Abstract
Introduction
Loss of HBeAg and development of anti-HBe (seroconversion) is seen as a milestone and endpoint in the treatment of HBeAg-positive patients with chronic hepatitis B (CHB). Among patients treated with nucleos (t)ide analogs (NA), recurrent viremia is common after discontinuation of therapy. Entecavir (ETV) and tenofovir (TDF) are highly potent NA. The durability of virological response and HBeAg seroconversion in patients treated with these agents is not well studied.
Methods
We retrospectively studied the outcomes of 54 HBeAg-positive CHB patients who were treated with either ETV (n = 30) or TDF (23) or both (n = 1) that achieved virological response and underwent seroconversion and consolidation therapy before cessation of treatment.
Results
Only 4 (7 %) patients had sustained virological, serological, and biochemical remission. Thirteen patients (24 %) continued to have HBV DNA levels below 2000 IU/mL and normal alanine aminotransferase activity (ALT). Thirty-seven patients (69 %) developed HBV DNA >2000 IU/mL, with 20 having elevated ALT. Among these 37 patients, 23 (62 %) remained HBeAg negative/anti-HBe positive, 12 (32 %) became HBeAg positive, and 2 (5 %) were HBeAg and anti-HBe negative. Duration of consolidation therapy did not correlate with low versus high level of virological relapse.
Conclusions
Durability of HBeAg seroconversion associated with ETV or TDF was not superior to that reported in patients treated with less potent NA. Our results, aggregated with others, suggest HBeAg seroconversion should not be considered as a treatment endpoint for most HBeAg-positive patients treated with NA. Future updates of treatment guidelines should reconsider HBeAg seroconversion as an endpoint to therapy.
Keywords: Remission, Relapse, Seroreversion
Introduction
Loss of hepatitis B e antigen (HBeAg) and development of antibodies to HBeAg (anti-HBe) (e Ag seroconversion) is viewed as a significant milestone and endpoint in the treatment of HBeAg-positive patients with chronic hepatitis B (CHB). Hepatitis B e Ag seroconversion is associated with undetectable serum HBV DNA, normalization in ALT levels with improvement in liver histology, and a lower incidence of the development of cirrhosis and hepatocellular carcinoma (HCC) [1–3]. Durable HBeAg seroconversion, as evidenced by undetectable HBV DNA and HBeAg non-reactivity/anti-HBe reactivity, is seen in 67 % of patients who seroconvert spontaneously [4, 5]. Data regarding the durability of response after nucleos (t)ide(NA)-induced HBeAg seroconversion followed by treatment cessation have been conflicting, potentially influenced by the heterogeneity of clinical and virological factors of study populations, and variable duration of treatment and follow-up. Most prior studies have examined post-seroconversion relapse rates and their predictors in patients treated with lamivudine (LAM). In predominantly Caucasian CHB cohorts, there have been reports of durable seroconversion in 80–90 % of patients [6, 7], whereas in Asian studies relapse rates as high as 68 % have been reported [8]. Overall, in Asian patients, NA-induced HBeAg seroconversion seems more difficult to achieve [9] and less durable after discontinuation of treatment, and disease relapse is more frequent [5, 8, 10–15]. Some data suggest that extending treatment with NAs following seroconversion, i.e., consolidation therapy for an additional period, increases durability [15–18].
A recent analysis of the durability of HBeAg seroconversion and virological response induced by NA therapy in 42 multiethnic patients followed up for a median of 59 months from the Netherlands found that only 13 (31 %) maintained remission as defined by persistent HBeAg negativity and low-level HBV replication. Among the 9 patients who stopped therapy (after at least 8 months of consolidation), only 2 demonstrated durable serologic and virological responses [19]. In a different study comprising Asian-American HBeAg-positive CHB patients treated with various NA, 90 % who discontinued therapy after seroconversion developed recurrent viremia. None of the patients who continued therapy experienced recurrent viremia [20]. While these studies suggest that only a minority of CHB patients can safely remain off therapy after NA-induced HBeAg seroconversion, they offer little guidance for predicting the durability of response for patients treated within currently recommended guidelines using TDF or ETV.
Based on drug potency and high barriers to resistance, entecavir (ETV) and tenofovir (TDF) monotherapy are considered to be the first-line NA for treatment of CHB. Long-term durability of HBeAg seroconversion after cessation of TDF or ETV compared to the older NAs has not been adequately studied. The study design of the registration trial for TDF required HBeAg-positive patients to continue treatment even after seroconversion and did not provide data regarding long-term durability after treatment cessation [21]. Many clinicians are continuing antiviral therapy indefinitely even after seroconversion. Furthermore, the rate of HBeAg seroconversion in the “realworld” clinical setting may be lower than the rate reported in clinical trials [9, 22]. Therefore, the number of HBeAg-positive CHB patients treated with either ETV or TDF who undergoes seroconversion followed by consolidation therapy is small.
Given the paucity of data to guide evidence-based recommendations, we studied the outcomes of HBeAg-positive CHB patients who were treated with more potent firstline agents, ETV or TDF, who discontinued therapy after seroconversion and consolidation. Predictors of outcome were also examined.
Patients and Methods
This was a retrospective study of HBeAg-positive CHB patients treated with either entecavir or tenofovir who achieved HBeAg seroconversion, in whom treatment was discontinued prior to loss of HBsAg. HBeAg seroconversion was defined as loss of HBeAg and development of anti-HBe with undetectable HBV DNA on 2 or more occasions, at least one month apart. Patients previously treated with interferon, co-infected with hepatitis C, hepatitis D, or HIV, were excluded. Patients were identified from chart reviews from 7 community and 3 academic gastroenterology/hepatology practices. Demographic data and laboratory tests including liver tests, HBeAg/anti-HBe status, HBV DNA level, and HBsAg were collected. Patients were followed up every 3–6 months. Although patients were drawn from multiple locations, all laboratory testing procedures were performed at certified facilities which met Clinical Laboratory Improvement Amendments (CLIA) using commercial kits approved by the US Food and Drug Administration (FDA).
Outcomes were defined as follows: remission was defined as persistently undetectable HBV DNA, durable HBeAg seroconversion, and normal alanine aminotransferase activities (ALT) (≤40 U/L). Low-level virological relapse was defined as reappearance of HBV DNA levels <2000 IU/mL. High-level relapse was defined as reappearance of HBV DNA ≥2000 IU/mL. HBeAg seroreversion was defined as reappearance of HBeAg.
The study was approved by the Institutional Review Boards at Saint Vincent Medical Center, University of Southern California, California Pacific Medical Center, and University of California, Los Angeles.
Statistical Analysis
Cumulative probability of high-level virological relapse was calculated according to Kaplan–Meier method. For the comparison between patients with low versus high level of HBV DNA at the time of relapse, the Kruskal–Wallis test for continuous variables and Chi-square test for categorical variables were used. Factors significantly associated with high HBV DNA level in univariate logistic regression analysis (unadjusted odds ratio in Table 3) at the level of P = 0.10 were included in multivariate logistic regression analysis (adjusted odds ratio in Table 3). The results of the analyses were expressed as unadjusted or adjusted odds ratios with their 95 % confidence intervals and associated P values. All reported P values were two-tailed. STATA version 12 (College Station, TX) was used in all statistical analyses.
Table 3.
Estimated odds ratio for high DNA level relapse using univariate and multivariate logistic regression analyses
| Variables | Levels | Unadjusted OR (95 % CI) | P value | Adjusted OR (95 % CI) | P value |
|---|---|---|---|---|---|
| Age | >45 vs.<45 | 4.81 (1.18–19.7) | 0.029 | 9.42 (1.80–49.3) | 0.008 |
| Prior treatment | Yes vs. no | 3.9 (0.95–15.9) | 0.058 | 3.61 (0.68–19.1) | 0.131 |
| Drug | TDF vs. ETV | 9.63 (1.91–48.4) | 0.006 | 13.1 (2.18–79.3) | 0.005 |
Results
A total of 54 HBeAg-positive CHB patients treated with ETV or TDF who underwent seroconversion and then discontinued therapy after a period of consolidation therapy without HBsAg loss were included in this study. Demographic and baseline clinical characteristics are shown in Table 1. Thirty-three patients were treatment naïve prior to receiving ETV (21 patients) or TDF (11 patients) or both NA (1 patient). Twenty-one patients were treated with other NAs before being switched to either ETV (9 patients, one of whom had been previously treated with lamivudine) or TDF (12 patients). There was no significant difference in mean levels of viremia between treatment-naïve and treatment-experienced patients prior to starting ETV or TDF.
Table 1.
Baseline characteristics of patients
| Baseline characteristics | n = 54 | ||
|---|---|---|---|
| Age (years) | 45.5 (24–81) median 43 | ||
| Gender, male (%) | 34 (63) | ||
| Ethnicity (%) | 51 Asian (94 %) | ||
| 3 Caucasian (6 %) | |||
| Foreign-born | 46 (85 %) | ||
| Treatment naïve (n = 33) | ETV | TDF | ETV/TDF |
| 21 | 11 | 1 | |
| Treatment experienced (n = 21) | ETV 9 | TDF 12 | |
| Mean baseline HBV DNA of treatment-naïve patients (log10 IU/mL) | 7.62 (median 7.40, range 2.94–8.72) | ||
| Mean HBV DNA of patients treated with other NAs at time of switch to ETV or TDF (log10 IU/mL) |
7.69 (median 4.85, range undetectable-8.80) | ||
| Time to undetectable HBV DNAa (months) | 11.4 (median 9.0, range 0–33.4) | ||
| Time to HBeAg seroconversiona (months) | 21.0 (median 18.3, 1.5–61.6) | ||
| Consolidation period (months)b | 16.8 (median 14.0, 1.5–55.3) | ||
| Follow-up period after discontinuation of therapy (months) | 30.3 (median 31.1, 1.9–71.4) | ||
After initiation of ETV or TDF
Length of therapy after seroconversion
Mean duration of treatment with ETV or TDF was 37.8 (range 4.6–100.5) months. HBV DNA undetectability occurred 11.4 (median 9.0, range 0–33.4) months after initiation of treatment with ETV/TDF. HBeAg seroconversion occurred 21.0 (median 18.3, range 1.5–61.6) months of ETV/TDF treatment. Treatment (consolidation therapy) was continued for an additional 16.8 (median 14.0, range 1.5–55.3) months after seroconversion. Thirtyeight (70 %) patients received at least 12 months of consolidation therapy. Mean follow-up after discontinuation of treatment was 30.3 (median 31.1, range 1.9–71.4) months.
Remission
After discontinuation of therapy, 4 of 54 patients (7 %) had persistently undetectable HBV DNA and normal ALT. Mean follow-up was 23.7 (2.0–47.9) months. None of these patients seroreverted and one patient became HBsAg negative and developed anti-HBs.
Low-Level Virological Relapse
A total of 13 of 54 patients (24 %) continued to have HBV DNA levels below 2000 IU/mL (range 0–1810) and normal alanine aminotransferase activity (ALT). Mean follow-up was 30.2 (2.0–71.4) months. Among these 13 patients, HBV DNA reappeared 8.3 (2.3–24.6) (mean) months after discontinuation of therapy. On follow-up visits, HBV DNA spontaneously became undetectable in 10 patients. No patient reverted to HBeAg-positive state although one patient lost anti-HBe.
High-Level Virological Relapse
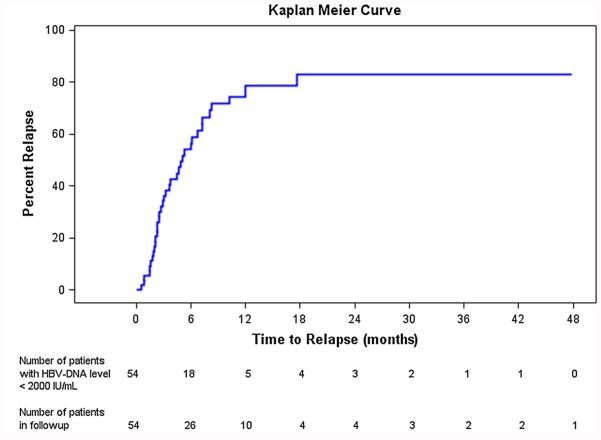
Thirty-seven patients (69 %) developed HBV DNA >2000 IU/mL 4.4 (0.5–17.6) months after discontinuation of ETV/TDF therapy. Mean follow-up was 30.4 (1.9–69.3) months. Figure 1 presents Kaplan–Meier curve estimating the time to high-level recurrent viremia. Cumulative highlevel virological relapse rates at 3, 6, 12 and 24 months were 35.6, 52.6, 67.9 and 69.9 %, respectively. Mean HBV DNA level at the time of reappearance was 7.5 (3.3–8.8) log10 IU/mL. Twenty of 37 (54 %) patients had elevated ALT which ranged from 46 to 2657 U/L with all having normal bilirubin levels. There was no significant difference in the levels of HBV DNA in patients who maintained normal ALT compared with patients who relapsed with abnormal ALT. Among these 37 patients, 23 (62 %) remained HBeAg negative/anti-HBe positive, 2 (5 %) were HBeAg and anti-HBe negative, and 12 (32 %) reverted to HBeAg positive (9 were anti-HBe negative, 3 anti-HBe positive).
Fig. 1.
Cumulative high-level virological relapse rate after discontinuation of ETV or TDF
When comparing patients who relapsed with high level of viremia to those who maintained low level of viremia, there was no significant difference in baseline HBV DNA level, time to undetectable HBV DNA level, duration of total treatment or consolidation therapy. Patients who were younger than age 45, treatment experienced prior to treatment with ETV/TDF, received ETV treatment were more likely to maintain low level of viremia after discontinuation of therapy (Table 2). In the multivariate analysis, the only significant risk factors for high-level virological relapse were age greater than 45 years and treatment with TDF (Table 3). Patients who relapsed with higher level of HBV DNA relapsed earlier than patients with low-level viremia after discontinuation of therapy (4.4 vs. 8.3 months, P = 0.026). There were no clinical (including age), biochemical, or virological factors that were associated with seroreversion. None of the patients developed liver decompensation or hepatocellular carcinoma during the follow-up period.
Table 2.
Factors associated with low (<2000 IU/mL) or high level (≥2000 IU/mL) of hepatitis B viremia after discontinuation of therapy
| Low viral load relapser | High viral load relapser | P value | ||
|---|---|---|---|---|
| Age | Mean ± SD (years) | 40.9 ± 6.0 | 47.4 ± 14.2 | 0.103 |
| Age (years) | ≤45 | 13 (81.3 %) | 18 (47.4 %) | 0.021 |
| >45 | 3 (18.7 %) | 20 (52.6 %) | ||
| Gender | Male | 9 (56.3 %) | 24 (63.2 %) | 0.634 |
| Race | Asian | 16 (100.0 %) | 35 (92.1 %) | 0.247 |
| Baseline HBV DNA level (log10) | Mean ± SD (IU/mL) | 7.41 ± 7.56 (n = 15) | 7.85 ± 8.15 (n = 32) | 0.561 |
| Prior treatment | None | 13 (81.3 %) | 20 (52.6 %) | 0.003 |
| Drug | ETV | 14 (87.5 %) | 16 (42.1 %) | 0.009 |
| TDF | 2 (12.5 %) | 21 (55.3 %) | ||
| ETV/TDF | 0 (0.0 %) | 1 (2.6 %) | ||
| Interval to negativity | Mean ± SD (mo) | 13.0 ± 8.1 (n = 15) | 11.4 ± 7.5 (n = 37) | 0.592 |
| Consolidation | <12 months | 7 (43.8 %) | 12 (31.6 %) | 0.392 |
| ≥12 months | 9 (56.2 %) | 26 (68.4 %) | ||
| Consolidation | <24 months | 11 (68.8 %) | 32 (84.2 %) | 0.198 |
| ≥24 months | 5 (31.2 %) | 6 (15.8 %) | ||
| Duration of Rx | Mean ± SD (mo) | 39.8 ± 17.7 (n = 16) | 37.0 ± 17.9 (n = 38) | 0.895 |
| Time to relapse | Mean ± SD (mo) | 8.3 + 7.1 (n = 13) | 4.4 + 3.5 (n = 37) | 0.026 |
Discussion
For HBeAg-positive CHB patients, HBeAg seroconversion is still cited as a milestone and potential treatment end-point, marking the transition from a high-replication, active disease state to low-replication, quiescent state. Although current guidelines recommend that NA therapy can be stopped with augmented benefits 6–12 months after HBeAg seroconversion [23–25], data presented here objectively question that treatment paradigm. Our study assessed the outcomes after achieving on-treatment HBeAg seroconversion in patients treated with the most potent NA (ETV and/or TDF) after treatment was stopped following a mean consolidation period of nearly 17 months, exceeding the 6–12 months specified by current guidelines. Among the 54 patients who underwent HBeAg seroconversion, only 4 (7 %) sustained absolute virological, serologic, and biochemical remission and 30 % retained low-level viremia (<2000 IU/mL), quiescent liver tests, and durable HBeAg negativity. In contrast, 70 % of patients relapsed with high-level viremia (<2000 IU/mL), with half of these having recurrent liver injury (ALT flares) and one third experiencing HBeAg seroreversion. Therefore, for most patients, durable remission following treatment cessation after HBeAg seroconversion is at best a temporary event.
Although AASLD HBV guidelines define normal ALT levels as 19 U/L for females and 30 U/L for males, many practitioners in the community still use higher ALT levels since many laboratories have upper limits of normal of 55–80 U/L [23, 26]. A higher value (>40 U/L) was chosen as the cut-off for abnormal ALT so that there would be no equivocation as to the definition of biochemical relapse. Indeed, there was only one additional patient (female) who relapsed with high-level viremia whose ALT was 37 U/L that was not counted as a flare.
A multicenter study also reported outcomes in patients treated with ETV or LAM for a finite period of 48 or 96 weeks, as determined by protocol-defined response criteria [27]. Among 96 patients who received ETV achieving HBeAg seroconversion (most of whom did not receive consolidation therapy), approximately 76 % maintained seroconversion during 6 months of follow-up, which is a similar proportion (78 %) to that observed in our study among patients who were followed up for longer (mean 30.3 months). Of note, in that study also, similar seroreversion and virological relapse rates were observed among 106 patients treated with ETV with HBeAg loss (10 without seroconversion) who achieved on-treatment undetectable HBV DNA levels (lower level of detection <57 IU/mL), and undetectable viremia was sustained for at least 6 months in only 32 % (34/106) of patients who had discontinued therapy. Identical virological relapse and seroreversion rates were noted in patients who were treated with LAM compared to patients treated with ETV [27].
Ridruejo et al. reported 15 patients who underwent HBeAg seroconversion who stopped ETV prior to under-going HBsAg seroconversion. Nine developed virological relapse including 3 with HBeAg seroreversion and 4 who became anti-HBe negative. In contrast, none of the 18 patients who underwent HBeAg and HBsAg seroconversion prior to stopping ETV experienced virological relapse [28]. In a study comprising HBeAg-positive CHB patients who were treated with either ETV or clevudine, 15 of 31 ETV-treated patients relapsed compared to 5 of 17 patients treated with clevudine. In their multivariate analysis, age ≤40 years and ≤15 months of consolidation treatment were predictors of virological relapse [29].
There are even less data regarding the durability of HBeAg seroconversion after discontinuation of TDF. To date, our study represents the largest reported experience with TDF. Patients treated with TDF had a higher rate of relapse with high level of viremia compared to patients treated with ETV. We have no plausible explanation for this difference and attribute this finding to relatively small sample sizes. ETV and TDF, which are more potent NAs and have a higher genetic barrier to resistance, have largely replaced older NAs as treatment for CHB patients. Our study, along with these comparative data, argue that a consolidation period within current guidelines, even using modern NA, may have little influence on the durability of post-treatment HBeAg suppression by the host immune response. The immunologic and potentially virological factors dichotomizing the two groups (durable vs. brittle seroconversion) are unknown but merit intensive study, which such well-characterized cohorts can facilitate.
Based primarily on studies with LAM therapy [5, 8, 15–17], current guidelines recommend additional consolidation therapy of 6–12 months after HBeAg seroconversion [21–23]. However, the relapse rates in these older studies may be underestimated because insensitive assays were used to measure HBV DNA [5, 8, 15, 17]. Seventy percent of the patients in our study received at least 12 months of consolidation therapy and had similar high virological relapse rates as patients who received less consolidation therapy. Other studies using PCR assays for HBV DNA detection report similar findings [10, 19].
Younger age at the time of spontaneous and NA-induced seroconversion is associated with more durable remission [5, 8, 10–17, 29]. Consistent with this finding, we found patients ≥45 years to be more likely to have high virological relapse. Interestingly, the rate of virological relapse after HBeAg seroconversion is significantly higher in patients treated with NAs compared to patients treated with interferon or those who spontaneously seroconvert [5]. We speculate that although ETV and TDF are highly potent, nevertheless, these agents like other NAs, suppress HBV replication and antigen production without directly influencing qualitative and quantitative aspects of the anti-HBV immune response. The likelihood of virological relapse and seroreversion, or conversely, durable virological remission seem to be influenced by differences in the host immune constitution when treatment is stopped. Although age-related differences in efficiency of the hepatic HBV immune response are well accepted and are beginning to be mechanistically dissected [30], such differences have focused on the gradient of natural outcome observed between neonates, children and adolescent-mature adults. Aggregated data from the current and prior HBeAg-positive CHB treatment studies argue that biologically important differences may also develop in the human hepatic immune response during adult life.
While we report one of the larger cohorts of HBeAg-positive patients treated with ETV/TDF through seroconversion and consolidation before treatment withdrawal, we recognize several limitations of this study: it is retrospective in design involving multiple sites that exhibit lack of uniform duration in consolidation therapy and lack of virological sequence information. The vast majority of patients in this study were Asian and our findings may not be applicable to non-Asian CHB patients. The number of patients in this study may not be large enough to identify additional factors that are predictive of durable off-treatment response.
The benefits of sustained viral suppression include the lower risk of disease progression [31, 32], regression of fibrosis [33, 34], and decreased incidence of HCC [35, 36]. On the other hand, these clinical benefits are negated in patients with virological relapse [31]. Most patients who relapsed with high HBV DNA levels did not serorevert and maintained normal aminotransferase activities. However, these individuals remain at increased risk of developing cirrhosis and HCC [37, 38]. Among cirrhotic CHB patients treated with ETV, lower probability of disease progression is only seen among patients with complete virological suppression [39].
In summary, the durability of HBeAg seroconversion associated with ETV and TDF is not superior to patients treated with earlier, less potent NA. Virological relapse was almost universal in our study, with 70 % having greater than 2000 IU/mL creating risk of liver injury. Our results coupled with other studies suggest HBeAg seroconversion without HBsAg seroconversion should not be considered as a treatment endpoint for most HBeAg-positive CHB patients treated with ETV and TDF, especially for those older than 45 years. Treatment guidelines should reconsider HBeAg seroconversion as an endpoint to therapy.
Acknowledgments
We thank Dr. Dat Nghiem for reviewing this manuscript and for his thoughtful comments and suggestions. Authors received the grant support from Gilead Sciences and Daughters of Charity Foundation.
Footnotes
Compliance with Ethical Standards
Conflict of interest Tse-Ling Fong and Myron J Tong: Gilead Sciences; Speaker Bureau, Advisory Board, Research Funding. BMS; Speaker Bureau, Advisory Board, Research Funding. Andy Tien, Kahee J. Jo, Wafa Mohammed, Andrew Velasco, Vinh-Huy LeDuc, Nickolas Nguyen, Yong-Won Cho and Stewart L. Cooper: No industry relationships or conflicts of interest. Danny Chu, Eddie Cheung, Edward A. Mena, Quang-Quoc Phan, Andy Yu, Steven-Bui Han, Ho S. Bae: Gilead Sciences; Speaker Bureau, Advisory Board. BMS; Speaker Bureau, Advisory Board. Mimi Chang: Gilead Sciences; Speaker Bureau. BMS; Speaker Bureau.
References
- 1.Fattovich G, Rugge M, Brollo L, et al. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology. 1986;6:167–172. doi: 10.1002/hep.1840060203. [DOI] [PubMed] [Google Scholar]
- 2.Hui CK, Leung N, Shek TW, et al. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690–698. doi: 10.1002/hep.21758. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Lau GK, Kao JH, Gane E. Hepatitis B e antigen seroconversion: a critical event in chronic hepatitis B virus infection. Dig Dis Sci. 2010;55:2727–2734. doi: 10.1007/s10620-010-1179-4. doi:10.1007/s10620-010-1179-4. [DOI] [PubMed] [Google Scholar]
- 4.Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 5.Kuo L-F, Lee C-M, Hung C-H, et al. High risk of hepatitis B virus reactivation in nucleos(t)e analogue-induced hepatitis B e antigen seroconverters older than 40 years. Dig Dis Sci. 2014;59:2580–2587. doi: 10.1007/s10620-014-3194-3. [DOI] [PubMed] [Google Scholar]
- 6.Dienstag JL, Cianciara J, Karayalcin S, et al. Durability of serologic response after lamivudine treatment of chronic hepatitis B. Hepatology. 2003;37:748–755. doi: 10.1053/jhep.2003.50117. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, Hou J-H, Chutaputti A, Manns M, Naoumov N. Sustained durability of HBeAg seroconversion in chronic hepatitis B patients after treatment with telbivudine. J Hepatol. 2008;48:S268. [Google Scholar]
- 8.Song B-C, Suh DJ, Lee HC, Chung Y-H, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology. 2000;32:803–806. doi: 10.1053/jhep.2000.16665. [DOI] [PubMed] [Google Scholar]
- 9.Jo K, Dodge JL, Wadley A, Wakil AE, Baron JL, Cooper S. HBeAg seroconversion is lower in Asian versus non-Asian patients during treatment of chronic hepatitis B with tenofovir or entecavir. Hepatology. 2013;58:669A. [Google Scholar]
- 10.Lee CM, Ong G-Y, Lin S-N, et al. Durability of lamivudine-induced HBeAg seroconversion for chronic hepatitis B patients with acute exacerbation. J Hepatol. 2002;37:669–674. doi: 10.1016/s0168-8278(02)00267-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Liu F, Li X-Y, Wang J-B, Zhang Z-H, Wang Y-Z. Stringent cessation criterion results in better durability of lamivudine treatment: a prospective clinical study in hepatitis B e antigen-positive chronic hepatitis B patients. J Viral Hepat. 2010;17:298–304. doi: 10.1111/j.1365-2893.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 12.Song BC, Cui XJ, Cho YK, et al. New scoring system for predicting relapse after lamivudine-induced hepatitis B e-antigen loss in chronic hepatitis B patients. Hepatol Res. 2009;39:1064–1071. doi: 10.1111/j.1872-034X.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 13.Fung J, Lai CL, Tanaka Y, et al. The duration of lamivudine therapy for chronic hepatitis B: cessation versus continuation of treatment after HBeAg seroconversion. Am J Gastroenterol. 2009;104:1940–1946. doi: 10.1038/ajg.2009.200. [DOI] [PubMed] [Google Scholar]
- 14.Jin YJ, Kim KM, Yoo DJ, et al. Clinical course of chronic hepatitis B patients who were off-treated after lamivudine treatment: analysis of 138 consecutive patients. Virol J. 2012;9:239. doi: 10.1186/1743-422X-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien R-N, Yeh C-T, Tsai S-L, Chu C-M, Liaw Y-F. Determinants for sustained HBeAg response to lamivudine therapy. Hepatology. 2003;38:1267–1273. doi: 10.1053/jhep.2003.50458. [DOI] [PubMed] [Google Scholar]
- 16.Byun KS, Kwon OS, Kim JH, et al. Factors related to post-treatment relapse in chronic hepatitis B patients who lost HBeAg after lamivudine therapy. J Gastroenterol Hepatol. 2005;20:1838–1842. doi: 10.1111/j.1440-1746.2005.03952.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuo YH, Chen CH, Wang JH, et al. Extended lamivudine consolidation therapy in hepatitis B e antigen-positive chronic hepatitis B patients improves sustained hepatitis B e antigen seroconversion. Scand J Gastroenterol. 2010;45:75–81. doi: 10.3109/00365520903394550. [DOI] [PubMed] [Google Scholar]
- 18.Lee HW, Lee HJ, Hwang JS, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010;51:415–421. doi: 10.1002/hep.23323. [DOI] [PubMed] [Google Scholar]
- 19.Reijnders JGP, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology. 2010;139:491–498. doi: 10.1053/j.gastro.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 20.Chaung KT, Ha NB, Trinh HN, et al. High frequency of recurrent viremia after hepatitis B e antigen seroconversion and consolidation therapy. J Clin Gastrol. 2012;46:865–870. doi: 10.1097/MCG.0b013e31825ceed9. [DOI] [PubMed] [Google Scholar]
- 21.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 22.Ahn J, Lee HM, Lim J, et al. Entecavir safety and effectiveness in a natural cohort of chronic hepatitis B patients in the United States. The ENUMERATE study. Hepatology. 2014;60:1100A. [Google Scholar]
- 23.Lok ASF, McMahon B. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 24.European Association for the Study of the Liver EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Liaw YF, Kao J-H, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 26.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 27.Gish RG, Lok AS, Chang TT, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Ridruejo E, Marciano S, Galdane O, et al. Relapse rates in chronic hepatitis B naïve patients after discontinuation of antiviral therapy with entecavir. J Viral Hepat. 2014;21:590–596. doi: 10.1111/jvh.12200. [DOI] [PubMed] [Google Scholar]
- 29.Song MJ, Song DS, Kim HY, et al. Durability of viral response after off-treatment in HBeAg positive chronic hepatitis B. World J Gastroenterol. 2012;18:6277–6283. doi: 10.3748/wjg.v18.i43.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Publicover J, Goodsell A, Nishimura S, et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest. 2011;121:1154–1162. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 32.Wong GL, Chan HL, Mak CW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 33.Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 34.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 35.Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 36.Wu CY, Lin JT, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147:143–151. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 37.Chen CJ, Yang HI, Su J, et al. REVEAL-HBV Study Group Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 38.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) study group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Zoutendijk R, Reijnders JG, Zoulim F, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62:760–765. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]