ABSTRACT
Case Study
Mrs. K is a 63-year-old woman who presented to the clinic with complaints of fatigue, bruising, weight loss of 20 pounds in the past 2 months, and decreased appetite. She stated that she had constant moderately severe joint pain and napped three times a day in an attempt to battle her fatigue. However, the naps resulted in no change in her fatigue. Her increase in fatigue interfered with her daily functioning, and she was unable to enjoy spending time with her family.
Mrs. K’s medical history included breast cancer diagnosed in 2006 and hypertension diagnosed in 2010. For her breast cancer, she was treated with chemotherapy and radiation therapy. She had no other pertinent medical history and no significant family history.
Physical examination revealed temperature of 38.9˚C, heart rate of 108 beats per minute, blood pressure of 158/68, respiratory rate of 22 breaths per minute, oxygen saturation of 96%; skin intact, pale, and cool to touch; scattered petechiae were present over both her lower extremities, abdomen, and back; and pale oral mucosa were observed. All other systems reviewed were unremarkable.
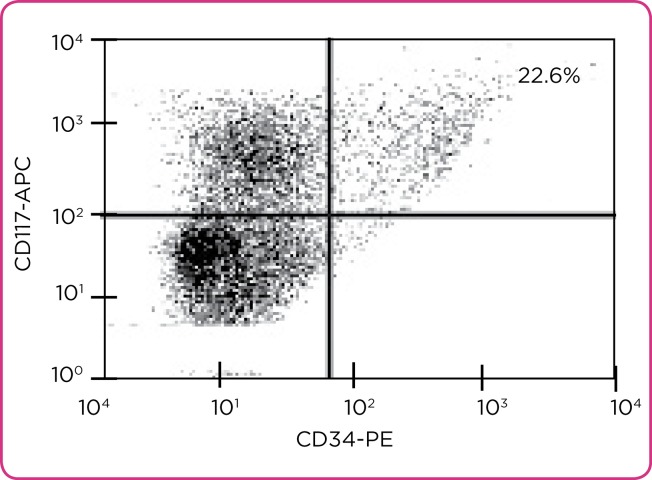
Laboratory workup revealed the following measures: peripheral blood with white blood cell count of 110,000 x 10₃/µL, hemoglobin of 8.6 g/dL, hematocrit of 28%, and a platelet count of 8,000 x 10₃/µL. A bone marrow biopsy evaluated by flow cytometry revealed 22.6% CD34⁺/CD117⁺ blasts (Figure 1).
Figure 1.
Flow cytometry depiction of blast cells. APC = allophycocyanin; PE = phycoerythrin.
ARTICLE
For advanced practitioners (APs) working with oncology patients, "22.6% CD34⁺/CD117⁺ blasts" on flow cytometry, as seen in Mrs. K’s case study above, may be familiar, but understanding and interpreting this laboratory result may be more challenging. Flow cytometry is a powerful, well-established method for effectively measuring attributes of cells, including cellular property functions, primarily by using cluster of differentiation (CD) properties of a cell.
The first flow cytometer was constructed roughly 60 years ago (Giaretti, 1997; Jaroszeski & Radcliff, 1999; Picot, Guerin, Le Van Kim, & Boulanger, 2012). The use of flow cytometry has expanded to provide invaluable information for many diseases and conditions (Davis et al., 2007; De Rosa, Brenchley, & Roederer, 2003). The purpose of this article is to provide a brief overview of the methodology of flow cytometry for the AP and to highlight some applications of flow cytometry in clinical practice.
Brief Overview
Flow cytometry is the process in which cells in an isotonic buffer suspension pass through a laser beam one by one (Macey, 2007). Quantifiable measurements of cellular attributes, such as cell size, granularity, DNA/RNA content, surface and intracellular receptors, and gene expression, are made using the principle of fluorescence, or excited light energy (Macey, 2007; Mach, Thimmesch, Orr, Slusser, & Pierce, 2010). The flow cytometer consists of four systems: fluidics, optics, electronics, and computer interface.
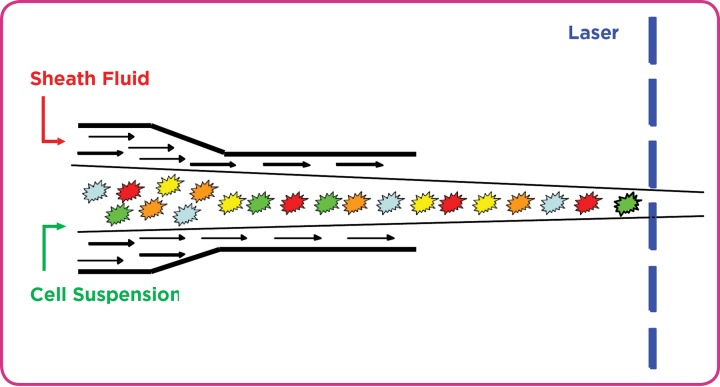
The first step is fluidics. Cellular suspensions, the most common source of material used in flow cytometry for disease characterization (Ibrahim& van den Engh, 2007) and usually derived from blood, tissue, or tumors, are labeled using fluorochrome-labeled monoclonal antibodies. Antibodies are proteins that recognize and bind to specific antigen structures on the cell surface, or intracellularly, to identify the measurement(s) of interest. Fluorochromes, compounds that emit light when excited, are bound to the antibodies (Ibrahim& van den Engh, 2007; Jaroszeski & Radcliff, 1999; Pedreira, Costa, Lecrevisse, van Dongen, & Orfao, 2013; Picot et al., 2012). The fluorochrome-labeled cells in liquid suspension then pass individually and rapidly through a laser beam–sensing area (Figure 2; Macey, 2007).
Figure 2.
The flow cytometer’s hydrodynamic force, created by sheath fluid, drives cell suspensions through a laser beam–sensing area one at a time.
Optics consists of the light-amplification source (laser). The laser emits light of a specified wavelength. There are several types of lasers, each capable of exciting specific fluorochromes and causing them to emit light ((Jaroszeski & Radcliff, 1999).
Once the suspension passes the laser, two initial detections are made. Forward scatter (FSC) is light that scatters in the same direction as the laser and is indicative of cellular morphology, or cell size. Side scatter (SSC) is light that is scattered at a 90˚ angle from the direction of the laser and is proportional to cell granularity (structures within the cell), or cell density ((Ibrahim& van den Engh, 2007; (Jaroszeski & Radcliff, 1999; (Macey, 2007; (Mach et al., 2010; (Pedreira et al., 2013; (Picot et al., 2012).
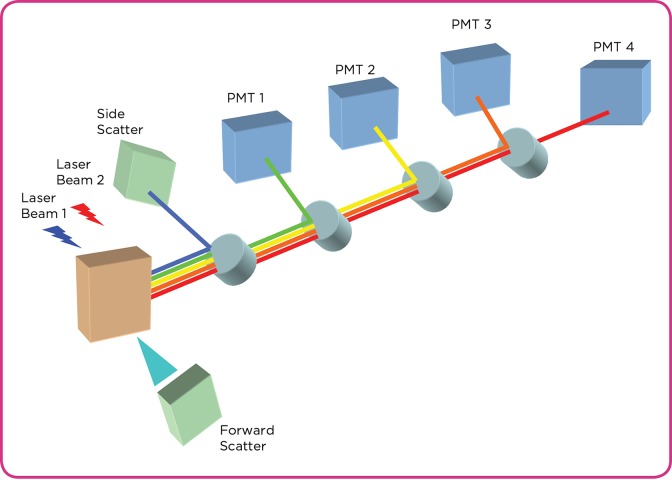
The electronics involve the conversion of photons into interpretable data. The flow cytometer’s photomultiplier tubes (PMTs) are semiconductors that generate electrical current based on light detection. The PMTs will convert the detected photons into electrical signals that can be interpreted (Figure 3; (Jaroszeski & Radcliff, 1999; (Macey, 2007; (Pedreira et al., 2013; (Picot et al., 2012).
Figure 3.
Basic schematic of flow cytometer optical system. PMT = photomultiplier tube.
Each signal detected by the cytometer is known as an "event," and all events for a prepared sample are acquired by the cytometer and stored on the computer. The computer is also responsible for regulating the function of the flow cytometer. Graphic analysis of the samples can be interpreted in one-, two-, or three-dimensional images using software designed for flow cytometry data analysis ((Macey, 2007).
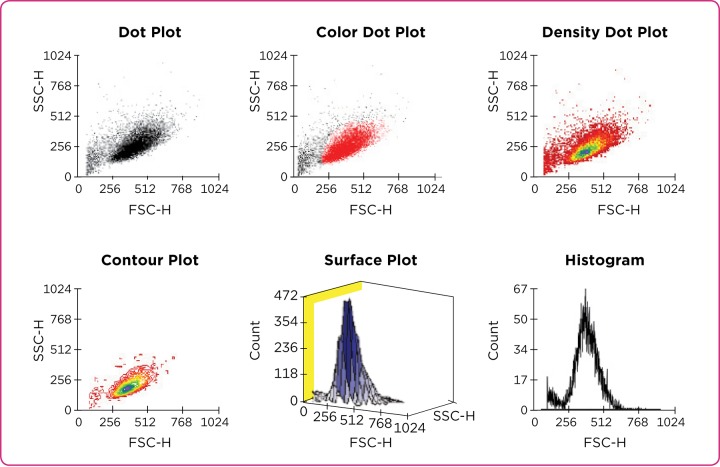
Data analysis is often depicted in graphic formats and accompanies patient test results. It can be displayed in a variety of ways. Histograms display one measurement of interest, whereas dot plots display two parameters of interest (Figure 4; (Herzenberg, Tung, Moore, Herzenberg, & Parks, 2006; (Ibrahim& van den Engh, 2007; (Jaroszeski & Radcliff, 1999; (Pedreira et al., 2013).
Figure 4.
Types of data-analysis plots. SSC-H = side scatter histogram; FSC-H = forward scatter histogram.
Many flow cytometers are capable of cell sorting. Cells or cell particles, such as lymphocytes or chromosomes, may be physically separated from the heterogeneous suspension and collected as a highly purified suspension ((Macey, 2007). This process allows for the observation and analysis of a uniform population for investigation. More clinically relevant, it allows for the selection of cells with particular features often used to diagnose or treat a disease or condition ((Koss & Melamed, 2006; (Macey, 2007).
Applications to Clinical Practice
Flow cytometry is often used to characterize diseases in clinical settings ((Barlogie et al., 1983; (Ibrahim & van den Engh, 2007). Peripheral blood, bone marrow aspirate, and cerebrospinal fluid are all specimens that can be analyzed using flow cytometry. However, only viable cells can be analyzed. If the sample does not contain viable cells, flow cytometry analysis is not an option ((Trewhitt, 2001).
Flow cytometry is most commonly indicated for both benign and malignant hematologic processes. It can aid in several clinical areas, including diagnosis, treatment plans, and monitoring residual or relapsed disease ((Craig & Foon, 2008; (Wood et al., 2007).
Measurement of DNA content was one of the earliest uses of flow cytometry ((Barlogie et al., 1983; (Giaretti, 1997). A 67% increase in DNA content was noted in malignant cells compared with nonmalignant cells ((Barlogie et al., 1983). Normal, healthy cells are diploid (two complete sets of chromosomes), whereas malignant cells most often have abnormalities in their chromosomes, which can be quantified by flow cytometry ((Friedlander, Hedley, & Taylor, 1984). Retrospective studies examined the relationship between DNA abnormalities and survival duration in patients with oncologic disease in an attempt to predict prognosis ((Barlogie et al., 1983; (Merkel, Dressler, & McGuire, 1987).
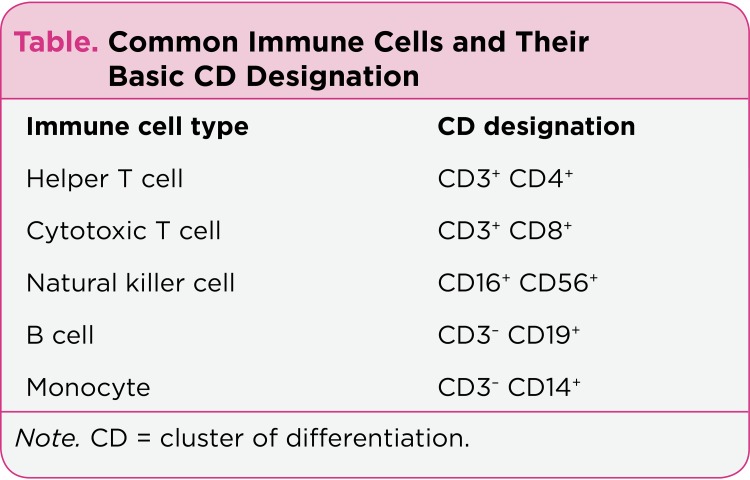
Phenotyping, the identification of specific observable characteristics, is another common use of flow cytometry in oncology. There are many phenotypic designations to differentiate healthy cells from tumor cells. The Table provides basic CD specification for common immune cell phenotypes ((Maecker, McCoy, & Nussenblatt, 2012).
Table 1.
Common Immune Cells and Their Basic CD Designation
Tumor cells often express distinguishable surface receptors. For example, flow cytometry analysis of patients with acute promyelocytic leukemia (APL) has been shown to demonstrate a myeloid phenotype as CD11b⁻ and CD11c⁻. In contrast, in normal myeloid cells, the phenotype is CD11b⁺ and CD11c⁺ ((Dong, Kung, Bhardwaj, & McGill, 2011).
Designation of T-cell or B-cell lineage in acute lymphoblastic leukemias (ALLs) is another example of phenotyping. Once denoted T-cell or B-cell ALL, each lineage can be refined by further immunoprofiling to determine subgroups and prognosis. Varma and Naseem ((2011) have described the phenotype of each subgroup in detail. A pre–B-cell leukemia will express CD19 and CD10, whereas a mature B-cell leukemia will express CD19 but not CD10. Understanding the specific origin of the B-cell leukemia assists in treatment options and prognosis. Craig and Foon ((2008) have described specific immunophenotypic characteristics of different hematologic cancers.
Several nurse-led research projects have used flow cytometry to enhance clinical understanding and to evaluate the physiologic effects of nursing interventions. It was used to evaluate the immune function of geriatric patients who received nutritional supplementation before and after flu shots ((Langkamp-Henken et al., 2006). Flow cytometry also has been used to examine the immune function of caregivers of patients with Alzheimer’s disease ((Thompson et al., 2004). In addition, Motzer and colleagues ((2002) used flow cytometry to examine natural killer and T-cell function in correlation to psychological distress of patients with irritable bowel syndrome. Lastly, since women experience immunosuppression during pregnancy, flow cytometry has been used to examine the immune state of postpartum women over time ((Gennaro et al., 1997).
Discussion
Flow cytometry has been used for many years in clinical practice. As flow cytometry has advanced, more complex questions and diseases have been diagnosed, explained, and monitored. It is important for APs to have a basic understanding of flow cytometry, since it is a prominent method used in clinical settings. When the AP sees complete white blood cell results, he/she can identify and understand critical values, thereby allowing the AP to anticipate patient needs. The same is true for flow cytometry results. If there is a basic understanding of what is being measured, the AP can anticipate the treatment, understand the implications of the results, and prepare and educate the patient.
Flow cytometry reports in clinical settings, to assist in diagnosis or disease monitoring, usually describe results by means of the actual numbers or percentages of the variable of interest. As previously described, acute myeloid leukemia (AML) and ALL have characteristic subtypes of the disease based on certain morphology, phenotype, and genetics. AML basic precursor cells are phenotypically positive for CD34, CD38, CD117, and HLA-DR (human leukocyte antigen D related). These phenotypic results are usually reported in percentages, with 10% to 20% or greater considered positive markers ((Dohner et al., 2010).
It is crucial to understand that flow cytometry analysis is often used in conjunction with other descriptive tests such as morphologic examination. Pathologists review the cellular morphology of the specimens. Often, hematologic neoplasms depict specific morphologic changes, and flow cytometry provides greater specificity. Flow cytometry often can detect recurrence of cancer before morphologic changes are detected.
Overall, best practices for proper diagnosis should include clinical presentation, morphologic analysis, flow cytometry analysis, and other pertinent testing, such as cytogenetics ((Wood et al., 2007). Limitations to flow cytometry include the facts that the laser can only analyze one cell at a time, cells must be in suspension to be analyzed (thereby restricting the analysis of tissue), highly trained operators are required, and cells must be viable to be analyzed.
Understanding flow cytometry will allow APs to prepare patients and families more effectively for forthcoming diagnoses and treatments. Furthermore, understanding the flow cytometry research involved in a disease or chronic condition, as well as flow cytometry laboratory results, will allow better patient assistance and education.
Revisiting Mrs. K, her bone marrow biopsy results revealed 22.6% CD34⁺/CD117⁺ blasts by flow cytometry, and more detailed flow cytometry and cytogenetic results diagnosed AML. The AP would be able to anticipate the appropriate treatment course for Mrs. K. Counseling and educating patients about AML can begin with both Mrs. K and her family well prepared.
Footnotes
The author has no potential conflicts of interest to disclose.
References
- 1.Barlogie B, Raber M N, Schumann J, Johnson T S, Drewinko B, Swartzendruber D E, Göhde W, Andreeff M, Freireich E J. Flow cytometry in clinical cancer research. Cancer research. 1983;43:3982–3997. [PubMed] [Google Scholar]
- 2.Craig Fiona E, Foon Kenneth A. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941–3967. doi: 10.1182/blood-2007-11-120535. [DOI] [PubMed] [Google Scholar]
- 3.Davis B H, Holden J T, Bene M C, Borowitz M J, Braylan R C, Cornfield D, Gorczyca W, Lee R, Maiese R, Orfao A, Wells D, Wood B L, Stetler-Stevenson M. 2006 Bethesda International Consensus recommendations on the flow cytometric immunophenotypic analysis of hematolymphoid neoplasia: medical indications. Cytometry. Part B, Clinical cytometry. 2007;72 Suppl 1:S5–13. doi: 10.1002/cyto.b.20365. [DOI] [PubMed] [Google Scholar]
- 4.De Rosa Stephen C, Brenchley Jason M, Roederer Mario. Beyond six colors: a new era in flow cytometry. Nature medicine. 2003;9:112–117. doi: 10.1038/nm0103-112. [DOI] [PubMed] [Google Scholar]
- 5.Döhner Hartmut, Estey Elihu H, Amadori Sergio, Appelbaum Frederick R, Büchner Thomas, Burnett Alan K, Dombret Hervé, Fenaux Pierre, Grimwade David, Larson Richard A, Lo-Coco Francesco, Naoe Tomoki, Niederwieser Dietger, Ossenkoppele Gert J, Sanz Miguel A, Sierra Jorge, Tallman Martin S, Löwenberg Bob, Bloomfield Clara D. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 6.Dong Henry Y, Kung Jia Xue, Bhardwaj Vatsala, McGill John. Flow cytometry rapidly identifies all acute promyelocytic leukemias with high specificity independent of underlying cytogenetic abnormalities. American journal of clinical pathology. 2011;135:76–84. doi: 10.1309/AJCPW9TSLQNCZAVT. [DOI] [PubMed] [Google Scholar]
- 7.Friedlander M L, Hedley D W, Taylor I W. Clinical and biological significance of aneuploidy in human tumours. Journal of clinical pathology. 1984;37:961–974. doi: 10.1136/jcp.37.9.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gennaro S, Fehder W, Gallagher P, Miller S, Douglas S D, Campbell D E. Lymphocyte, monocyte, and natural killer cell reference ranges in postpartal women. Clinical and diagnostic laboratory immunology. 1997;4:195–201. doi: 10.1128/cdli.4.2.195-201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giaretti W. Origins of ... flow cytometry and applications in oncology. Journal of clinical pathology. 1997;50:275–277. doi: 10.1136/jcp.50.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzenberg Leonore A, Tung James, Moore Wayne A, Herzenberg Leonard A, Parks David R. Interpreting flow cytometry data: a guide for the perplexed. Nature immunology. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim Sherrif F, van den Engh Ger. Flow cytometry and cell sorting. Advances in biochemical engineering/biotechnology. 2007;106:19–39. doi: 10.1007/10_2007_073. [DOI] [PubMed] [Google Scholar]
- 12.Jaroszeski M J, Radcliff G. Fundamentals of flow cytometry. Molecular biotechnology. 1999;11:37–53. doi: 10.1007/BF02789175. [DOI] [PubMed] [Google Scholar]
- 13.Koss L. G., Melamed M. R. Philadelphia: Lippincott: Williams & Wilkins; 2006. Koss’ diagnostic cytology and its histopathologic bases. [Google Scholar]
- 14.Langkamp-Henken Bobbi, Wood Steven M, Herlinger-Garcia Kelli A, Thomas Debra J, Stechmiller Joyce K, Bender Bradley S, Gardner Elizabeth M, DeMichele Stephen J, Schaller Joseph P, Murasko Donna M. Nutritional formula improved immune profiles of seniors living in nursing homes. Journal of the American Geriatrics Society. 2006;54:1861–1870. doi: 10.1111/j.1532-5415.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 15.Macey M. G. Flow cytometry: Principles and applications. Totowa, NJ: Humana Press; 2007. [Google Scholar]
- 16.Mach William J, Thimmesch Amanda R, Orr James A, Slusser Joyce G, Pierce Janet D. Flow cytometry and laser scanning cytometry, a comparison of techniques. Journal of clinical monitoring and computing. 2010;24:251–259. doi: 10.1007/s10877-010-9242-4. [DOI] [PubMed] [Google Scholar]
- 17.Maecker Holden T, McCoy J Philip, Nussenblatt Robert. Standardizing immunophenotyping for the Human Immunology Project. Nature reviews. Immunology. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkel D E, Dressler L G, McGuire W L. Flow cytometry, cellular DNA content, and prognosis in human malignancy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1987;5:1690–1703. doi: 10.1200/JCO.1987.5.10.1690. [DOI] [PubMed] [Google Scholar]
- 19.Motzer Sandra Adams, Jarrett Monica, Heitkemper Margaret M, Tsuji Joyce. Natural killer cell function and psychological distress in women with and without irritable bowel syndrome. Biological research for nursing. 2002;4:31–42. doi: 10.1177/1099800402004001005. [DOI] [PubMed] [Google Scholar]
- 20.Pedreira Carlos E, Costa Elaine S, Lecrevisse Quentin, van Dongen Jacques J M, Orfao Alberto. Overview of clinical flow cytometry data analysis: recent advances and future challenges. Trends in biotechnology. 2013;31:415–425. doi: 10.1016/j.tibtech.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Picot Julien, Guerin Coralie L, Le Van Kim Caroline, Boulanger Chantal M. Flow cytometry: retrospective, fundamentals and recent instrumentation. Cytotechnology. 2012;64:109–130. doi: 10.1007/s10616-011-9415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson Russel L, Lewis Sharon L, Murphy Margaret R, Hale Jennifer M, Blackwell Paula H, Acton Gayle J, Clough Dorothy H, Patrick Graham J, Bonner Peter N. Are there sex differences in emotional and biological responses in spousal caregivers of patients with Alzheimer's disease? Biological research for nursing. 2004;5:319–330. doi: 10.1177/1099800404263288. [DOI] [PubMed] [Google Scholar]
- 23.Trewhitt K. G. Bone marrow aspiration and biopsy: Collection and interpretation. Oncology Nursing Forum. 2001;28:1409–1417. [PubMed] [Google Scholar]
- 24.Varma Neelam, Naseem Shano. Application of flow cytometry in pediatric hematology-oncology. Pediatric blood & cancer. 2011;57:18–29. doi: 10.1002/pbc.22954. [DOI] [PubMed] [Google Scholar]
- 25.Wood Brent L, Arroz Maria, Barnett David, DiGiuseppe Joseph, Greig Bruce, Kussick Steven J, Oldaker Teri, Shenkin Mark, Stone Elizabeth, Wallace Paul. 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry. Part B, Clinical cytometry. 2007;72 Suppl 1:S14–22. doi: 10.1002/cyto.b.20363. [DOI] [PubMed] [Google Scholar]