Abstract
Older donor age is associated with lower graft and patient survival among all recipients of liver transplantation (LT). Among patients with hepatitis C virus (HCV), donor age is one of the strongest predictors of fibrosis severity and graft loss. We evaluated the implementation of a donor age restriction policy for LT patients with HCV at a single center and the effects that this policy had on wait-list (WL) and post-LT outcomes for HCV and non-HCV patients. This was a cohort study of 2388 WL patients and 1015 LT recipients between March 2002 and January 2013 and reflected 3 different eras of donor age policies. With the donor age restriction, the median donor age was reduced in LT recipients with HCV versus LT recipients without HCV (30 versus 48 years, P < 0.001) without differences in the WL time (10.6 versus 8.0 months, P = 0.23). According to a competing risks regression, those with HCV and those without HCV had lower subhazard ratios (SHRs) of dropout or death on the WL during the donor age restriction era versus the era without donor age restriction [SHR = 0.68 (P < 0.01) and SHR = 0.64 (P = 0.01), respectively]. No differences were seen in early post-LT survival for patients with or without HCV between eras (P = 0.7 and P = 0.88, respectively). In conclusion, we show that donor age restriction for HCV results in a lower donor age for HCV recipients without obvious adverse WL consequences. Although additional studies are needed, our results demonstrate the feasibility of donor age restriction for LT recipients with HCV, and such information may be relevant to programs with limited access to new antiviral therapies for which modifying the risk of severe disease remains of paramount importance.
Liver transplantation (LT) is the best treatment for complications of cirrhosis, including small hepatocellular carcinoma (HCC).1,2 The increasing disparity between the organ supply and the needs of patients is well recognized, and every year, 20% of patients either die or are removed from the LT waiting list.3 To increase the number of available donors, a gradual expansion of what were thought to be suitable criteria for deceased donors has occurred.1 Included in this group of extended criteria donors are liver donors of advanced age. An older donor age has long been recognized as a factor associated with worse recipient outcomes for patients undergoing LT of any etiology.4 The patient with hepatitis C virus (HCV), in particular, has a significant risk of more rapid progression of HCV fibrosis and decreased patient and graft survival with an older donor.4-11 Patients with other liver diseases that recur after transplantation, such as autoimmune liver disease and nonalcoholic liver disease, may be at higher risk of progressive recurrent disease with older donors also, although this has not been studied.
HCV remains the most common indication for LT in the United States,1 and strategies for improving graft survival and minimizing recurrent HCV disease continue to evolve. A policy of donor age restriction for HCV patients has not been widely adopted because of concerns about reduced access to LT for HCV patients with consequently higher wait-list (WL) mortality or, conversely, concerns about an increased risk of graft loss for non-HCV patients receiving a high proportion of grafts from older donors. Notwithstanding these issues, in our transplant program, concerns about poor outcomes for HCV patients with older donors led to a policy of donor age restriction for HCV-positive recipients in 2009. This study represents a critical assessment of outcomes for both HCV and non-HCV recipients associated with the implementation of this policy change. The findings have relevance for LT programs with limited access to new antiviral therapies for HCV patients.
PATIENTS AND METHODS
Data Source and Assembly of Cohorts
Cohorts of WL patients and LT patients at the University of California San Francisco (UCSF) were assembled from the Organ Procurement and Transplantation Network/United Network for Organ Sharing database. Those in the WL cohort had an initial listing date between March 1, 2002 and January 25, 2013, and those in the LT cohort had an LT date from the same time period. Once the 2 cohorts were identified, they were divided into 3 eras to reflect our center-specific changes in organ allocation based on donor age. Era 1 (donor age unrecognized), from March 1, 2002 to December 31, 2005, represented a time during which the effect of donor age on HCV graft survival was unrecognized. Era 2 (no specific policy), from January 1, 2006 to September 30, 2009, represented a time when there was evidence suggesting worse graft outcomes for HCV-positive patients with an older donor age,4-7 but no specific organ allocation policy based on donor age was in place at our center. Although studies on the association between donor age and mortality were published as early as 2002, we chose January 1, 2006 as the starting point to allow the uptake of published data to enter into clinical practice. Era 3 (donor age restriction), from October 1, 2009 to January 25, 2013, represented a period with a center-specific policy in place that restricted the donor age to ≤50 years for HCV-positive patients. The decision to use a donor age ≤50 years as the cutoff was based on the available data at the time, including the recommendation by the International Liver Transplantation Society that HCV-infected recipients should receive grafts from donors less than 50 years old.4-12 It should be noted that the final decision regarding the acceptance of a graft for an individual patient remained at the discretion of the surgeon. Therefore, for cases in which death was thought to be imminent without transplantation, the donor age policy could be overruled.
For the analysis, HCV patients were compared to non-HCV patients. Individuals were considered to be HCV-infected if their primary or secondary listing diagnosis codes were related to HCV (4104, 4206, 4216, 4104, and 4106). Approximately 20% of the total HCV population (253 individuals who were listed and 90 individuals who underwent transplantation) also had significant alcohol use and were grouped in the HCV category. Patients included in the non-HCV group had primary listing diagnosis codes for alcoholrelated cirrhosis (4215), autoimmune hepatitis (4212), nonalcoholic steatohepatitis (4214), cryptogenic cirrhosis (4209 and 4213), hepatitis B virus (4102 and 4202), primary biliary cirrhosis (4220), primary sclerosing cholangitis (4240, 4241, 4242, and 4245), alpha-1-antitrypsin deficiency (4300), Wilson’s disease (4301), or hemochromatosis (4302). Patients with HCC were included in both the HCV and non-HCV groups. Patients were excluded if they had a living donor; were listed for retransplantation; were status 1A, 1B, or were <18 years old at listing. Patients were followed from their initial WL date until (1) LT, (2) WL death or dropout, or (3) administrative censoring on January 25, 2013.
Statistical Approach: Demographics, Primary, and Secondary Outcomes
Differences in demographic characteristics between the groups were compared with the Kruskal-Wallis test and the chi-square test for continuous and categorical variables, respectively. The primary outcome of the study was WL death or dropout among WL candidates. The explanatory variable of interest was the era of listing with stratification by HCV status. We defined an individual as dying or dropping off the WL if the removal code from the database indicated removal due to death (code 8) or a deteriorated medical status (code 13). A competing risks regression analysis, as previously defined by Fine and Gray,13 was used, with LT (code 4) and other reasons for WL removal, including transfer to or transplantation at another center (codes 5, 6, 7, 9, 12, 14, 16, and 24), acting as competing risks. Predictors of WL death/dropout were chosen on the basis of previously documented associations and demographic factors, with the primary predictor being the WL era. Those predictors with a P value < 0.05 in the univariate analysis were evaluated for the multivariate model with forward and backward selection. Secondary outcomes included the median donor age and WL time for LT recipients by the era of transplantation and the HCV status. Post-LT survival was determined with the Kaplan-Meier method, and groups were compared with the log-rank test. Predictors of death were assessed on the basis of previously documented associations and patient demographics with univariate and multivariate Cox proportional hazards regression methods; predictors with a P value < 0.05 in the univariate analysis were chosen for evaluation in the multivariate model with forward and backward selection. Select interactions were evaluated, and tests for violations of the proportional hazards assumption were performed with Schoenfeld residuals. All analyses were performed with Stata 12 (StataCorp, College Station, TX.) The study was reviewed and approved by the UCSF institutional review board (institutional review board protocol 11-08062).
RESULTS
Cohorts and Demographics
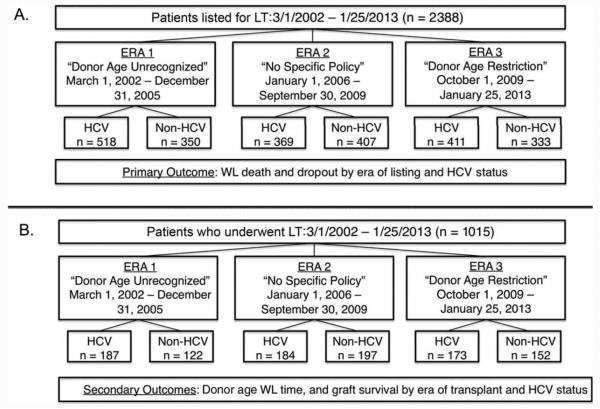
In all, 2388 WL candidates and 1015 LT recipients were identified during the study period (Fig. 1). The demographic characteristics of the 2 cohorts are shown in Table 1. During the study period, more than half of the WL patients had a diagnosis of HCV at listing; the median age was 56 years [interquartile range (IQR)=50-61 years], and 69.9% were male. The number of individuals listed with an HCC exception remained constant during the study period at approximately 20%. When we compared WL patients by the era of listing, we found that patients were more likely to be listed with HCV in era 1 versus eras 2 and 3, and the median Model for End-Stage Liver Disease (MELD) score at listing increased over time. Conversely, the median MELD score at WL removal was lower in era 3 versus eras 1 and 2.
Figure 1.
Organization of (A) patients listed for transplantation and (B) patients undergoing transplantation by the eras during the study period.
TABLE 1.
Patient Demographics
| WL Patients | All Patients (n=2388) | Era 1 (n=868) | Era 2 (n=776) | Era 3 (n=744) | P Value* |
|---|---|---|---|---|---|
| HCV-positive [n (%)] | 1298 (54.4) | 518 (59.7) | 369 (47.6) | 411 (55.2) | <0.001 |
| Age at listing (years)† | 56 (50-61) | 53 (48-59) | 55 (50-60) | 57 (52-62) | <0.001 |
| Male sex [n (%)] | 1668 (69.8) | 613 (70.6) | 544 (70.1) | 511 (68.7) | 0.69 |
| African American [n (%)] | 132 (5.5) | 42 (4.8) | 51 (6.6) | 39 (5.2) | 0.28 |
| HCC exception [n (%)] | 430 (18.0) | 139 (16.0) | 153 (19.7) | 138 (18.6) | 0.13 |
| MELD score at listing† | 14 (11-19) | 14 (11-18) | 14 (10-20) | 15 (11-21) | 0.01 |
| MELD score at WL removal† | 27 (16-33) | 28 (18-33) | 28 (18-32) | 25 (13-33) | 0.01 |
| Follow-up time (months)† | 11.7 (4.7-28.4) | 15.9 (5.6-49.6) | 12.6 (4.8-35.0) | 9.2 (3.9-16.3) | <0.001 |
|
| |||||
| Transplant Patients | All Patients (n=1015) | Era 1 (n=309) | Era 2 (n=381) | Era 3 (n=325) | P Value* |
|
| |||||
| HCV-positive [n (%)] | 544 (53.6) | 187 (60.5) | 184 (48.3) | 173 (53.2) | 0.01 |
| Age at transplant (years)† | 56.3 (50.6-61.4) | 54.7 (49.8-59.6) | 56.6 (51.0-61.8) | 57.3 (52.0-62.0) | 0.01 |
| Male sex [n (%)] | 734 (72.3) | 220 (71.2) | 277 (72.7) | 237 (72.9) | 0.87 |
| African American [n (%)] | 65 (6.4) | 24 (7.8) | 29 (7.6) | 12 (3.7) | 0.05 |
| HCC exception [n (%)] | 365 (36.0) | 107 (34.6) | 146 (38.3) | 112 (34.5) | 0.48 |
| MELD score at listing† | 15 (10-24) | 14 (10-23) | 15 (11-23) | 15 (10-26) | 0.72 |
| MELD score at transplant† | 31 (27-35) | 31 (26-35) | 29 (25-34) | 31 (28-37) | <0.001 |
| Donor risk index† | 1.42 (1.18-1.71) | 1.45 (1.19-1.76) | 1.42 (1.19-1.72) | 1.40 (1.18-1.66) | 0.52 |
| Follow-up time (months)† | 8.7 (2.8-18.2) | 8.3 (3.7-21.5) | 8.5 (3.0-18.2) | 9.6 (1.8-16.1) | 0.44 |
P values compare differences between eras 1, 2, and 3.
The data are presented as medians and IQRs.
As for individuals who underwent transplantation during the study period, the majority underwent transplantation for HCV-related complications; 36.0% had an HCC exception status. The median MELD score at LT was 31 (IQR=27-35). There were no differences seen in the donor risk index between the eras. Although the difference was not statistically significant, there appeared to be higher proportions of HCV transplant recipients who received grafts from HCV antibody–positive donors in the more recent eras (era 1, 3.2%; era 2, 8.7%; era 3, 7.5%; P = 0.09). During the study period, the median donor age was higher for patients undergoing transplantation at UCSF versus other region 5 centers (P = 0.01); however, the proportion of transplants performed at UCSF versus other centers in region 5 did not change over time (P = 0.13; Table 2).
TABLE 2.
Donor Age and Number of Transplants in Region 5 From 2002 to 2012
| Donor Age (Years)* |
Transplants [n (%)] |
|||
|---|---|---|---|---|
| Year of Transplantation | UCSF | Other Transplant Centers† | UCSF | Other Transplant Centers‡ |
| 2002 | 41 (26-55) | 36 (21-51) | 105 (12.5) | 734 (87.5) |
| 2003 | 43 (24-53) | 39 (23-50) | 127 (15.1) | 715 (84.9) |
| 2004 | 41 (28-55) | 38 (23-50) | 124 (14.1) | 755 (85.9) |
| 2005 | 44 (26-56) | 37 (23-49) | 160 (16.9) | 786 (83.1) |
| 2006 | 40 (24-53) | 38 (23-50) | 149 (15.8) | 794 (84.2) |
| 2007 | 40 (24-53) | 41 (25-51) | 127 (13.5) | 814 (86.5) |
| 2008 | 41 (27-51) | 42 (25-52) | 147 (16.1) | 767 (83.9) |
| 2009 | 39 (24-53) | 41 (26-51) | 153 (17.7) | 713 (82.3) |
| 2010 | 40 (25-51) | 39 (26-51) | 139 (15.1) | 781 (84.9) |
| 2011 | 41 (24-51) | 40 (25-53) | 151 (16.0) | 793 (84.0) |
| 2012 | 36 (23-51) | 42 (27-53) | 114 (15.1) | 642 (84.9) |
The data are presented as medians and IQRs.
P=0.01 for comparisons between UCSF and other region 5 centers.
P=0.13 for comparisons between UCSF and other region 5 centers.
Donor Age and WL Time by Era and HCV Status
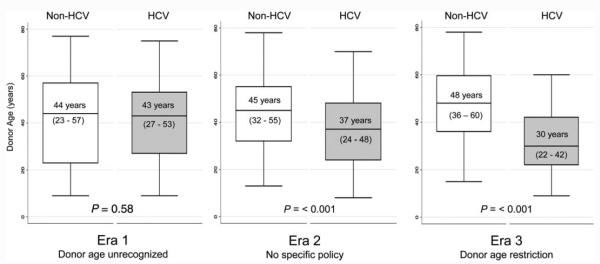
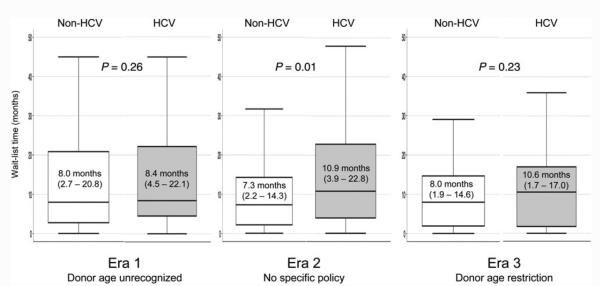
The median donor ages of HCV and non-HCV patients in each era of listing are shown in Fig. 2. In era 1, before the effect of donor age on post-LT outcomes was known, there was no difference in the median donor ages of those with HCV and those without HCV (43 and 44 years, P = 0.575). However, during era 2, when there was increased recognition of the adverse consequences of donor age on HCV outcomes, the median donor age of HCV LT recipients decreased significantly, and the median donor age of HCV recipients was 8 years less than that of their non-HCV counterparts (37 versus 45 years, P < 0.001). This effect was even more pronounced in the era of a formalized donor age restriction policy (era 3), with an 18-year difference in the median donor ages of recipients with HCV and recipients without HCV (30 versus 48 years, P < 0.001). During era 3, there were 7 patients with HCV who received grafts from donors older than 50 years (range=51-60 years). We looked specifically at the median donor age of young transplant recipients (≤40 years at the time of LT) without HCV, and we found that the donor age in this group did not change significantly during the 3 eras [era 1, 42.5 years (IQR=23-53 years); era 2, 38 years (IQR=32-42 years); era 3, 46 years (IQR=40-51 years); P = 0.34]; this suggested that the increase in donor age occurred mostly in older LT recipients. A comparison of times on the WL for HCV and non-HCV patients by era showed that non-HCV patients had a shorter median WL time in era 2, but there was no significant difference in WL times between HCV patients and non-HCV patients during era 3 (Fig. 3).
Figure 2.
Secular trends in the median donor age according to the HCV status. IQRs are shown in parentheses.
Figure 3.
Secular trends in the median WL time according to the HCV status. IQRs are shown in parentheses.
WL Death and Dropout by Era and HCV Status
During the study period, 658 WL patients (27.6%) were removed from the WL because of death or medical deterioration requiring delisting [58% of the HCV patients (n=383) and 42% of the non-HCV patients (n=275)] after a median WL time of 10.6 months (IQR=3.9-25.2 months). In the univariate analysis, HCV patients listed in eras 2 and 3 had significantly lower subhazard ratios (SHRs) of death/dropout in comparison with patients in era 1 [SHR for era 2 versus era 1 as the reference = 0.78, 95% confidence interval (CI)=0.62-0.98, P = 0.03; SHR for era 3 versus era 1 as the reference = 0.67, 95% CI=0.52-0.88, P = 0.01; Table 3]. Other factors predictive of death/dropout were a higher MELD score at the transplant listing (SHR per unit increase in the MELD score = 1.02, 95% CI=1.01-1.25, P < 0.001) and the absence of an HCC exception (SHR=0.12, 95% CI=0.07-0.21, P < 0.001 for the presence of an HCC exception). After multivariate adjustments, the cumulative incidence of WL death and dropout remained lower in eras 2 and 3 versus era 1 (SHR for era 2 versus era 1 as the reference = 0.78, 95% CI=0.62-0.98, P = 0.04; SHR for era 3 versus era 1 as the reference = 0.68, 95% CI=0.52-0.89, P = 0.01; Fig. 4).
TABLE 3.
Competing Risks Regression Analysis for WL Dropout/Death by HCV Status
| Univariate Model |
Multivariate Model |
|||
|---|---|---|---|---|
| Variable | SHR (95% CI) | P Value | SHR (95% CI) | P Value |
| HCV patients (n=1298) | ||||
| WL era | ||||
| 1. Donor age unrecognized | Reference | — | Reference | — |
| 2. No specific policy | 0.78 (0.62-0.98) | 0.003 | 0.78 (0.62-0.98) | 0.036 |
| 3. Donor age restriction | 0.67 (0.52-0.88) | 0.033 | 0.68 (0.52-0.89) | 0.006 |
| Age at listing (per 5-year increase) | 0.97 (0.91-1.05) | 0.486 | — | |
| Female sex (versus male sex) | 1.01 (0.81-1.25) | 0.936 | — | |
| Listing MELD score (per unit increase) | 1.02 (1.01-1.03) | 0.001 | 1.01 (0.99-1.02) | 0.091 |
| HCC exception (yes versus no) | 0.12 (0.07-0.21) | <0.001 | 0.12 (0.07-0.22) | <0.001 |
| African American race | 1.02 (0.69-1.50) | 0.914 | — | |
| Non-HCV patients (n=1090) | ||||
| WL era | ||||
| 1. Donor age unrecognized | Reference | — | Reference | — |
| 2. No specific policy | 0.71 (0.54-0.92) | 0.009 | 0.75 (0.58-0.98) | 0.039 |
| 3. Donor age restriction | 0.64 (0.46-0.87) | 0.005 | 0.64 (0.46-0.88) | 0.007 |
| Age at listing (per 5-year increase) | 1.05 (0.99-1.11) | 0.08 | — | |
| Female sex (versus male sex) | 1.07 (0.83-1.37) | 0.602 | — | |
| Listing MELD score (per unit increase) | 1.02 (1.01-1.03) | 0.001 | 1.01 (0.99-1.02) | 0.317 |
| HCC exception (yes versus no) | 0.08 (0.03-0.19) | <0.001 | 0.08 (0.03-0.21) | <0.001 |
| African American race | 1.42 (0.79-2.55) | 0.246 | — | |
Figure 4.
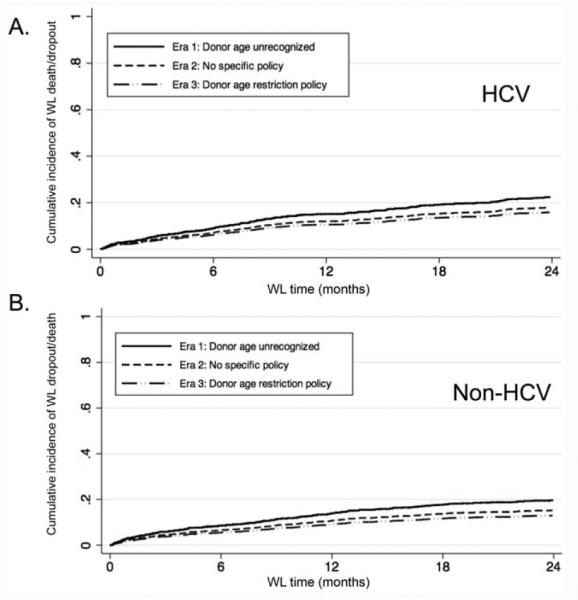
Adjusted competing risks regression analysis for WL dropout or death by the era of listing and the HCV status: (A) HCV and (B) non-HCV. Both graphs have been adjusted for the MELD score at listing and the presence of an HCC exception.
A similar analysis was performed for individuals without HCV (Table 3 and Fig. 4), and the results were consistent with those for the HCV patients. The multivariate analysis again showed that the SHRs for WL death/dropout were lower in eras 2 and 3 versus era 1 (SHR for era 2 versus era 1 as the reference = 0.75, 95% CI=0.58-0.98, P = 0.04; SHR for era 3 versus era 1 as the reference = 0.64, 95% CI=0.46-0.88, P = 0.01). No differences in WL death/dropout were observed between individuals with HCV and individuals without HCV by the era of listing (non-HCV patients versus HCV patients: era 1, SHR = 1.15, 95% CI=0.92-1.45, P = 0.22; era 2, SHR = 1.26, 95% CI=0.96-1.66, P = 0.09; and era 3, SHR = 1.14, 95% CI=0.81-1.61, P = 0.45).
Posttransplant Survival by Era and HCV Status
One hundred ninety-six transplant recipients (19.3%) died after LT (125 HCV patients and 71 non-HCV patients) after a median follow-up of 36.5 months (IQR = 12.4 – 69.9 months); the overall 1- and 3-year post-LT survival rates were 91.6% and 83.3%, respectively. No differences were seen in post-LT survival by the era of listing between those with HCV and those without HCV (Fig. 5). According to the univariate analysis of factors associated with post-LT survival in the HCV cohort, female sex [hazard ratio (HR) = 1.83, 95% CI=1.27-2.65, P < 0.001], African American race (HR = 1.63, 95% CI=1.00-2.66, P = 0.049), a higher donor age (HR per decade = 1.12, 95% CI=1.00-1.26, P = 0.05), an absence of an HCC exception at transplantation (HR for the presence of an exception = 0.66, 95% CI=0.45-0.98, P = 0.04), and a split liver graft (HR=2.07, 95% CI=1.08-3.95, P = 0.03) were statistically significant, but the era of transplantation was not (HR for era 2 with era 1 as the reference = 0.88, 95% CI=0.58-1.32, P = 0.53; HR for era 3 with era 1 as the reference = 1.10, 95% CI=0.63-1.91, P = 0.75; Table 4). Even after we controlled for these potential confounders, the era of transplantation was not associated with post-LT survival (HR for era 2 with era 1 as the reference = 0.90, 95% CI=0.60-1.37, P = 0.63; HR for era 3 with era 1 as the reference = 1.31, 95% CI=0.73-2.32, P = 0.36; Table 4). Only female sex (HR=1.73, 95% CI=1.19-2.52, P = 0.01), an older donor age (HR per decade = 1.17, 95% CI=1.03-1.33, P = 0.01), and a split liver graft (HR=2.50, 95% CI=1.26-4.98, P = 0.01) remained associated with post-LT survival (Table 4). The assumption of proportional hazards was not violated in the final model (P = 1.00). Among the non-HCV individuals, only an older age at transplantation was associated with post-LT survival (HR=1.44, 95% CI=1.10-1.88, P = 0.01; Table 4). The proportional hazards assumption was not violated (P = 0.58).
Figure 5.
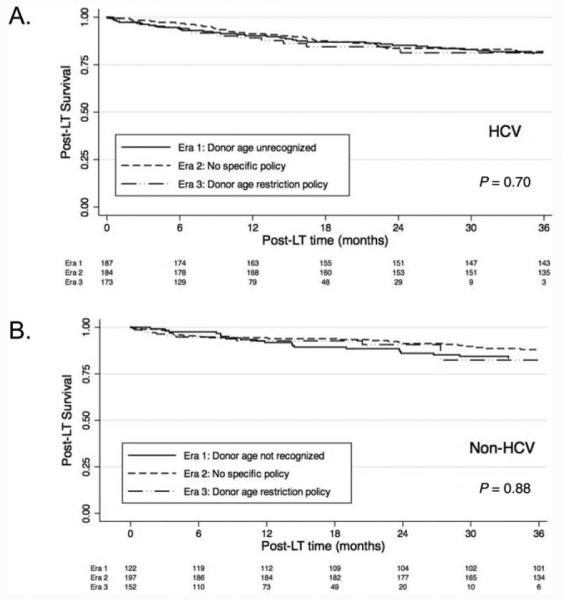
Kaplan-Meier analysis of posttransplant survival for (A) patients with HCV and (B) patients without HCV according to the era of transplantation.
TABLE 4.
Recipient and Donor Factors Associated With Post-LT Survival by HCV Status
| Univariate Model |
Multivariate Model |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P Value | HR (95% CI) | P Value |
| HCV patients (n=544) | ||||
| WL era | ||||
| 1. Donor age unrecognized | Reference | — | Reference | — |
| 2. No specific policy | 0.88 (0.58-1.32) | 0.53 | 0.90 (0.60-1.37) | 0.63 |
| 3. Donor age restriction | 1.10 (0.63-1.91) | 0.75 | 1.31 (0.73-2.32) | 0.36 |
| Age at transplant (per 10 years) | 1.16 (0.90-1.50) | 0.25 | — | |
| Female sex (versus male sex) | 1.83 (1.27-2.65) | <0.001 | 1.73 (1.19-2.52) | 0.01 |
| African American race | 1.63 (1.00-2.66) | 0.049 | 1.43 (0.87-2.35) | 0.16 |
| HCC exception at LT | 0.66 (0.45-0.98) | 0.04 | 0.72 (0.49-1.07) | 0.10 |
| MELD score at transplant (per 5 units) | 1.06 (0.99-1.14) | 0.10 | — | |
| Donor age (per 10 years) | 1.12 (1.00-1.26) | 0.05 | 1.17 (1.03-1.33) | 0.01 |
| Donor height (cm) | 0.99 (0.97-1.00) | 0.17 | — | |
| Cold ischemia time (per hour) | 0.96 (0.77-1.19) | 0.68 | — | |
| Donation after cardiac death | 1.27 (0.56-2.89) | 0.57 | — | |
| Donor race: African American | 0.86 (0.43-1.69) | 0.66 | — | |
| Split liver graft | 2.07 (1.08-3.95) | 0.03 | 2.50 (1.26-4.98) | 0.01 |
| Non-HCV patients (n=471) | ||||
| WL era | ||||
| 1. Donor age unrecognized | Reference | — | Reference | — |
| 2. No specific policy | 0.87 (0.51-1.48) | 0.61 | 0.87 (0.52-1.48) | 0.62 |
| 3. Donor age restriction | 1.03 (0.49-2.19) | 0.94 | 1.01 (0.47-2.13) | 0.99 |
| Age at transplant (per 10 years) | 1.44 (1.10-1.89) | 0.01 | 1.44 (1.10-1.88) | 0.01 |
| Female sex (versus male sex) | 1.23 (0.76-2.01) | 0.39 | — | |
| African American race | 0.82 (0.20-3.32) | 0.78 | — | |
| HCC exception at LT | 1.30 (0.81-2.08) | 0.27 | — | |
| MELD score at transplant (per 5 units) | 1.01 (0.92-1.11) | 0.86 | — | |
| Donor age (per 10 years) | 0.90 (0.78-1.02) | 0.11 | — | |
| Donor height (cm) | 1.01 (0.99-1.03) | 0.31 | — | |
| Cold ischemia time (per hour) | 0.75 (0.48-1.17) | 0.20 | — | |
| Donation after cardiac death | 1.53 (0.38-6.26) | 0.55 | — | |
| Donor race: African American | 1.27 (0.63-2.56) | 0.50 | — | |
| Split liver graft | 0.91 (0.33-2.49) | 0.85 | — | |
DISCUSSION
HCV remains the most common indication for LT in the United States, and the proportion in need of LT for complications of cirrhosis is expected to increase over the next decade.14 Graft and patient losses due to recurrent HCV disease continue to be a major issue, and although new anti-HCV therapies may yield substantial benefits in the future,15,16 an important immediate goal of management is to minimize deaths due to severe and progressive disease. Donor age is a potentially modifiable risk factor, and selecting younger donors for recipients with HCV may be an important strategy for improving posttransplant outcomes. We have shown that restricting the donor age to ≤50 years for WL patients with HCV is not associated with longer waiting times or a higher risk of WL mortality, and this suggests that the implementation of such a policy is feasible even in programs with long wait times such as ours in region 5.
We found that over the past decade, the median donor age of transplant recipients with HCV decreased from 43 to 30 years, and the median donor age of transplant recipients without HCV increased from 44 to 48 years, with the greatest shift in median donor ages occurring with the implementation of our donor age policy for HCV patients. With an older age evident for non-HCV patients, an equally important aim of the study was to examine the effects of this policy on post-LT outcomes for non-HCV patients. Importantly, we showed improved survival for the non-HCV patients during the eras, and donor age was not predictive of post-LT survival for non-HCV patients in our competing risks analysis; this lack of harm with the use of older donors for the non-HCV population has also been suggested by other investigators.17,18 These results are reassuring, but we acknowledge that the replication of these results may be center-dependent and influenced by the median age of the donor population. Interestingly, we noted in both HCV and non-HCV individuals that the risk of WL death/dropout was significantly less during the most recent eras despite the differences in the donor ages that we observed. We believe that this may reflect overall improvements over the past decade in the medical management of patients with decompensated liver disease and advances in therapies for bridging patients with HCC within the Milan criteria to transplantation.
The rationale for restricting HCV patients to younger donors is a reduction of the risk of severe and/or progressive HCV disease after LT. Thus, the secondary aims of the study were to assess post-LT survival as a surrogate for severe HCV disease. We did not see any significant improvements in post-LT survival in the HCV population despite the significant decrease in the median donor age with our restriction policy. There are several potential reasons that a difference is not evident. First, the median donor age in era 1 before the donor age began to decrease was 43 years for HCV recipients. In a study by Lake et al.,4 the risk of posttransplant death increased in HCV individuals once the donor age was greater than 40 years (41-50 years, HR=1.67, P < 0.01); however, it continued to increase as the donor age increased (51-60 years, HR=1.86, P < 0.01; >60 years, HR=2.21, P < 0.01). Therefore, the magnitude of the benefit that we would expect may be lower than what would be expected if the median donor age had been greater than 50 years before the donor age restriction began. Second, our 3-year post-LT survival rate was already high, and the limited number of post-LT events diminished our power to detect a survival difference. Moreover, because the median time to the development of recurrent HCV cirrhosis is 8 to 10 years, a longer period of follow-up may be required before the survival benefit associated with a lower donor age would become apparent. Ideally, this question would be best addressed by an assessment of fibrosis severity in HCV patients according to the era of transplantation; however, the database used for this study did not contain information on post-LT histology. Finally, the interleukin-28B status of the recipients and donors was not known because the study began in 2002 before the effect of the interleukin-28B status on HCV outcomes was appreciated. This may also be confounding our post-LT survival analysis. Therefore, although we were able to demonstrate a lack of adverse outcomes while patients were on the transplant WL, we will be unable to conclude that a donor age restriction policy for HCV patients translates into improved posttransplant outcomes until a longer period of follow-up with histological outcomes has been assessed.
Notably, previous work has suggested that patients with cirrhosis due to a combination of HCV and alcohol use and patients with cirrhosis due to HCV or alcohol alone may not have similar clinical outcomes on the WL or during the post-LT period.19-21 As previously described, approximately 20% of the HCV population in this study was concurrently using alcohol. We, therefore, conducted sensitivity analyses that excluded those individuals and looked at both WL and post-LT outcomes in the HCV-alone population. We found no differences in the point estimates in either model (data not shown); therefore, we feel that it was appropriate, at least in this study, to include both HCV patients with alcohol as a cofactor and HCV patients without alcohol as a cofactor in the main analyses because the presence of HCV, regardless of the alcohol status, would have led to donor age restriction.
The results of our study will continue to be relevant even as we enter the new era of antiviral therapy for HCV. Although the success of the new treatment regimens is expected to improve the management of HCV disease after LT, with a greater proportion of patients attaining a sustained virological response before LT and with post-LT antiviral therapy having greater efficacy, the optimal antiviral strategy is still evolving, and recurrent disease will continue to be an issue in the years to come. For example, one strategy for improving access to deceased donors for patients with HCV involves the use of anti-HCV–positive donors. These recipients will be at risk for HCV infection after LT, and the course will be influenced by the donor age. Moreover, access to new drug therapies will be limited for some patients and in many countries, so an effort to minimize disease progression through donor selection is a potentially useful management strategy. Furthermore, even if the large majority of HCV patients enter LT virus-free, an older donor age has been shown to be associated with worse graft and patient outcomes even in those without HCV; therefore, donor age restriction may continue to be a strategy that can be implemented in the future in attempt to optimize post-LT outcomes for specific high-risk populations.
There are several limitations to our study. We do not have information on several posttransplant factors that also could affect post-LT survival, including HCV treatment and treated acute rejection; therefore, we were not able to adjust for these factors in our survival model. Additionally, we recognize that factors other than the donor age restriction policy that we have not accounted for in the era effect may have been changing during the study period. As mentioned previously, this is likely responsible for the decreased dropout/death hazard seen in the more recent eras. Additionally, this is a single-center experience, so others must consider center-specific characteristics in applying these findings. Finally, the limited posttransplant follow-up is a limitation and likely contributed to our inability to demonstrate a post-LT survival benefit in the more recent eras in which donor age restriction was used. However, because of the well-established association between donor age and post-transplant HCV disease outcomes, the information provided by this study will be useful to transplant physicians in their efforts to improve posttransplant outcomes.
In conclusion, we have shown that a significant decrease in donor age can be achieved for LT candidates with HCV without obvious adverse WL outcomes for patients with or without HCV. Moreover, the modest increase in donor age seen for non-HCV patients with this policy did not affect post-LT survival with a median follow-up of 36.5 months. Although long-term follow-up is needed to capture the full benefits and potential risks of this policy, our results provide support for those programs that are using donor age restriction as a strategy to improve post-LT outcomes for their HCV patients.
Acknowledgments
This work was funded by the Canadian Association for the Study of the Liver/Merck Fellowship in Hepatology (to Jennifer A. Flemming).
Abbreviations
- CI
confidence interval
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- SHR
subhazard ratio
- UCSF
University of California San Francisco
- WL
wait list
Footnotes
Potential conflict of interest: Nothing to report.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation by the Organ Procurement and Transplantation Network or the US government.
REFERENCES
- 1.Kim WR, Stock PG, Smith JM, Heimbach JK, Skeans MA, Edwards EB, et al. OPTN/SRTR 2011 annual data report: liver. Am J Transplant. 2013;13(suppl 1):73–102. doi: 10.1111/ajt.12021. [DOI] [PubMed] [Google Scholar]
- 2.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. for OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC, Feng S, Roberts JP. An examination of liver offers to candidates on the liver transplant wait-list. Gastroenterology. 2012;143:1261–1265. doi: 10.1053/j.gastro.2012.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lake JR, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am J Transplant. 2005;5:549–557. doi: 10.1111/j.1600-6143.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 5.Berenguer M, Prieto M, San Juan F, Rayo'n JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36:202–210. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 6.Wali M, Harrison RF, Gow PJ, Mutimer D. Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut. 2002;51:248–252. doi: 10.1136/gut.51.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machicao VI, Bonatti H, Krishna M, Aqel BA, Lukens FJ, Nguyen JH, et al. Donor age affects fibrosis progression and graft survival after liver transplantation for hepatitis C. Transplantation. 2004;77:84–92. doi: 10.1097/01.TP.0000095896.07048.BB. [DOI] [PubMed] [Google Scholar]
- 8.Alonso O, Loinaz C, Moreno E, Jime'nez C, Abradelo M, Go'mez R, et al. Advanced donor age increases the risk of severe recurrent hepatitis C after liver transplantation. Transpl Int. 2005;18:902–907. doi: 10.1111/j.1432-2277.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- 9.Mutimer DJ, Gunson B, Chen J, Berenguer J, Neuhaus P, Castaing D, et al. Impact of donor age and year of transplantation on graft and patient survival following liver transplantation for hepatitis C virus. Transplantation. 2006;81:7–14. doi: 10.1097/01.tp.0000188619.30677.84. [DOI] [PubMed] [Google Scholar]
- 10.Rayhill SC, Wu YM, Katz DA, Voigt MD, Labrecque DR, Kirby PA, et al. Older donor livers show early severe histological activity, fibrosis, and graft failure after liver transplantation for hepatitis C. Transplantation. 2007;84:331–339. doi: 10.1097/01.tp.0000270313.31328.63. [DOI] [PubMed] [Google Scholar]
- 11.Lai JC, Verna EC, Brown RS, Jr, O’Leary JG, Trotter JF, Forman LM, et al. for Consortium to Study Health Outcomes in HCV Liver Transplant Recipients (CRUSH-C). Hepatitis C virus-infected women have a higher risk of advanced fibrosis and graft loss after liver transplantation than men. Hepatology. 2011;54:418–424. doi: 10.1002/hep.24390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesner RH, Sorrell M, Villamil F. for International Liver Transplantation Society Expert Panel. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1–S9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 15.Burton JR, Jr, O’Leary JG, Verna EC, Saxena V, Dodge JL, Stravitz RT, et al. A US multicenter study of hepatitis C treatment of liver transplant recipients with proteaseinhibitor triple therapy. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlton MR, Gane EJ, Manns MP, Brown RS, Curry MP, Kwo PY, et al. Sofosbuvir and ribavirin for the treatment of established recurrent hepatitis C infection after liver transplantation: preliminary results of a prospective, multicenter study [abstract] Hepatology. 2013;58(6):1378A. [Google Scholar]
- 17.Faber W, Seehofer D, Puhl G, Guckelberger O, Bertram C, Neuhaus P, Bahra M. Donor age does not influence 12-month outcome after orthotopic liver transplantation. Transplant Proc. 2011;43:3789–3795. doi: 10.1016/j.transproceed.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 18.Darius T, Monbaliu D, Jochmans I, Meurisse N, Desschans B, Coosemans W, et al. Septuagenarian and octogenarian donors provide excellent liver grafts for transplantation. Transplant Proc. 2012;44:2861–2867. doi: 10.1016/j.transproceed.2012.09.076. [DOI] [PubMed] [Google Scholar]
- 19.Lucey MR, Schaubel DE, Guidinger MK, Tome S, Merion RM. Effect of alcoholic liver disease and hepatitis C infection on waiting list and posttransplant mortality and transplant survival benefit. Hepatology. 2009;50:400–406. doi: 10.1002/hep.23007. [DOI] [PubMed] [Google Scholar]
- 20.Aguilera V, Berenguer M, Rub'ın A, San-Juan F, Rayo'n JM, Prieto M, Mir J. Cirrhosis of mixed etiology (hepatitis C and alcohol): posttransplantation outcome—comparison with hepatitis C virus–related cirrhosis and alcoholic-related cirrhosis. Liver Transpl. 2009;15:79–87. doi: 10.1002/lt.21626. [DOI] [PubMed] [Google Scholar]
- 21.Singal AK, Hmoud BS, Guturu P, Kuo YF. Outcome after liver transplantation for cirrhosis due to alcohol and hepatitis C: comparison to alcoholic cirrhosis and hepatitis C cirrhosis. J Clin Gastroenterol. 2013;47:727–733. doi: 10.1097/MCG.0b013e318294148d. [DOI] [PubMed] [Google Scholar]