2 | 5/10 | dioxane | <5 |
| 7 | Ni(COD)2 | 5/10 | 2-Me-THF | 52 |
| 8 | NiCl2 | 5/10 | DME | 35 |
| 9 | NiBr2 | 5/10 | DME | 51 |
| 10 | Ni(COD)2 | 7.5/15 | DME | 90 (88)[c] |
Reactions conducted on a 0.1 mmol scale.
Yields determined by 1H NMR spectroscopy of the crude reaction mixtures.
Isolated yield after chromatographic purification.
With the optimized conditions in Table 1 (entry 10), we probed the scope of diphenylmethane derivatives (Table 2). The reaction with 4-fluoro diphenylmethane (1b) afforded the desired product 3ba in 67% yield (entry 2). With 4-chloro and 4-bromo diphenylmethane NaN(SiMe3)2 proved to be a better base, providing products 3ca and 3da in 98% and 89% yield, respectively (entries 3–4). It is remarkable that generation of the Ni(π-allyl) is faster than the oxidative addition of C–Cl and C–Br bonds under our conditions. 4-Methyl diphenylmethane gave 3ea in 61% yield (entry 5). Sterically hindered 2-methyl diphenylmethane reacted to provide 3fa in 65% yield (entry 6). Fluorene derivatives are interesting components in material and photochemistry.[32] Due to the increased acidity of fluorene, 1.5 equiv LiOtBu could be used with 1.2 equiv of allylOBoc to provided 3ga in 83% yield (entry 7). Unfortunately, due to the higher pKa of 4-methoxy diphenylmethane, poor yields were obtained despite additional optimization.
Table 2.
Scope of Diarylmethanes in Allylic Alkylation Reactions.[a]

| |||||
|---|---|---|---|---|---|
| Entry | Ar | Base | Ratio (1:base:2a) | Product | Yield (%) |
| 1 | Ph | KHMDS | 1:5:3 | 3aa | 88 |
| 2 | 4-C6H4-F | KHMDS | 1:4:3 | 3ba | 67 |
| 3 | 4-C6H4-Cl | NaHMDS | 1:5:3 | 3ca | 98 |
| 4 | 4-C6H4-Br | NaHMDS | 1:5:3 | 3da | 89 |
| 5 | 4-C6H4-Me | KHMDS | 1:5:3 | 3ea | 61 |
| 6 | 2-C6H4-Me | KHMDS | 1:5:3 | 3fa | 65 |
|
| |||||
| 7 |
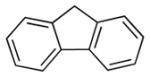
|
LiOtBu | 1:1.5:1.2 | 3ga | 83 |
Reactions conducted on a 0.1 mmol scale.
Isolated yield after chromatographic purification.
We next turned our attention to biologically relevant heterocyclic pronucleophiles (4a–f, Table 3). Pleasingly, a lower catalyst loading could be applied to these more acidic pronucleophiles. Pyridine containing diarylmethanes are useful in drug discovery.[33] 2-Benzylpyridine underwent coupling under the standard conditions to afford 5aa in 91% yield (entry 1). Likewise, 3- and 4-benzylpyridine provided desired products 5ba and 5ca in 91% and 93% yield, respectively (entries 2 and 3). 3,3′-Dipyridylmethane was also a viable substrate, generating 5da in 90% yield (entry 4). Thiophene containing products are important in agrochemicals and pharmaceuticals.[34] 2-Benzylthiophene rendered coupling product 5ea in 82% yield (entry 5). Xanthene derivatives are building blocks for the synthesis of dyes.[32] Application of our standard reaction conditions to xanthene furnished 5fa in 81% yield (entry 6).
Table 3.
Scope of Heterocyclic Diarylmethanes in Allylic Alkylation Reactions.[a]
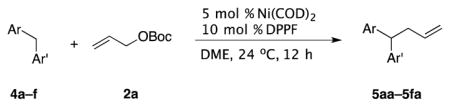
| ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | Ar′ | Base | Ratio (4:base:2a) | Product | Yield (%) |
| 1 | 2-Py | Ph | NaHMDS | 1:2:1.2 | 5aa | 91 |
| 2 | 3-Py | Ph | NaHMDS | 1:3:1.2 | 5ba | 91 |
| 3 | 4-Py | Ph | LiHMDS | 1:2:1.2 | 5ca | 93 |
| 4 | 3-Py | 3-Py | LiHMDS | 1:3:1.2 | 5da | 90 |
| 5 | 2-thienyl | Ph | NaHMDS | 1:2:1.2 | 5ea | 82 |
|
| ||||||
| 6 |
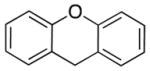
|
LiHMDS | 1:3:1.2 | 5fa | 81 | |
Reactions conducted on a 0.1 mmol scale.
Diallylation to construct quaternary carbon centers was achieved using excess allyl electrophile with 5 mol % Ni and 10 mol % DPPF (4a–f, Table 4). Presumably, the products could be cyclized using ring-closing metathesis.[35] 2-Benzyl, 3-benzyl, or 4-benzyl pyridine all gave good yields (75–84%, entries 1–3). 2-Benzylthiophene provided diallylation product 6da in 83% yield under the standard reaction conditions. Fluorene and xanthene were also good substrates, leading to products in 89–90% yield (entries 5 and 6).
Table 4.
Scope of Diallylation of Diarylmethanes

| ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | Ar′ | Base | Ratio (4:base:2a) | Product | Yield (%) |
| 1 | 2-Py | Ph | KHMDS | 1:5:3 | 6aa | 84 |
| 2 | 3-Py | Ph | KHMDS | 1:5:3 | 6ba | 78 |
| 3 | 4-Py | Ph | KHMDS | 1:5:3 | 6ca | 75 |
| 4 | 2-thienyl | Ph | KHMDS | 1:5:3 | 6da | 83 |
|
| ||||||
| 5 |
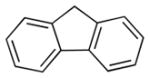
|
KOtBu | 1:5:3 | 6ea | 90 | |
| 6 |
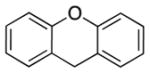
|
NaHMDS | 1:5:3 | 6fa | 89 | |
Reactions conducted on a 0.1 mmol scale.
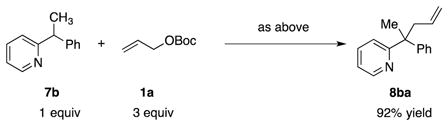
After exploring the diallylation, we wanted to determine if other tertiary C–H’s could be allylated using our method. Thus, with triphenylmethane (7a), the allylated product 8aa was isolated in 90% yield [Eq. (1)]. Similarly, 2-(1-phenylethyl)pyridine (7b) also underwent allylation to form 8ba in 92% yield [Eq. (2)]. These initial results bode well for further development of Ni-catalyzed allylic substitutions.
 |
(1) |
 |
(2) |
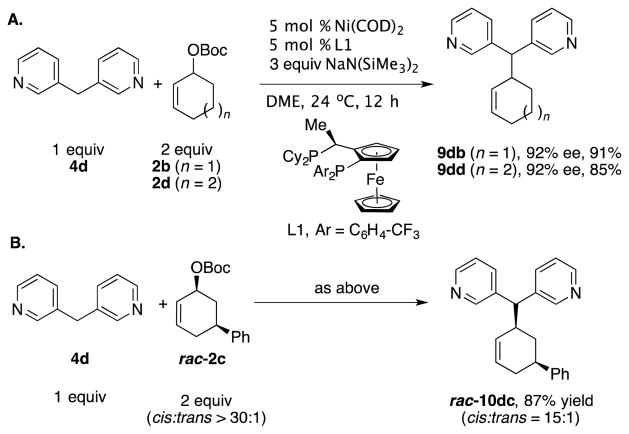
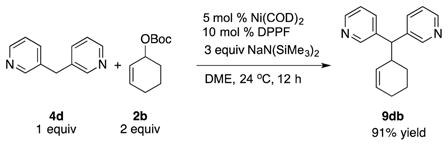
As outlined in the introduction, Ni-catalyzed allylic substitutions with hard nucleophiles, such as Grignard reagents, undergo reactions predominantly via transmetallation followed by reductive elimination (Scheme 1).[6,19] The nucleophiles employed in Tables 2–4 are organopotassium, -sodium, or -lithium derivatives, which would be predicted to undergo reaction via the hard nucleophile pathway. To probe this key step, we initially explored cyclic 2b to determine if it was viable in nickel-catalyzed allylic substitution reactions. Thus, employing electrophile 2b with NaN(SiMe3)2 afforded the substitution product 9db in 91% yield [Eq. (3)].
 |
(3) |
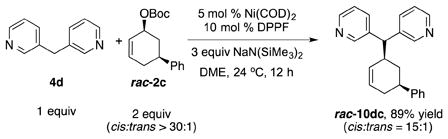
To determine if the nucleophile derived from 3,3′-dipyridylmethane (4d) and NaN(SiMe3)2 behaves as a hard or soft nucleophile, we employed the stereoprobe rac-2c [Eq. (4)]. If the reaction proceeds with a single inversion, the trans-diastereomer will predominate, leading to the conclusion that reaction took place via the hard nucleophile pathway (Scheme 1). In contrast, formation of the cis-product would indicate a double inversion, where the nucleophile attacks the allyl moiety opposite the nickel (soft nucleophile pathway, Scheme 1). Conducting the allylic substitution under the standard conditions led to formation of the product 10dc in 89% yield [Eq. (4)]. Analysis of the 1H NMR coupling constants of the product[14] led to its assignment as the cis-diastereomer arising from a double inversion pathway. The stereochemistry of the product, therefore, indicates that the reaction proceeded by nucleophilic attack directly on the Ni(allyl) (soft nucleophile pathway). It is surprising that this basic nucleophile behaves as a soft nucleophile with catalysts derived from either nickel or palladium.[13]
 |
(4) |
The AAA with diarylmethane pronucleophiles is challenging because selectivity is usually difficult to control with highly reactive nucleophiles. We therefore screened 178 enantioenriched mono and bidentate phosphine ligands with 3 equiv of base, 3,3′-dipyridylmethane and 2 equiv of cyclohexenyl-OBoc (2b) in the Ni-catalyzed AAA. We identified a Josiphos derivative (L1, Scheme 3) as the best hit with 75% assay yield and 70% ee. After optimization (see Supporting Information), we were able to obtain 9db in 91% yield with 92% ee (Scheme 3A). Likewise, with the 7-membered ring (n = 2), we obtained the product 9dd in 85% yield with 92% ee. In order to determine if this catalyst/nucleophile combination also reacts via the soft nucleophile pathway, we performed the reaction with stereoprobe rac-2c. We observed predominately cis product, which indicates the nucleophile reacts by the soft nucleophile pathway (Scheme 3B).
Scheme 3.
Asymmetric Allylic Alkylation and Mechanistic Study with L1.
In summary, we have developed the first Ni-catalyzed allylic alkylation with diarylmethane pronucleophiles. The protocol is robust with different nucleophiles including diphenylmethane derivatives and heteroaryl containing diarylmethanes. We have demonstrated that this method can be used to construct quaternary centers. In addition, the first Ni-catalyzed asymmetric allylic alkylation (AAA) of soft nucleophiles with high ee has been demonstrated. These results indicate that Ni-catalyzed asymmetric allylic alkylation (AAA) is not limited to hard nucleophiles and that this area warrants further investigation and development.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIGMS 104349) and National Science Foundation (CHE-1464744) for financial support. H.J. thanks China Scholarship Council [201406350156] for financial support.
References
- 1.Trost BM, VanVranken DL. Chem Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- 2.Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- 3.Trost BM, Machacek MR, Aponick A. Acc Chem Res. 2006;39:747–760. doi: 10.1021/ar040063c. [DOI] [PubMed] [Google Scholar]
- 4.Trost BM. J Org Chem. 2004;69:5813–5837. doi: 10.1021/jo0491004. [DOI] [PubMed] [Google Scholar]
- 5.Lu Z, Ma S. Angew Chem Int Ed. 2008;47:258–297. doi: 10.1002/anie.200605113. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2008;120:264–303. [Google Scholar]
- 6.Consiglio G, Waymouth RM. Chem Rev. 1989 [Google Scholar]
- 7.Trost BM, Toste FD. J Am Chem Soc. 1999;121:4545–4554. [Google Scholar]
- 8.Zhang P, Morken JP. J Am Chem Soc. 2009;131:12550–12551. doi: 10.1021/ja9058537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Le H, Kyne RE, Morken JP. J Am Chem Soc. 2011;133:9716–9719. doi: 10.1021/ja2039248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misale A, Niyomchon S, Luparia M, Maulide N. Angew Chem Int Ed. 2014;53:7068–7073. doi: 10.1002/anie.201309074.Angew Chem. 2014;126:7188–7193.for a recent AAA with Cu catalysts and hard nucleophiles see: You H, Rideau E, Sidera M, Fletcher SP. Nature. 2015;517:351–355. doi: 10.1038/nature14089.
- 11.Trost BM, Thaisrivongs DA. J Am Chem Soc. 2008;130:14092–14093. doi: 10.1021/ja806781u. [DOI] [PubMed] [Google Scholar]
- 12.Trost BM, Malhotra S, Olson DE, Maruniak A, Du Bois J. J Am Chem Soc. 2009;131:4190–4191. doi: 10.1021/ja809697p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sha SC, Zhang J, Carroll PJ, Walsh PJ. J Am Chem Soc. 2013;135:17602–17609. doi: 10.1021/ja409511n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Stanciu C, Wang B, Hussain MM, Da CS, Carroll PJ, Dreher SD, Walsh PJ. J Am Chem Soc. 2011;133:20552–20560. doi: 10.1021/ja208935u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiyama T, Wakasa N. Tetrahedron Lett. 1985;26:3259–3262. [Google Scholar]
- 16.Gomez-Bengoa E, Heron NM, Didiuk MT, Luchaco CA, Hoveyda AH. J Am Chem Soc. 1998;120:7649–7650. [Google Scholar]
- 17.Didiuk MT, Morken JP, Hoveyda AH. Tetrahedron. 1998;54:1117–1130. [Google Scholar]
- 18.Consiglio G, Piccolo O, Roncetti L, Morandini F. Tetrahedron. 1986;42:2043–2053. [Google Scholar]
- 19.Consiglio G, Morandini F, Piccolo O. J Am Chem Soc. 1981;103:1846–1847. [Google Scholar]
- 20.Kobayashi Y, Ikeda E. J Chem Soc, Chem Commun. 1994:1789. [Google Scholar]
- 21.Farthing CN, Koćovský P. J Am Chem Soc. 1998;120:6661–6672. [Google Scholar]
- 22.Smith SW, Fu GC. J Am Chem Soc. 2008;130:12645–12647. doi: 10.1021/ja805165y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son S, Fu GC. J Am Chem Soc. 2008;130:2756–2757. doi: 10.1021/ja800103z. [DOI] [PubMed] [Google Scholar]
- 24.(a) Kobayashi Y, Tokoro Y, Watatani K. Tetrahedron Lett. 1998;39:7537–7540. [Google Scholar]; (b) Kobayashi Y, Mizojiri R, Ikeda E. J Org Chem. 1996;61:5391–5399. [Google Scholar]; (c) Kobayashi Y, Watatani K, Kikori Y, Mizojiri R. Tetrahedron Lett. 1996;37:6125–6128. [Google Scholar]; (d) Kobayashi Y, Takahisa E, Usmani SB. Tetrahedron Lett. 1998;39:597–600. [Google Scholar]; (e) Kobayashi Y, Tokoro Y, Watatani K. Eur J Org Chem. 2000;23:3825–3834. [Google Scholar]; (f) Usmani SB, Takahisa E, Kobayashi Y. Tetrahedron Lett. 1998;39:601–604. [Google Scholar]
- 25.Trost BM, Spagnol MD. J Chem Soc, Perkin Trans1. 1995:2083–2097. [Google Scholar]
- 26.Srinivas HD, Zhou Q, Watson MP. Org Lett. 2014;16:3596–3599. doi: 10.1021/ol5016724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wisniewska HM, Swift EC, Jarvo ER. J Am Chem Soc. 2013;135:9083–9090. doi: 10.1021/ja4034999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsubara R, Jamison TF. J Am Chem Soc. 2010;132:6880–6881. doi: 10.1021/ja101186p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shields JD, Ahneman DT, Graham TJA, Doyle AG. Org Lett. 2014;16:142–145. doi: 10.1021/ol4031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh PJ, Lurain AE, Balsells J. Chem Rev. 2003;103:3297–3344. doi: 10.1021/cr0000630. [DOI] [PubMed] [Google Scholar]
- 31.Bricout H, Carpentier J-F, Mortreux A. Tetrahedron Lett. 1996;37:6105–6108. [Google Scholar]
- 32.Griesbaum K, Behr A, Biedenkapp D, Voges H-W, Garbe D, Paetz C, Collin G, Mayer D, Höke H. Hydrocarbons. Wiley-VCH Verlag GmbH & Co; KGaA, Weinheim, Germany: 2000. [Google Scholar]
- 33.Roughley SD, Jordan AM. J Med Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 34.Swanston J. Thiophene. Wiley-VCH Verlag GmbH & Co; KGaA, Weinheim, Germany: 2000. [Google Scholar]
- 35.Kotha S, Manivannan E, Ganesh T, Sreenivasachary N, Deb A. Synlett. 1999;10:1618–1620. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.