Abstract
Patients with T1 hepatocellular carcinoma (HCC; 1 lesion < 2 cm) are currently not eligible for priority listing for liver transplantation (LT). A common practice is to wait without locoregional therapy (LRT) until tumor growth occurs from T1 to T2 (1 lesion 2-5 cm or 2-3 lesions ≤ 3 cm) to be eligible for listing with Model for End-Stage Liver Disease exception. We aimed to evaluate the intention to treat outcome of the “wait and not ablate” approach for nonresection candidates with T1 HCC until tumor growth to T2. The study included 114 patients with T1 HCC 1.0-1.9 cm followed by serial imaging every 3 months. Two investigators performed independent imaging reviews to confirm the diagnosis. Median increase in total tumor diameter was 0.14 cm/month. Probabilities of progression from T1 to directly beyond T2 without LT listing were 4.4% at 6 months and 9.0% at both 12 and 24 months. The 1- and 3-year survival was 94.5% and 75.5%. In multivariate analysis, predictors of rapid tumor progression, defined as a >1 cm increase in total tumor diameter over 3 months, included alcoholic liver disease (odds ratio [OR], 6.52; P = 0.02) and Hispanic race (OR, 3.86; P = 0.047), whereas hepatitis B appeared to be protective (OR, 0.09; P = 0.04). By competing risks regression, predictors of exclusion from LT (with or without listing for LT under T2) were alpha-fetoprotein (AFP) ≥ 500 ng/mL (HR, 12.69; 95% confidence interval, 2.8-57.0; P = 0.001) and rapid tumor progression (HR, 5.68; P < 0.001). In conclusion, the “wait and not ablate” approach until tumor growth from T1 to T2 before LT listing is associated with a <10% risk of tumor progression to directly beyond T2 criteria. However, patients with AFP ≥ 500 ng/mL and rapid tumor progression are at high risk for wait-list dropout and should receive early LRT.
Worldwide, hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths, and within the United States, the incidence has nearly tripled in the past 2 decades.1,2 Resection is typically considered first-line therapy in those without evidence of portal hypertension or liver dysfunction.3 Excellent 5-year posttransplant survival approaching 75% with recurrence rates of less than 10%-15% has been seen in patients undergoing liver transplantation (LT) for HCC within the Milan criteria.4-6 Although the Model for End-Stage Liver Disease (MELD) score has been used since 2002 for the prioritization of patients with end-stage liver disease for LT,7-9 patients with HCC are at risk for tumor progression and dropout from the waiting list independent of their MELD score.10 Consequently, a MELD priority exception system for HCC has been in place for those meeting the Milan criteria.
In the United Network for Organ Sharing (UNOS) staging classification, the Milan criteria4 are further divided into stage T1 and T2 HCC, where T1 patients have a single tumor of <2 cm and T2 patients have a single tumor measuring 2-5 cm or 2-3 lesions with none greater than 3 cm. There have been several refinements to the MELD exception system, and currently, patients with T2 HCC initially receive 22 MELD exception points and are eligible for exception upgrades every 3 months while they remain on the waiting list within Milan criteria. Since 2004, patients with T1 HCC have not been eligible for priority listing for LT. This is in large part due to the low risk for waitlist dropout in these patients and a sizeable proportion of misdiagnosis of HCC (no HCC in the explants) in patients transplanted for presumed T1 HCC.11-13
As LT with deceased donors for T1 HCC is no longer an immediate treatment option, the management of these patients is not standardized, and 2 competing and quite dissimilar approaches have emerged. One strategy is to treat T1 HCC patients with immediate locoregional therapy (LRT) of which radiofrequency ablation (RFA) is the preferred modality.14 Five-year tumor-free survival with immediate RFA of T1 HCC is reported to be 38%-60%.15-17 An alternative strategy in LT candidates is to carefully observe patients without LRT until the tumor grows to reach stage T2, at which point the patients are eligible for priority listing for LT with MELD exception before undergoing LRT. The risk of tumor progression directly from stage T1 to beyond conventional transplant criteria using this “wait and not ablate” approach is unknown. The primary aim of the present study was to evaluate the intention to treat (ITT) outcome of the “wait and not ablate” approach for nonresection candidates with T1 HCC being considered for LT and to determine risk factors for rapid tumor progression or exclusion from LT in this patient population.
PATIENTS AND METHODS
Study Design and Patient Population
This study included consecutive patients aged 18 years and older who were evaluated at our institution from March 2004 to January 2012 with an initial solitary tumor measuring 1.0 to 1.9 cm in largest diameter. This start date was chosen to encompass the UNOS HCC policy change in which patients with T1 HCC were no longer eligible for priority listing for LT with MELD exception. Patients were initially identified by reviewing records from our center’s multidisciplinary tumor board attended by transplant hepatologists, transplant surgeons, oncologists, interventional radiologists, and diagnostic radiologists with an expertise in diagnostic abdominal imaging. The diagnosis of T1 HCC was based on either quadruple-phase contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) showing arterial phase hyperenhancement and washout on the delayed phases of imaging,18 or if a lesion showed interval growth by ≥3 mm with the same imaging technique within 6 months.19 All patients with suspected T1 HCC but without typical radiologic characteristics such as a lack of arterial hyperenhancement and/or delayed washout were independently reviewed by 2 investigators (Nicholas Fidelman and Neil Mehta). In these cases, a retrospective review of all subsequent scans was performed and those who developed typical HCC characteristics or >3 mm growth were included in the study. Patients were excluded from this study if they were resection candidates without any evidence of liver dysfunction and without portal hypertension or if they had lack of tumor visualization or interval growth on all follow-up imaging. The latter were considered not to have HCC. Hepatic lesions < 1 cm in diameter were also not counted as HCC. Percutaneous biopsy was not routinely performed for the diagnosis of HCC at our institution.
All patients in the “wait and not ablate” cohort received HCC surveillance with contrast-enhanced CT or MRI every 3 months until tumor progression to beyond T1 criteria at which point LRT was undertaken. There was 100% compliance with this surveillance protocol. All included patients had a minimum follow-up of 6 months after the diagnosis of T1 HCC. The variables collected included demographic data (age, sex, race/ethnicity), liver-related factors (etiology of liver disease and Child-Turcotte-Pugh [CTP] score), baseline laboratory data at diagnosis of T1 HCC (alpha-fetoprotein [AFP] and MELD score), and radiologic characteristics including maximal tumor diameter and presence or absence of arterial hyperenhancement and delayed washout.
Among patients who underwent LT, the freshly explanted livers were processed according to a routine clinical protocol and were sliced serially at 10-mm intervals. Macroscopically visible neoplastic nodules were evaluated with microscopy after hematoxylineosin staining. The histologic grade of differentiation was based on the modified Edmondson criteria (grade 1, well-differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated).20 Explant tumor stage and the presence of microvascular or macrovascular tumor invasion were also recorded. Explant tumor staging in this study was based only on the maximal diameter and number of viable tumors.
Outcomes
The primary outcome of interest was progression from T1 directly to beyond T2 criteria. A secondary outcome, exclusion from LT, was directed toward those who progressed to within T2 and listed for LT but then had wait-list dropout due to liver-related death or tumor progression beyond T2. In patients who progressed to within T2 but were not listed for LT for various reasons such as no longer being interested in undergoing LT or being noncompliant with our center’s transplant policies, follow-up was censored at the date the patient was first noted to be within T2 criteria. Date of death was obtained from our transplant database and confirmed using the Social Security Death Index.
Statistical Analysis
Patient characteristics were summarized using median and interquartile range (IQR) for continuous variables and proportions for categorical variables. Kaplan-Meier ITT survival and 95% confidence intervals (CIs) were estimated from the time of T1 diagnosis to death or last follow-up. Survival was compared between patients with and without immediate LRT using the log-rank test.
Tumor growth rate was calculated based on initial tumor size at T1 HCC diagnosis and size of tumor(s) at time of progression beyond T1 (or at last imaging if still within T1 at end of study follow-up) divided by the time interval between these 2 cross-sectional studies. Logistic regression models with odds ratios (ORs) were used to assess predictors of rapid tumor progression defined as the upper 20% of tumor progression within the study population. This rapid tumor progression cutoff was equivalent to >1 cm increase in total tumor diameter over 3 months. Univariate analyses included baseline clinical characteristics (age, sex, race/ethnicity, etiology of liver disease, and MELD score) and tumor characteristics at the time of T1 HCC diagnosis (AFP level, tumor size, and presence or absence of arterial phase hyperenhancement and delayed washout). Predictors of rapid tumor progression with a univariate P value < 0.1 were evaluated in the multivariate model.
We estimated the cumulative probability of progression from T1 diagnosis directly to beyond T2 while accounting for competing risks (CRs). Patients were simultaneously at risk of dying while within T1 criteria (modeled as a CR) and were otherwise censored at the date of LT, wait-list dropout, or last follow-up. Follow-up time was measured from the date of T1 diagnosis. We also estimated the cumulative probability of exclusion from LT. We accounted for competing events (LT or death while within T1 criteria). Patients were censored at the date of LT, last follow-up, or wait-list dropout if the reason for dropout was unrelated to liver disease or tumor progression. Follow-up time was measured from the date of T1 diagnosis. Univariate and multivariate hazard ratios (HRs) for predictors of exclusion from LT were determined by CR regression using the Fine and Gray method21 with predictors having a P value of < 0.1 further evaluated in the multivariate analysis.
Post-LT outcomes, patient survival (event defined as post-LT death), and HCC recurrence-free survival (event defined as the first of HCC recurrence or death) were evaluated using the Kaplan-Meier method. Patients were followed from transplant to the first event of interest or last follow-up. Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC) and Stata/IC 11.1 (Statacorp, College Station, TX).
RESULTS
Baseline Clinical and Tumor Characteristics
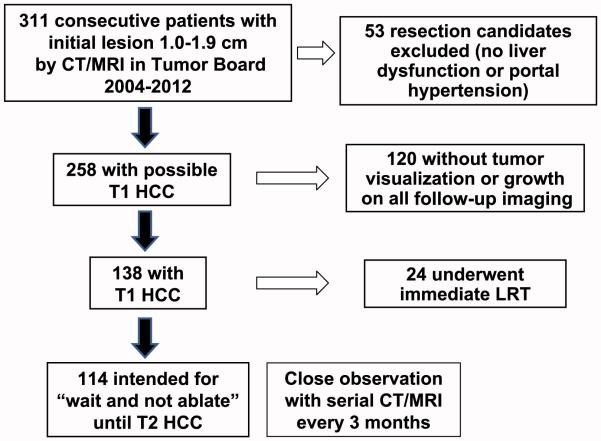
Among the 311 patients initially identified, 53 patients were resection candidates without liver dysfunction or portal hypertension and were therefore excluded. Of the remaining 258 patients, 120 (46.5%) either had lack of tumor visualization or growth on all subsequent imaging (median follow-up 2.6 years [IQR, 1.4-3.4]). These patients were considered not to have HCC and were also excluded. Of the 138 patients with T1 HCC, 24 (17.4%) underwent immediate LRT (Fig. 1). The most common reasons for undergoing immediate LRT instead of the “wait and not ablate” approach included patient preference and contraindications to LT including advanced age.
Figure 1.
Flow diagram with exclusion criteria describing the formation of the “wait and not ablate” cohort.
The remaining 114 patients comprised the “wait and not ablate” cohort. Median age at diagnosis of T1 HCC was 60 years (IQR, 55-65 years), and 85 (74.6%) were men. The most common race/ethnicities were Caucasian (47.4%), Asian (31.6%), and Hispanic (14.0%) with few African Americans (4.4%). Hepatitis C virus (HCV) was the most common etiology of liver disease (57.0%), followed by hepatitis B virus (HBV; 22.8%), nonalcoholic fatty liver disease (NAFLD; 10.5%), and alcoholic liver disease (8.8%). At the time of diagnosis of T1 HCC, the median MELD score was 11 (IQR, 9-13), and there was a roughly equal proportion with Child class A (48.2%) and Child class B/C (51.8%) cirrhosis (Table 1).
TABLE 1.
Baseline Clinical Characteristics of the “Wait and Not Ablate” Cohort (n=114)
| Characteristic | Value |
|---|---|
| Age, years | 60 (55-65) |
| Sex, male | 85 (74.6) |
| Race/ethnicity | |
| Caucasian | 54 (47.4) |
| Asian | 36 (31.6) |
| Hispanic | 16 (14.0) |
| African American | 5 (4.4) |
| Etiology of liver disease | |
| HCV | 65 (57.0) |
| HBV | 26 (22.8) |
| NAFLD | 12 (10.5) |
| Alcoholic liver disease | 10 (8.8) |
| Hemochromatosis | 1 (0.9) |
| MELD score | 11 (9-13) |
| Child class (n5112) | |
| A | 54 (48.2) |
| B | 48 (42.9) |
| C | 10 (8.9) |
| Initial tumor size, cm | 1.4 (1.2-1.6) |
| AFP at T1 diagnosis (n=103) | |
| ≤20 | 62 (60.2) |
| 21-99 | 28 (27.2) |
| 100-499 | 8 (7.8) |
| ≥500 | 5 (4.8) |
| Presence of arterial hyperenhancement | 104 (91.2) |
| Presence of delayed washout | 84 (73.7) |
NOTE: Data are given as median (IQR) or n (%).
Median initial tumor diameter was 1.4 cm (IQR, 1.2-1.6 cm), and 52.6% had a lesion measuring 1.0-1.4 cm in maximal diameter at diagnosis. The median AFP level was 12 ng/mL (IQR, 4.7-41.0 ng/mL); 60.2% of patients had an AFP less than 20 ng/mL and 4.8% had an AFP greater than 500 ng/mL. Arterial hyperenhancement was observed in 91.2%, and delayed washout was present in 73.7% of patients (Table 1).
ITT Outcome
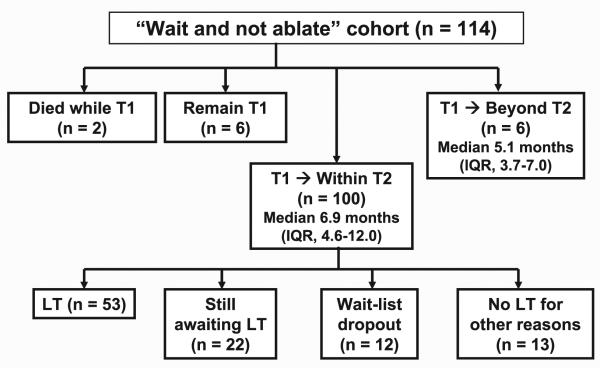
Median follow-up from date of diagnosis of T1 HCC was 2.4 years (IQR, 1.4-4.4 years). Progression from T1 to within T2 criteria was seen in 100 (87.7%) patients at a median of 6.9 months (IQR, 4.6-12.0 months). Six (5.3%) patients progressed from T1 directly to beyond T2 criteria at a median of 5.1 months (IQR, 3.7-7.0 months). An additional 6 patients remained within T1 criteria at the end of study follow-up, and 2 patients died while within T1 criteria due to non-HCC causes (Fig. 2).
Figure 2.
ITT outcomes of the 114 patients in the “wait and not ablate” cohort.
Of the 100 patients progressing from T1 to within T2 criteria, all underwent at least 1 LRT at a median of 1.8 months (IQR, 0.7-2.6 months) from diagnosis of T2 HCC to receipt of first LRT; 53% underwent LT after receiving MELD exception, 22% were still awaiting LT at end of study follow-up, and 12% dropped off the LT wait list because of subsequent tumor progression to beyond T2. The remaining 13% were never listed for LT for such reasons as being noncompliant with our program’s transplant requirements or no longer being interested in LT (Fig. 2). After a median follow-up of 2.4 years, the Kaplan-Meier ITT survival for the entire “wait and not ablate” cohort at 1, 3, and 5 years was 94.5% (95% CI, 88.1%-97.5%), 75.5% (95% CI, 64.5%-83.5%), and 55.0% (95% CI, 41.6%-66.4%), respectively.
Tumor Progression
Median increase in overall total tumor diameter was 0.14 cm per month (IQR, 0.08-0.29 cm). The median time to tumor progression beyond T1 was significantly longer in patients with an initial lesion measuring 1.0-1.4 cm than in those with an initial lesion measuring 1.5-1.9 cm (9.0 months [IQR, 5.2-14.8 months] versus 6.1 months [IQR, 4.3-9.7 months], respectively; P = 0.02). This reflects a longer period of time for smaller tumors to grow to beyond T2 criteria. At the time of progression beyond T1, 71.7% had a single lesion measuring at least 2 cm in diameter (median, 2.3 cm; IQR, 2.0-2.9 cm) and 22.6% had 2 lesions (median larger tumor diameter, 2.0 cm; IQR, 1.7-2.4 cm). Only 1 patient had more than 3 lesions at the time of progression beyond T1. Median AFP at time of tumor progression beyond T1 was 18 ng/mL (IQR, 5.0-63.0 ng/mL).
Predictors of Rapid Tumor Progression
Rapid tumor progression, defined as >1 cm increase in total tumor diameter over 3 months, was observed in 23 (20.2%) patients. In addition to the 6 patients who rapidly progressed from stage T1 directly to beyond T2, there were an additional 3 patients with rapid tumor progression who progressed from stage T1 to within T2 but then dropped out because of tumor progression. They accounted for 25% (3/12) of all patients who dropped out after listing with MELD exception once within stage T2. On multivariate analysis, significant predictors of rapid tumor progression included Hispanic race/ethnicity (OR, 3.86; P = 0.047) and alcoholic liver disease (OR, 6.52; P = 0.02), whereas hepatitis B was associated with reduced odds of rapid tumor progression compared to HCV (OR, 0.09; P = 0.04). We were unable to detect a statistically significant association between rapid tumor progression and age, sex, MELD score, CTP score, and tumor characteristics at T1 diagnosis including size, AFP, and presence of arterial enhancement or delayed washout (Table 2).
TABLE 2.
Univariate and Multivariate Analyses of Predictors of Rapid Tumor Progression*
| Predictor Variables | OR (95% CI) | P Value |
|---|---|---|
| Univariate analysis | ||
| Age at T1 diagnosis (per year) | 1.02 (0.96-1.08) | 0.56 |
| Sex, female | 0.76 (0.26-2.28) | 0.63 |
| Race/ethnicity (versus Caucasian) | ||
| Hispanic | 4.47 (1.29-15.46) | 0.02 |
| African American | 3.83 (0.55-26.68) | 0.17 |
| Asian | 1.19 (0.37-3.78) | 0.77 |
| Diagnosis of liver disease (versus HCV) | ||
| Alcohol | 6.67 (1.49-29.91) | 0.01 |
| NASH | 0.30 (0.04-2.54) | 0.27 |
| HBV | 0.13 (0.02-1.07) | 0.06 |
| CTP score (per point) | 1.06 (0.81-1.38) | 0.66 |
| MELD score (per point) | 1.04 (0.92-1.17) | 0.57 |
| Tumor size at T1 diagnosis (per cm) | 1.98 (0.40-9.90) | 0.41 |
| AFP at T1 diagnosis | ||
| ≥20 (versus<20) | 1.87 (0.71-4.92) | 0.20 |
| ≥100 (versus<100) | 1.18 (0.30-4.75) | 0.81 |
| Hypovascularity | 1.78 (0.42-7.49) | 0.43 |
| Absence of washout | 1.09 (0.38-3.12) | 0.87 |
| Multivariate analysis | ||
| Hispanic (versus Caucasian) | 3.86 (1.02-14.65) | 0.047 |
| Alcohol (versus HCV) | 6.52 (1.34-31.66) | 0.02 |
| HBV (versus HCV) | 0.09 (0.01-0.93) | 0.04 |
Defined as>1 cm increase in total tumor diameter over 3 months.
Progression Directly From T1 to Beyond T2
Six patients (5.3%) were found to have progressed from stage T1 HCC directly to beyond T2 criteria. More detailed information on these 6 patients are presented in Table 3. Four of these patients progressed directly beyond T2 criteria based on tumor burden alone at a median of 6.5 months from T1 diagnosis. Median total tumor diameter in these 4 patients was 7.8 cm (range, 5.7-11.0 cm). A fifth patient had portal vein tumor thrombus 3 months after T1 diagnosis and a final patient had an adrenal metastasis 7 months after T1 diagnosis. Median AFP at time of progression from T1 directly beyond T2 was 6.5 ng/mL (range, 4.4-32). CR cumulative probability of progression from T1 HCC directly to beyond T2 criteria was 4.4% (95% CI, 1.4-10.0) within 6 months and 9.0% (95% CI, 3.2-18.5) within 1 and 2 years.
TABLE 3.
Characteristics of the 6 Patients Who Progressed Directly From Stage T1 to Beyond T2 Criteria
| Patient | Age, Years |
Liver Disease Etiology |
Size at T1 Diagnosis, cm |
AFP at T1 Diagnosis, ng/mL |
Time to Progression Directly Beyond T2, Months |
Tumor Burden When Directly Beyond T2 |
AFP When Directly Beyond T2, ng/mL |
|---|---|---|---|---|---|---|---|
| 1 | 63 | HCV | 1.0 | 11.2 | 5.9 | 7 lesions up to 2.3 cm | 6.1 |
| 2 | 57 | HCV | 1.9 | 41.0 | 2.1 | 2 lesions up to 2.9 cm+portal vein tumor invasion |
32 |
| 3 | 56 | HCV | 1.4 | 13.7 | 3.6 | 3 lesions up to 4.9 cm | 15 |
| 4 | 55 | Alcohol | 1.9 | 4.0 | 4.3 | 2 lesions up to 4 cm | 4.7 |
| 5 | 58 | HBV | 1.6 | 34.3 | 12.0 | Single 11-cm lesion | 6.9 |
| 6 | 66 | NAFLD | 1.5 | 4.0 | 6.9 | Single 3-cm lesion and adrenal metastasis |
4.4 |
Predictors of Exclusion From LT
There were 18 patients excluded from LT, which included the 6 who progressed directly from T1 to beyond T2 as well as 12 additional patients who experienced tumor progression after listing with MELD exception for LT at the T2 tumor stage before progression to beyond T2 criteria. CR cumulative probability of exclusion from LT was 4.5% (95% CI, 1.7-9.4) within 6 months, 7.3% (95% CI, 3.4-13.2) within 1 year, and 15.6% (95% CI, 9.1-23.6) within 2 years from T1 HCC diagnosis. Significant predictors of exclusion from LT by CR included an AFP ≥ 500 ng/ mL at the time of diagnosis of T1 HCC (HR, 12.69; P = 0.001) and rapid tumor progression (HR, 5.68; P < 0.001). We were unable to detect a statistically significant association between exclusion from LT and age, sex, race/ethnicity, etiology of liver disease, MELD score, CTP score, tumor diameter, AFP, and presence of arterial enhancement or delayed washout (Table 4).
TABLE 4.
CRs Univariate and Multivariate Analyses of Predictors of Exclusion From LT
| Predictor Variables | HR (95% CI) | P Value |
|---|---|---|
| Univariate analysis | ||
| Age at T1 diagnosis (per year) | 0.99 (0.95-1.04) | 0.73 |
| Sex, female | 0.68 (0.23-2.00) | 0.49 |
| Race/ethnicity (versus Caucasian) | ||
| Hispanic | 1.90 (0.57-6.35) | 0.30 |
| African American | 1.64 (0.22-12.24) | 0.63 |
| Asian | 0.96 (0.32-2.90) | 0.94 |
| Diagnosis of liver disease (versus HCV) | ||
| Alcohol | 1.99 (0.41-9.73) | 0.40 |
| HBV | 0.91 (0.29-2.81) | 0.86 |
| NASH | 0.47 (0.06-3.81) | 0.48 |
| CTP score (per point) | 0.92 (0.68-1.24) | 0.60 |
| MELD score (per point) | 0.95 (0.82-1.11) | 0.55 |
| Tumor size at T1 diagnosis (per cm) | 1.64 (0.29-9.37) | 0.58 |
| AFP at T1 diagnosis | ||
| ≥20 (versus<20) | 2.09 (0.72-6.11) | 0.18 |
| ≥100 (versus<100) | 2.07 (0.58-7.30) | 0.26 |
| ≥500 (versus<500) | 6.90 (1.66-28.70) | 0.008 |
| Hypovascularity | 1.30 (0.31-5.46) | 0.72 |
| Absence of washout | 0.82 (0.27-2.47) | 0.72 |
| Rapid tumor progression* | 4.12 (1.61-10.53) | 0.003 |
| Multivariate analysis | ||
| AFP≥500 (versus<500) | 12.69 (2.82-57.01) | 0.001 |
| Rapid tumor progression* | 5.68 (2.20-14.64) | <0.001 |
Defined as>1 cm increase in total tumor diameter over 3 months.
Explant Characteristics and Post-LT Outcomes
Fifty-three patients underwent LT after being listed with MELD exception once within T2 criteria. Median AFP at the time of LT was 10.6 ng/mL (IQR, 4.1-39.0 ng/mL). In the explant, the tumor(s) showed complete necrosis with no residual tumors as a result of LRT in 19 (35.8%) patients, within T1 or T2 criteria in 28 (52.8%) patients, and understaging to beyond T2 criteria in 6 (11.3%) patients. No patients had macrovascular invasion, and 3 (5.7%) patients had microvascular invasion. Among 34 patients with viable tumors in the explant, most had either well-differentiated (26.5%) or moderately differentiated HCC (64.7%), whereas only 8.8% had poorly differentiated tumor grade. There were no other tumor histologies seen on explant besides HCC. Transplanted patients had a median post-LT follow-up time of 2.9 years (IQR, 1.0-4.8 years). Post-LT survival at 1 and 5 years was 90.0% (95% CI, 77.5%-95.7%) and 77.0% (95% CI, 58.7%-88.0%), respectively. HCC recurrencefree post-LT survival at 1 and 5 years was 87.9% (95% CI, 75.0%-94.4%) and 75.6% (95%, CI 59.9%-85.5%), respectively.
Immediate LRT and Resection
Twenty-four patients did not undertake the “wait and not ablate” approach and instead underwent immediate LRT at the time of T1 HCC diagnosis. Reasons for this alternate approach included contraindication to LT due to advanced age (n = 9), patient preference (n = 8), and having an AFP > 1000 ng/mL (n = 2). No specific reason for immediate LRT could be identified in 5 patients. None of the patients who underwent immediate LRT had undergone LT by the end of study follow-up. Kaplan-Meier ITT survival for the immediate LRT cohort at 1 and 5 years was 100.0% and 79.5%, respectively. There were no significant differences in the overall ITT survival for those with immediate LRT as compared to the “wait and not ablate” approach (P = 0.15).
Of the 53 patients identified as resection candidates who were excluded from the “wait and not ablate” protocol, 24 underwent resection at our center with confirmed HCC in the resection specimen. HCC recurrence-free survival at 1 and 5 years after resection was 91.7% and 62.5%, respectively. Among the other 29 patients, 5 underwent resection at other centers; 7 chose not to undergo resection and underwent laparoscopic RFA instead; 14 did not have confirmed diagnosis of HCC and did not undergo further treatment; and 3 patients were lost to follow-up. None of these patients received LT.
DISCUSSION
HCC is being detected at an earlier stage of disease22 presumably because of increased screening of at-risk populations23 and improved imaging/detection methods. Because patients with unresectable T1 HCC (single lesion < 2 cm) are not eligible for priority listing for LT under the current system of organ allocation, it is important to evaluate the outcomes of the 2 management strategies: immediate LRT and “wait and not ablate.” In this largest study of the “wait and not ablate” strategy of close observation until tumor growth from T1 to T2 stage before LT listing and LRT, the probability of progression from T1 directly to beyond T2 criteria by CR was just 4.4% by 6 months and 9% by 1 and 2 years. Of those with tumor growth to within T2 criteria, 53% had received LT and 22% were still awaiting LT at the end of study follow-up.
Our findings indicate a generally slow rate of tumor growth in patients with T1 HCC, based on a median increase in total tumor diameter of 0.4 cm every 3 months. This rate appears to be significantly slower than the commonly reported HCC doubling time of 3-6 months.24,25 Among patients in our “wait and ablate” cohort who underwent LT, only 9% had poorly differentiated tumor grade, 4% had microvascular invasion, and 11% had tumor understaging to beyond T2 criteria in the liver explant. In contrast, there was a nearly 20% rate of understaging to beyond T2 criteria in the explant of patients with initial diagnosis of HCC at T2 stage according to a previous report from our center.26 These findings suggest that allowing for the growth of T1 HCC to meet T2 criteria before LT listing and subsequent LRT generally does not result in the development of unfavorable tumor characteristics that are associated with poor post-LT outcomes. Additionally, it appears that the majority of patients with T1 HCC do not need urgent LT. A previous study from our institution has suggested that patients with a single 2-3 cm HCC listed with MELD exception are at decreased risk of wait-list dropout compared to those with either a single tumor 3-5 cm or multiple tumors within Milan criteria.26 The present study similarly showed that in the absence of a significantly elevated AFP level, patients with T1 HCC typically have a slow rate of tumor growth with <10% risk of progression to directly beyond T2 and may not derive a significant benefit from immediate LT. Therefore, even though the only avenue to LT in these patients may be living donor liver transplantation (LDLT) because they do not qualify for MELD exception listing, it is our opinion not to advocate for LDLT in this population.
Although the majority of T1 patients showed slow tumor growth, 20% exhibited rapid tumor progression, defined in this study as a >1 cm increase in total tumor diameter over 3 months. Rapid tumor progression and AFP≥ 500 ng/mL at the time of T1 diagnosis were the only significant predictors of exclusion from LT. Rapid tumor progression was associated with alcoholic cirrhosis and Hispanic race. The explanation for this association is unclear. It has been shown that Hispanics have a higher prevalence of NAFLD associated with diabetes and obesity, which are also risk factors for HCC.27-29 It is possible that the concurrent risk factors for fatty liver in Hispanic patients contributed to more rapid HCC progression, but this is speculative and requires further study. Additionally, the probability of rapid tumor progression was also lower among patients with HBV compared to HCV, which may relate to the use of nucleos(t)ide analogues in patients with HBV cirrhosis. Treatment with nucleos(t)ide analogues in HBV cirrhosis has been shown to reduce the risk of HCC development30,31 as well as HCC recurrence.32
Given limited organ availability, one might question the “wait and not ablate” strategy to transplant all patients with T1 HCC. There are 30,000 new patients with HCC per year in the United States,33 and only 1300 LTs performed yearly for HCC.34 Immediate RFA for a single lesion of <2 cm has a 5-year tumor-free survival in the range of 38%-60%.15-17 To date, no prospective studies have directly compared these 2 strategies. A recent study using Markov modeling did compare immediate RFA or transarterial chemoembolization versus a “wait and not ablate” approach in 1000 theoretical T1 nonresection candidates with compensated cirrhosis.35 The authors found that overall median survival was similar between the immediate RFA group and the monitored group at 5.2 years from diagnosis of T1 HCC, but the strategy of immediate RFA was significantly more cost-effective per life-year gained ($92,094 versus $144,427). Only 5.3% of patients in the present study progressed from T1 directly to beyond T2 with a CR cumulative probability of 4.4% at 6 months, whereas the Markov model used a higher estimated risk of 7% of tumor progression to beyond T2 criteria at 6 months. Furthermore, 87% of our patients were listed for LT, but the Markov model was constructed using a listing rate of only 50% (for theoretical patients progressing to within T2). Given the overestimated risk of progression from T1 directly to beyond T2, and the underestimated LT listing rate among the “wait and not ablate” group, it is not entirely clear if one modality is clearly superior to the other.
Even before deciding on a treatment strategy, it is important to establish whether a small liver lesion actually represents HCC. Less than 20% of arterially enhancing hepatic lesions under 2 cm are in fact HCC.11,36 MRI is only 52%-62% sensitive for the diagnosis of T1 HCC,37,38 and smaller tumors appear to show less pronounced delayed washout compared to larger HCC.39 Furthermore, 31% of patients who underwent LT for T1 HCC have been found to have no HCC in the explant,12 an important reason why patients with T1 HCC are no longer eligible for priority listing for LT. As the diagnosis of T1 HCC is often in doubt, biopsy may be needed to confirm the diagnosis, but even biopsy has a limited sensitivity of only 70% for lesions of < 2 cm,38 and carries a risk of needle track seeding.40 The diagnosis of HCC in smaller lesions is undeniably more difficult than in larger lesions. This has prompted the Liver Imaging Reporting and Data System (LI-RADS),41 which distinguishes lesions ≥ 2 cm from those < 2 cm, for determining UNOS priority listing for LT. The diagnostic difficulty for small lesions <2 cm lends itself nicely to the “wait and not ablate” approach. Following lesions every 3 months with cross-sectional imaging may allow for improved diagnostic assessment based on interval growth and developing of radiographic features that are more characteristic of HCC.
In this study, we screened a large cohort of patients with a suspicious nodule, but only included those with a high degree of certainty in the HCC diagnosis. Patients without interval growth or disappearance of the lesion on extensive follow-up imaging were not considered to have HCC and were excluded. The median follow-up was 2.6 years during which these patients were monitored by imaging every 3 months without radiologic evidence of HCC diagnosis. It is therefore unlikely that we have missed HCC cases that would significantly impact our overall results. We recognize that the use of interval growth >3 mm as a diagnostic criterion is somewhat arbitrary, but even the proposed 50% threshold growth under the LI-RADS system has not yet been validated. Of the 38 patients who were reviewed by the 2 investigators in the current study, only 11 were included solely based on interval growth. Of these 11 patients, 7 had HCC on explant, and 4 dropped out due to tumor progression. Consequently, we believe that our assessment using the >3 mm growth cutoff has a low false-positive rate in this study.
There are a number of limitations with the present study. As already noted, the diagnosis of small HCC lesions is often very difficult. In the present study, imaging studies not showing typical characteristics of HCC were independently reviewed by 2 of the investigators to reduce diagnostic errors. Nevertheless, the possibility of misdiagnosis still exists. The present study was also not designed to compare the ITT outcomes between the “wait and not ablate” versus immediate LRT strategies due to selection bias. A randomized controlled study comparing these 2 approaches is unlikely to be undertaken. Our center is in a region with prolonged wait-list time, and thus the results may not be generalized. Finally, our study is limited by a small number of events which reduces our power to detect statistically significant associations and contributes to imprecision in our point estimates. The small number of patients (n = 6) who progressed from T1 directly to beyond T2 limits our ability to determine factors associated with this primary outcome. This group was combined with those who progressed from T1 to within T2 with subsequent dropout due to tumor progression (n = 12) to identify predictors of exclusion from LT, but these 2 groups may be very different with respect to tumor behavior and pattern of progression. These limitations notwith-standing, our results suggest that “wait and not ablate” is an acceptable treatment strategy for patients with T1 HCC.
Until a prospective, multicenter, ITT comparison of these 2 strategies is undertaken, the best approach remains a tailored decision based on patient characteristics. Patients with either rapid tumor progression or an AFP ≥ 500 ng/mL at the time of T1 diagnosis appear to be at high risk for wait-list dropout and should receive early intervention with LRT. It has been argued that LT for HCC in Child class A cirrhosis results in a very low survival benefit and may not constitute optimal use of a scarce resource.42 In studies evaluating RFA as a primary treatment for HCC, those with Child A cirrhosis and a solitary tumor <2 cm (T1 HCC) had the best longterm outcome.14,43,44 These patients are also predicted to have a low risk of developing recurrent HCC beyond Milan criteria when compared to single or multiple tumors of >2 cm after primary RFA.45 Consequently, patients with T1 HCC and Child A cirrhosis may be considered for primary RFA, and then LT in the event of recurrent or de novo HCC.45,46
In summary, the “wait and not ablate” approach until tumor growth from T1 to T2 before LT listing carries a <10% risk of tumor progression directly to beyond T2 at 1 and 2 years, and it appears to be a viable treatment strategy under the current system of organ allocation for LT. The decision to “wait and not ablate” or to proceed immediately with LRT depends on a number of factors, particularly the severity of underlying liver disease. There is a subgroup with T1 HCC with rapid tumor progression who are at high risk for exclusion from LT due to aggressive tumor biology. Additionally, those with T1 HCC and an initial AFP ≥ 500 ng/mL are at increased risk for exclusion from LT and should receive early intervention with LRT.
Abbreviations
- AFP
alpha-fetoprotein
- CI
confidence interval
- CR
competing risk
- CT
computed tomography
- CTP
Child-Turcotte-Pugh
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IQR
interquartile range
- ITT
intention to treat
- LDLT
living donor liver transplantation
- LI-RADS
Liver Imaging Reporting and Data System
- LRT
locoregional therapy
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- RFA
radiofrequency ablation
- UNOS
United Network for Organ Sharing
Footnotes
Presented in part in a plenary session at the 64th Annual American Association for the Study of Liver Diseases meeting; Washington, DC; November 2013 (Hepatology 2013;58(suppl 1):212-213A).
REFERENCES
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Fuster J, Bruix J. for Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(suppl 1):S115–S120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM. Evidence-based medicine in the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17(suppl 3):S428–S433. doi: 10.1046/j.1440-1746.17.s3.40.x. [DOI] [PubMed] [Google Scholar]
- 7.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 8.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 9.Freeman RB, Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, et al. for UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 10.Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9:684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 11.Jeong YY, Mitchell DG, Kamishima T. Small (<20 mm) enhancing hepatic nodules seen on arterial phase MR imaging of the cirrhotic liver: clinical implications. AJR Am J Roentgenology. 2002;178:1327–1334. doi: 10.2214/ajr.178.6.1781327. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(suppl 1):S261–S267. doi: 10.1053/j.gastro.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Freeman RB, Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4(suppl 9):114–131. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 14.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 15.Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–1033. doi: 10.1148/radiol.11110817. [DOI] [PubMed] [Google Scholar]
- 16.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofre-quency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–418. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Hung HH, Chiou YY, Hsia CY, Su CW, Chou YH, Chiang JH, et al. Survival rates are comparable after radiofre-quency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol. 2011;9:79–86. doi: 10.1016/j.cgh.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. for American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: Long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968–1977. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581–586. doi: 10.1023/a:1022505203786. [DOI] [PubMed] [Google Scholar]
- 25.Koteish A, Thuluvath PJ. Screening for hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13(pt 2):S185–S190. doi: 10.1016/s1051-0443(07)61785-0. [DOI] [PubMed] [Google Scholar]
- 26.Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19:1343–1353. doi: 10.1002/lt.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Konishi I, Hiasa Y, Shigematsu S, Hirooka M, Furukawa S, Abe M, et al. Diabetes pattern on the 75 g oral glucose tolerance test is a risk factor for hepatocellular carcinoma in patients with hepatitis C virus. Liver Int. 2009;29:1194–1201. doi: 10.1111/j.1478-3231.2009.02043.x. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 31.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. for Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 32.Tan ZM, Sun BC. Effects of antiviral therapy on preventing liver tumorigenesis and hepatocellular carcinoma recurrence. World J Gastroenterol. 2013;19:8895–8901. doi: 10.3748/wjg.v19.i47.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Cancer Society. 2014 Available from: http://www.cancer.org/cancer/livercancer/detailedguide/liver-cancer-what-is-key-statistics. Accessed November 21, 2015.
- 34.Kim WR, Smith JM, Skeans MA, Schladt DP, Schnitzler MA, Edwards EB, et al. OPTN/SRTR 2012 annual data report: liver. Am J Transplant. 2014;14(suppl 1):69–96. doi: 10.1111/ajt.12581. [DOI] [PubMed] [Google Scholar]
- 35.Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl. 2010;16:1186–1194. doi: 10.1002/lt.22129. [DOI] [PubMed] [Google Scholar]
- 36.Marrero JA, Hussain HK, Nghiem HV, Umar R, Fontana RJ, Lok AS. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl. 2005;11:281–289. doi: 10.1002/lt.20357. [DOI] [PubMed] [Google Scholar]
- 37.Krinsky GA, Lee VS, Theise ND, Weinreb JC, Morgan GR, Diflo T, et al. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8:1156–1164. doi: 10.1053/jlts.2002.35670. [DOI] [PubMed] [Google Scholar]
- 38.Forner A, Vilana R, Ayuso C, Bianchi L, Sole' M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 39.van den Bos IC, Hussain SM, Dwarkasing RS, Hop WC, Zondervan PE, de Man RA, et al. MR imaging of hepato-cellular carcinoma: relationship between lesion size and imaging findings, including signal intensity and dynamic enhancement patterns. J Magn Reson Imaging. 2007;26:1548–1555. doi: 10.1002/jmri.21046. [DOI] [PubMed] [Google Scholar]
- 40.Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 41.Purysko AS, Remer EM, Coppa CP, Leão Filho HM, Thupili CR, Veniero JC. LI-RADS: a case-based review of the new categorization of liver findings in patients with end-stage liver disease. Radiographics. 2012;32:1977–1995. doi: 10.1148/rg.327125026. [DOI] [PubMed] [Google Scholar]
- 42.Berry K, Ioannou GN. Are patients with Child’s A cirrhosis and hepatocellular carcinoma appropriate candidates for liver transplantation? Am J Transplant. 2012;12:706–717. doi: 10.1111/j.1600-6143.2011.03853.x. [DOI] [PubMed] [Google Scholar]
- 43.Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?: surgeon’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:422–424. doi: 10.1007/s00534-009-0239-7. [DOI] [PubMed] [Google Scholar]
- 44.Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, et al. for Barcelona Clínic Liver Cancer Group. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–1360. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 45.Tsuchiya K, Asahina Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, et al. Risk factors for exceeding the Milan criteria after successful radiofrequency ablation in patients with early-stage hepatocellular carcinoma. Liver Transpl. 2014;20:291–297. doi: 10.1002/lt.23798. [DOI] [PubMed] [Google Scholar]
- 46.Yao FY. Conundrum of treatment for early-stage hepato-cellular carcinoma: radiofrequency ablation instead of liver transplantation as the first-line treatment? Liver Transpl. 2014;20:257–260. doi: 10.1002/lt.23848. [DOI] [PubMed] [Google Scholar]