Abstract
In mammals, rewarding properties of drugs depend on their capacity to activate appetitive motivational states. With the underlying mechanisms strongly conserved in evolution, invertebrates have recently emerged as a powerful new model in addiction research. In crayfish natural reward has proven surprisingly sensitive to human drugs of abuse, opening an unlikely avenue of research into the basic biological mechanisms of drug addiction. In a series of studies we first examined the presence of natural reward systems in crayfish, then characterized its sensitivity to a wide range of human drugs of abuse. A conditioned place preference (CPP) paradigm was used to demonstrate that crayfish seek out those environments that had previously been paired with the psychostimulants cocaine and amphetamine, and the opioid morphine. The administration of amphetamine exerted its effects at a number of sites, including the stimulation of circuits for active exploratory behaviors (i.e., SEEKING). A further study examined morphine-induced reward, extinction and reinstatement in crayfish. Repeated intra-circulatory infusions of morphine served as a reward when paired with distinct visual or tactile cues. Morphine-induced CPP was extinguished after repeated saline injections. Following this extinction phase, morphine-experienced crayfish were once again challenged with the drug. The priming injections of morphine reinstated CPP at all tested doses, suggesting that morphine-induced CPP is unrelenting. In an exploration of drug-associated behavioral sensitization in crayfish we concurrently mapped measures of locomotion and rewarding properties of morphine. Single and repeated intra-circulatory infusions of morphine resulted in persistent locomotory sensitization, even 5 days following the infusion. Moreover, a single dose of morphine was sufficient to induce long-term behavioral sensitization. CPP for morphine and context-dependent cues could not be disrupted over a drug free period of 5 days.
This work demonstrates that crayfish offer a comparative and complementary approach in addiction research. Serving as an invertebrate animal model for the exposure to mammalian drugs of abuse, modularly organized and experimentally accessible nervous systems render crayfish uniquely suited for studying (1) the basic biological mechanisms of drug effects, (2) to explore how the appetitive/seeking disposition is implemented in a simple neural system, and (3) how such a disposition is related to the rewarding action of drugs of abuse. This work aimed to contribute an evolutionary, comparative context to our understanding of a key component in learning, and of natural reward as an important life-sustaining process.
Keywords: Drug reward, Amphetamine, Cocaine, Heroin, Motivation, Decapoda, Crustacea
The development of an addictive cycle depends on two separate components. First, psychoactive drugs, acting as reinforcers, are able to strengthen behaviors that promote continued drug consumption. Then, drug-seeking behaviors become compulsive, even when paired with negative consequences (Wise, 1998). A common misconception holds that only humans possess a susceptibility for these phenomena and it may explain why evolutionary factors have received scant attention in addiction research compared to cultural, environmental, biological, or pathological concerns (Nesse and Berridge, 1997; Panksepp et al., 2002; Panksepp et al., 2004). It has become clear that a wide range of mammals will work long and hard to obtain access to human drugs of abuse through compulsive self-administration (Johanson et al., 1976). Structured by selective pressures, natural reward systems in all mammals usually align with an individual's adaptive needs, enticing it to satisfy inherent motivations for nourishment, sex, or “contact comfort” (Panksepp, 2005). Across a wide range of mammals neural reward circuits have proven highly sensitive to a wide spectrum of substances, including psychostimulants, opioids, and alcohol. Several lines of evidence indicate that drug-induced sensitization is associated with a continued and enduring amplification of reinforcement following drug intake (Robinson and Becker, 1986; Vanderschueren and Kalivas, 2000; Vanderschueren et al., 1997). The degree of drug-induced behavioral sensitization depends on the precise patterns of drug regimes (Shaham et al., 1994). For instance, repeated drug exposure, separated by long intervals, is thought to be more effective in inducing sensitization than a chronic regime of high or escalating doses at short intervals (Vanderschueren and Kalivas, 2000; Vanderschueren et al., 1997; Robinson and Becker, 1986). Repeated, intermittent, or chronic exposure to amphetamine, cocaine, and morphine causes long-lasting behavioral sensitization in rats (Vanderschueren et al., 1999; Pierce and Kalivas, 1997; Zarrindast et al., 2007; White, 1995). Opioids in mammals either reduce or enhance locomotor activity, depending on dose and time protocols (Timára et al., 2005; Zhao et al., 2004). Parallels to the general phenomena of acute response, tolerance, withdrawal and sensitization have been identified in many mammals (Morgan and Sedensky, 1995; Singh and Heberlein, 2000; Schafer, 2004; Scholz, 2005; Nichols, 2006; Morozova et al., 2006; Raffa et al., 2006; Feng et al., 2006).
Mammalian analogs specifically center on the function of SEEKING systems (Panksepp, 1998), a set of brain mechanisms that underly anticipation, curiosity, excitement and pursuit. Full capitalization for primary-process, emotional systems (e.g., SEEKING) is a nomenclature used for mammals (Panksepp, 1998, 2005), and does not necessarily apply fully to the present review of addictive tendencies in invertebrates.
Hypotheses for the underlying neural pathways focus on the importance of mesolimbic, dopaminergic neurons (Alcaro et al., 2007; Panksepp, 1998). Traditionally the role of this SEEKING pathway is viewed via the generation of hedonic affect (Wise, 1982). Mounting evidence, however, challenges a simple, unitary “pleasure” view of dopamine function. Instead, the role of mesolimbic dopamine circuits in behavior has recently been conceptualized within more ethological domains (Ikemoto and Panksepp, 1999; Panksepp, 1998; Robinson and Berridge, 2001) as well as operant-anticipatory learning contexts (Schultz, 1997). In particular, the attribution of incentive salience to rewarding or aversive events figures prominently in current perceptual/sensory hypotheses of dopamine function (Robinson and Berridge, 1993; Spanagel and Weiss, 1999), while SEEKING urges are phrased in more action-oriented terms (Panksepp, 1998, 2005; Panksepp et al., 2002, 2004). Dopamine's role in behavioral control is supported by the existence of residual reward capacity even after substantial depletion (Berridge and Robinson, 1998). Modulation of the motivational value of reward-related stimuli, separable from traditional concepts of hedonia, have been re-conceptualized in terms of ‘wanting’ (Berridge and Robinson, 1998) and SEEKING (Panksepp, 1998). Mesolimbic and neostriatal dopaminergic processes may thereby foster the expression of rewarded behavior via enhanced perceptual salience of stimuli and invigorated action terms. Dopamine systems may be essential for ‘wanting’/SEEKING in centives through an increase in perceived attractiveness, rather than ‘liking’ them via the mediation of pleasurable internal states (Robinson and Berridge, 2001, 2003). ‘Reward’ is not a unitary process but rather a system into which an assembly of constituent elements feed, many of which can be separately identified and manipulated (Berridge and Robinson, 1998; Robinson and Berridge, 2003). Drugs alter brain functions, and the resulting drug-associated behaviors can, in turn, be activated and maintained when a particular environmental cue is associated with the effect of the drug. In the absence of the drug, the conditioned stimulus can sustain, and even re-establish drug-seeking behavior (Davis and Smith, 1976; Cervo et al., 2003; Burbassi and Cervo, 2008).
Widely conserved throughout mammalian evolution, dopaminergic pathways of ventral tegmental area, medial forebrain bundle, and nucleus accumbens are integral elements for feelings of desire in humans (Alcaro et al., 2007). Moreover, compulsive aspects of addictive behaviors map onto motivational, subcortical neural circuits, with strong anatomical, neurochemical, and possibly motivational homologies (Butler and Hodos, 1996; Vincent et al., 1998; Panksepp and Panksepp, 2000). Alternative hypotheses for mammalian dopamine function in reward concern a more direct role in the learning process (Schultz, 1997). Single unit recordings in monkeys, combined with theoretical considerations from formal learning theory, implicate dopamine specifically in the modulation of memory formation or expression for cues predictive of positive reinforcement. Initially, mesolimbic dopamine neurons fire phasically and unconditionally to the presentation of novel, natural rewards (Schultz, 2001). When a reward is paired with a cue in repeated learning trials, however, preferential dopamine neuron firing shifts from the presentation of the reward itself to the presentation of the predictive cue. Upon successful prediction of the reward, these neurons no longer respond to the predictive cue. Such findings underscore the fundamental importance of novelty in natural activity patterns of dopamine neurons and suggest a role of mesolimbic, dopamine circuits in the coding of prediction errors that can serve as a global teaching signal (Waelti et al., 2001). Behavior indicative of reward is controlled by three distinct sub-processes: the ‘Pavlovian labeling’ of an object's attractiveness (i.e., its incentive value), the learning of a relationship between potentially predictive cues and the object of attraction maintaining approach behavior, and the object's ability to produce hedonic affect. Although hypotheses of dopamine function have been advanced for each one of these sub-processes underlying reward, ethological hypotheses ultimately converge on a dopamine-based neurochemical signal that fosters reinforcement (Wise, 1998; Ikemoto and Panksepp, 1999; Kelley, 1999; Everitt et al., 2001; Panksepp et al., 2004). Compulsive components of addiction hinge on motivational subcortical neural circuits, with anatomical, neurochemical and motivational similarities shared across all vertebrates (Panksepp and Burgdorf, 2000; Butler and Hodos, 1996). The overarching theme strongly suggests that addictive compounds act on evolutionarily conserved brain substrates for reward that we share with much of our phylogenetic lineage.
The search for the evolutionary antecedents of drug-induced reinforcement, compulsive drug seeking, withdrawal, reinstatement, and continued drug consumption has now broadened. Invertebrate model systems, where exposure to mammalian drugs of abuse causes enduring effects on nervous system and behavior, have emerged as powerful new tools in drug addiction research (see review in Wolf and Heberlein, 2003). Invertebrate taxa share significant commonalities with mammals in key neurochemical properties of reward including sequence homologies in crucial receptor elements (Hen, 1992, 1993), neuropharmacology (Tierney, 2001), methods of inactivation (Poerzgen et al., 2001), general modes of action (Vernier et al., 1995, 1997), and association with similar behavioral contexts (Kravitz et al., 1980; Kravitz, 2000). Aminergic systems using G protein-coupled, metabotropic monoamine receptors emerged during an early evolutionary transition to multicellular life (Vernier et al., 1995). Presumably, early metazoans adopted monoamine systems to coordinate functions of individual cells in different parts of the body for orchestrating an adaptive response towards environmental perturbations (Gillette, 2006). Emergence of dopamine and serotonin receptors, for example, predate that of a chordate lineage (Hen, 1992; Peroutka and Howell, 1994; Vernier et al., 1995; Walker et al., 1996), such that invertebrates and vertebrates share homologies within functional sets of receptor families. Since the pre-Cambrian, however, independent evolutionary paths have given rise to the present-day diversity of (ortho-and paralogous) subtypes, each with a particular pharmacological profile.
In invertebrates repeated application of addictive drugs produces a number of behavioral stereotypies (Palladini et al., 1996; McClung and Hirsh, 1998; Torres and Horowitz, 1998), that are both dose-dependent and subject to sensitization (McClung and Hirsh, 1999). Fruit flies exhibit a form of behavioral sensitization (McClung and Hirsh, 1998, 1999) that resembles the protracted neurobehavioral effects of psychostimulants in the mammalian brain (reviewed in Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999; Pierce and Kalivas, 1997). Behavioral sensitization is thought to reflect an intensification of drug craving in mammals (Robinson and Berridge, 1993) and its relevance broadly extends to a wide range of rewards (Nocjar and Panksepp, 2002). The demonstration of sensitization in fruit flies presents an opportunity to study the neurobiology of addiction in a genetically tractable species (Wolf, 1999; Wolf and Heberlein, 2003). Exhibiting striking parallels to sensitization in mammals, catecholamine circuits appear to be a common neurochemical denominator in this process. Consistent with antagonist and lesion studies in mammals (e.g., Kelly and Iversen, 1976; Vezina and Stewart, 1989), dopamine modulates the behavioral efficacy of cocaine administration (Bainton et al., 2000; Li et al., 2000; Palladini et al., 1996; Torres and Horowitz, 1998). In both flies (Li et al., 2000) and rats (Kalivas, 1995), stimulation of pre-synaptic sites is essential for the initiation of behavioral sensitization. The integral role of post-synaptic monoamine receptors in cocaine-induced fruit fly behavior has been characterized in strains of mutant flies that exhibit either compensatory under- or over-expression of such receptors (Li et al., 2000). Flies that under-express such receptors display a blunted response to cocaine upon initial exposure, whereas the opposite is true of over-expressing flies. Neither mutant sensitizes. Flies with reduced dopamine levels lack the characteristic, cocaine-induced motor behaviors (Bainton et al., 2000), and selective vertebrate dopamine receptor antagonists block cocaine-induced behavioral stereotypies in fruit flies (Torres and Horowitz, 1998) and planarians (Palladini et al., 1996). Consistent with work in both mammals and flies, pre-synaptic catecholaminergic mechanisms appear to modulate the formation of behavioral sensitization, while post-synaptic elements act in its maintenance.
Besides underscoring the neurochemical similarities of vertebrate and invertebrate lineages, work with psychostimulants in fruit flies has also contributed new hypotheses and insights concerning the roles of trace amines and circadian mechanisms in drug addictions. Tyramine, the monohydroxyphenol analog of dopamine, is essential for behavioral sensitization of Drosophila. Mutants with reduced levels of this trace amine exhibit a normal initial response to cocaine exposure, but do not sensitize, while an increase in tyramine mirrors behavioral sensitization of wild-type flies (McClung and Hirsh, 1999). Behavioral sensitization in flies also depends on tyramine interactions with members of the circadian gene family. In contrast to wild-type flies, those lacking the per gene do not exhibit a normal sensitization response when challenged with post-synaptic stimulation of a vertebrate D2 agonist (Andretic et al., 1999; Andretic and Hirsh, 2000). With attention focused on these processes in mammals, recent work has suggested that the validity of such findings could well span wide, taxonomic borders. Tyramine occurs at trace levels in the mammalian brain (Durden and Davis, 1993), mostly supplied through food intake. Its pharmacological profile in vertebrates is “amphetamine-like”, as it augments synaptic catecholamines through inhibition of membrane transporter uptake (Sitte et al., 1998). Moreover, some of the 15 trace amine receptors that have since been cloned in both rats and humans, are located in the ventral tegmental area (Borowsky et al., 2001). Finally, up-regulated transcription of per, a circadian gene required for sensitization in flies, has been demonstrated in mammalian, dorsal striatal regions receiving input from midbrain dopamine neurons (Nikaido et al., 2001). Such work highlights how an exploration of basic processes in simpler systems can advance unanticipated hypotheses for a study of related phenomena in mammals.
To date, natural reward processes have received relatively little attention in invertebrates. Rewarding properties for invertebrate taxa have been demonstrated for psychostimulants (Wolf, 1999; Kusayama and Watanabe, 2000; Panksepp and Huber, 2004), opioids (Srivastava and Singh, 2006; Nathaniel et al., 2009, 2010), alcohol (Parsons, 1979; Bellen, 1998; Cadieu et al., 1999; Abramson et al., 2000, 2004), nicotine (Singaravelan et al., 2005), and caffeine (Singaravelan et al., 2005). Initial work in fruit flies (McClung and Hirsh, 1998; Torres and Horowitz, 1998) and planarians (Palladini et al., 1996) characterized unconditioned, stereotypical motor behaviors that accompany cocaine administration. Psychostimulant sensitivity in stereotypical movements, increased locomotor activity, and consummatory behaviors (Bellen, 1998; Torres and Horowitz, 1998; Wolf, 1999; Kusayama and Watanabe, 2000) is similar to the behavioral effects of cocaine in mammals (reviewed in Wise and Bozarth, 1987). Due to their small size, fruit flies have mainly been used in studies of reflexive motor sequences rather than for more complex studies of behavioral reward. Despite similar mechanisms of behavioral sensitization in invertebrates and vertebrates, it remains unclear whether a conditioned stimulus can be attractive, salient, and directly induce behavioral sensitization to promote drug-seeking behavior in an invertebrate system. It is also not known whether the elimination of the response-contingent drug in an invertebrate model decreases drug seeking behavior. Evidence from land snails indicates that individuals will readily self-administer electrical current into brain regions related to sexual behavior (Balaban and Chase, 1991), while avoiding such treatment for areas controlling escape. Thus, snails appear to have distinct neuroanatomical substrates for reward or punishment (Balaban, 1993; Balaban and Maksimova, 1993). A study of natural substrate preference in planarians demonstrated a switch to the non-preferred environment when it was paired with methamphetamine, an effect that was blocked by pretreatment with selective vertebrate D1 and D2 antagonists (Kusayama and Watanabe, 2000).
In crayfish, a complex behavioral repertoire combines with detailed knowledge of neurochemical systems and a body size that supports in vivo handling. This offers distinct advantages for a dissection of drug-sensitive reward. Crayfish have proven to be a useful model for exploring the proximate neural mechanism of behavioral decisions (Mullonev, 2003), and neurochemical mechanisms in neuroethological studies (Panksepp and Huber, 2002), suggesting that crayfish may contribute more as an invertebrate model to explore the neurochemical basis of drug addiction apart from showing reward to psychostimulants. The comparative simplicity of their nervous system facilitates the recognition of the biological underpinnings of behavioral responses to drugs. Decapod crustaceans, such as crayfish and lobsters, have been used extensively in neuroethology (Livingstone et al., 1981; Edwards et al., 2003), making them great models for studies of invertebrate drug reward. Due to their anatomical and physiological characteristics (Ma et al., 1992; Beltz, 1999; Heinrich et al., 2002), as well as their accessibility to pharmaco-behavioral manipulations (Huber and Delago, 1998; Panksepp and Huber, 2002), monoamine neuromodulatory systems have received particular emphasis in previous research with decapod crustaceans. The aminergic system in crayfish is mapped onto fewer than 1000 large and accessible neurons (Elofsson et al., 1966; Cournil et al., 1994, 1995; Tierney et al., 2003). About 30–35 dopamine neurons are located in the brain and nerve cord of crayfish (Furshpan and Potter, 1959; Tierney et al., 2003), including neurons such as the Br and L cells with large somata (up to 100 μm diameter) and far-reaching axonal projections (Tierney et al., 1999). Furthermore, evidence for conserved, monoamine re-uptake mechanisms in invertebrates (Corey et al., 1994; Demchyshyn et al., 1994; Poerzgen et al., 2001) suggests that crayfish offer an opportunity to characterize the fundamental neural mechanisms for reward to amphetamine, cocaine and morphine.
Systemic injections of cocaine and amphetamine were accompanied by characteristic, unconditioned changes in crayfish behavior (Panksepp and Huber, 2004). Intracardial infusions of cocaine produced rapid backwards walking, waving of the claws, and a series of tailflips. The crayfish then assumed a static posture with an overall flexion of the abdomen and walking legs, and claws that pointed towards the front and slightly downwards. The duration of the cocaine-induced posture was dose dependent. Following an infusion of a large dose of amphetamine, crayfish moved to the corner of the aquarium and investigated the perimeter walls with their antennae. Muscle tremors were observed in walking legs. These drug-induced, stereotyped behavior patterns are reminiscent of those commonly seen in mammals following high doses of psychostimulants. Videos of the elicited behavior patterns are available at <http://caspar.bgsu.edu/∼crayfish/psychostimulant/reward/>.
Reinforcing properties of psychostimulants were tested in a series of conditioned place preference experiments. Crayfish were connected to an infusion cannula and isolated in a specific quadrant with a removable Plexiglas enclosure for 30 min (see Fig. 1). Amphetamine (5 μg/g body weight) or cocaine (2.5 μg/g body weight) was infused for the first 5 min of the session. On 5 successive days each animal received two conditioning sessions per day (separated by 8–12 h), one in each environment in random order (i.e., psychostimulant-treated crayfish received one drug and one vehicle infusion/day while crayfish in the control group received two vehicle infusions/day). For the test trial on day 6, the crayfish was placed into the center of the aquarium and was allowed access to the entire aquarium in a drug-free state for 60 min. To confirm successful treatment during CPP testing, an application of a 50 μg cocaine bolus produced robust postural effects. Amphetamine and, to a lesser extent, cocaine both served as rewards when paired with a distinct visual environment (Panksepp and Huber, 2004). Treated animals showed an increased preference (+46.3% for amphetamine; +24.9% for cocaine) for the area that had been paired with the drug (Fig. 1).
Fig. 1.
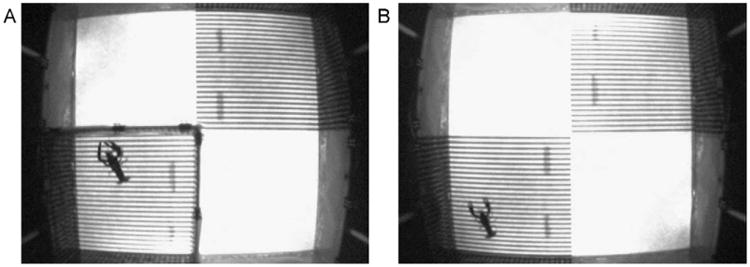
Overhead view of the arena for CPP experiments which contained four quadrants of two different visual areas, arranged diagonally from each other. The arena was lit from below with distinct, alternating black/white stripes in one area. Free-ware, Java-based software was used to track the crayfish in a video feed from a camera mounted above the tank. In a conditioning session a crayfish was isolated in a specific quadrant (A) with a removable Plexiglas enclosure for 30 min where amphetamine or cocaine was infused for the first 5 min. When allowed to move freely about the entire arena (B) following 5 successive days of psychostimulant conditioning, crayfish exhibited a strong preference for the environment that had been paired with amphetamine or cocaine.
Subsequent work was designed to investigate the fundamental, biological mechanisms of drug effects, to explore how the appetitive/seeking disposition is implemented in a simple neural system, and how such disposition is related to the rewarding action of drugs of abuse. To explore whether amphetamine stimulates exploration, novelty and SEEKING in crayfish, we identified three distinct exploratory behaviors (i.e., locomotion, rearing up on the side of the tank, and antennae movements) when crayfish were placed into a novel arena. Dose-response curves for changes in these behaviors were obtained when amphetamine was infused systemically or directly into the head ganglion. In order to distinguish between novelty and the unconditioned effects of the drug, a subset of experiments administered amphetamine in an environment to which crayfish had previously been habituated. When applied systemically, infusions of amphetamine as low as 0.5 μg/g increased activity, including use of antennae and rearing. Stimulant effects of amphetamine were demonstrated at even smaller amounts and with a faster time course when the drug infusion was applied directly to the supraesophageal ganglion (Fig. 2). When habituated to their environments, crayfish typically remain stationary for extended periods of time, however, systemic administration of various doses of amphetamine strongly aroused active exploratory behaviors. This effect was dose dependent and greatly enhanced locomotion, antenna movements, and rearing. A number of other behaviors, including excavating a depression and grooming, were unaffected by amphetamine. This suggests the presence of selective effects towards specific behavioral patterns associated with exploration, rather than a more generalized arousal state. It appears that enhanced locomotion and exploratory sampling arises from brain networks for appetitive motivational states that can be recruited by psychostimulants such as amphetamine.
Fig. 2.
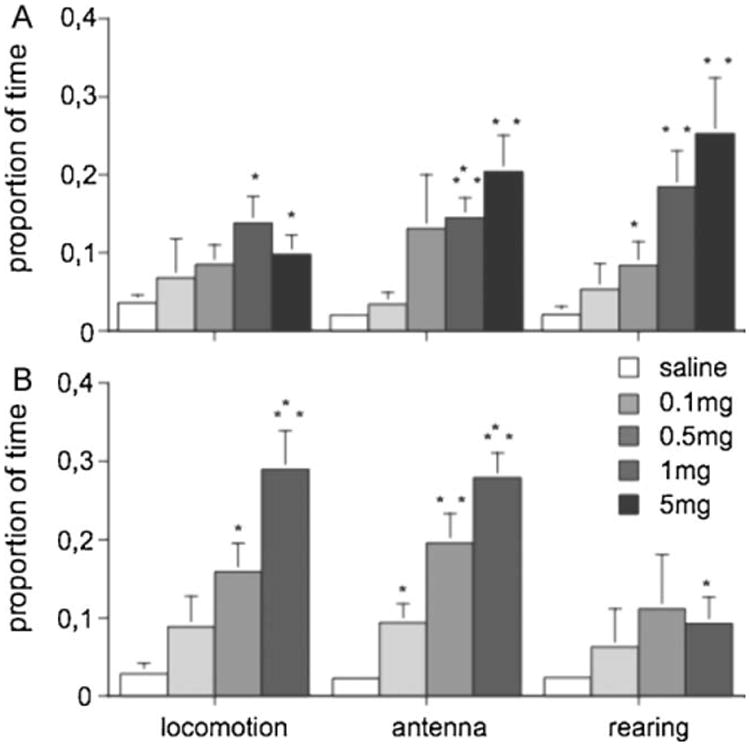
Dose-dependent changes in crayfish exploratory behaviors over a 40 min period as a function of infusions of amphetamine into the systemic circulation (A) or the supraesophageal ganglion (B). Results are expressed as changes in mean percent-age of time (±SE) spent in each behavioral category compared to saline. Significance levels from post hoc tests are listed as *P < 0.05, **P < 0.01, ***P <0.001.
Exploratory behaviors in crayfish are driven by tactile and olfactory information (McMahon et al., 2005; Patullo and Macmillan, 2006), detected primarily via antennae and antennules, and conveyed mainly to the olfactory lobe of the brain (Mellon, 2000; Sullivan and Beltz, 2005). Modulated by serotonin and dopamine transmission (Sandeman and Sandeman, 1987; Sandeman et al., 1995, Schmidt, 1997), the olfactory lobe of crayfish may represent the site of action of amphetamine, and perhaps other drugs as well. In sum, theses data are consistent with rewarding properties of amphetamine in crayfish as determined by CPP procedures (Panksepp and Huber, 2004), and such reward components may arise from the activation of explorative and approach dispositions within crayfish brains. Integrative control of such adaptive responses may be to promote the ability of organisms to search for favorable, life-supporting environmental conditions.
Morphine, as an unconditioned stimulus, was presented in a particular area marked by visual and tactile cues (Nathaniel et al., 2009). In crayfish, multiple administration of morphine produced behavioral sensitization, manifested in enhancing locomotion behavior at low doses. Higher doses suppressed locomotor activity in crayfish suggesting the development of tolerance. A study of a conditioned place preference paradigm in crayfish explored morphine-induced reward, extinction and reinstatement (Nathaniel et al., 2009). After testing for the presence of the CPP we aimed to determine: (1) whether it could be extinguished by repeated pairing with saline and (2) whether, following extinction, CPP could be reinstated by priming injections of different doses of morphine given prior to the test session. Regardless of the doses used, morphine acted as a powerful reward when paired with a novel environment. Extinction trials, where the previously reinforced environment was repeatedly paired with saline alone, reduced the previous established CPP. This reduction in compulsive drug-seeking behavior likely resulted from a declining significance of drug-paired stimuli. Behavioral sensitization to opiates enhanced the behavioral effects upon their re-administration (Heidbreder et al., 1996; Mattingly et al., 1997). In crayfish behavioral response to morphine resulted from a direct pharmacological effect of the drug as well as learned associations of distinct stimuli with the drug rewarding experience. These data suggest that, as in mammals (Lubman et al., 2007; Robinson and Berridge, 1993; Lu et al., 2002; Fattore et al., 2005; Bartoletti et al., 1987; Tao et al., 2006), the link between environmental cues and behavioral sensitization in opiate relapse also exists in crayfish.
Nathaniel et al. (2010) examined whether behavioral sensitization can be evoked by single or repeated drug pre-treatment regimes in an open field test. Systemic infusions of morphine produced distinct postural displays, grooming behavior, a series of tail-flipping, movement of mouthparts, continuous exploration of the corners of the aquarium, and mild leg tremor. Regardless of the dose, a single systemic injection of morphine produced an enduring enhancement of locomotion compared to saline during the entire challenge period (Fig. 3). In the repeated dose regimen, locomotor activity was even higher. Five days later, the amount of locomotion was remeasured to determine the presence of long-lasting effect of morphine on behavioral sensitization. Locomotion of crayfish, pretreated with single or repeated morphine, remained sensitized to different doses of morphine, while pretreatments with saline were unaffected when injected with saline. This finding indicated that a single dose of morphine is sufficient to induce long-term behavioral sensitization in an invertebrate system. The induction of behavioral sensitization with a single dose of morphine had previously been observed in mammals (Smith, 1985, 1991; Zarrindast et al., 2007) and such motivational similarities appear to extend to invertebrates (Panksepp and Burgdorf, 2000; Poerzgen et al., 2001). Although sensitization (or reverse tolerance) is progressively enhanced, sensitization is not only a direct pharmacological action of the drug, but also of learned associations with drug experience (Pierce and Kalivas, 1997).
Fig. 3.
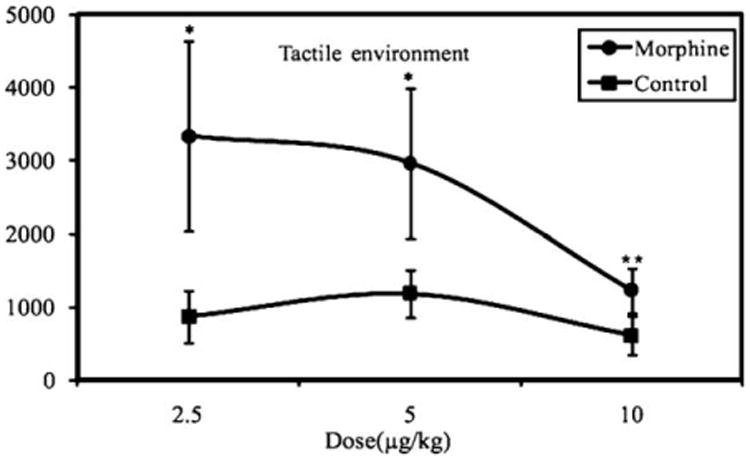
Mean distance traveled in crayfish pretreated with 2.5 μg/g, 5.0 μg/g and 10.0 μ/g doses of morphine (n = 7) or saline (n = 7) for textured compartments with tactile stimuli. Injection of morphine significantly increased locomotion when compared with saline injection. Locomotion was high at 2.5 μg/g and 5.0 μg/g doses (*P<=0.05; **P<0.01).
Altogether these studies demonstrate that research into drug-sensitive reward in crayfish offers significant new insights into the complex interactions of behavioral sensitization and neurochemical changes in the development of drug addiction. Not particularly known for their cognitive abilities, crayfish continue to surprise with behavioral phenomena indicating powerful effects for drug-sensitive reward, behavioral sensitization, and drug dependence. In short, the basic motivational and emotional processes that are so evidently coded by specific neurochemical systems, apparently already had their start long before vertebrates walked (or swam) the face of the earth.
Footnotes
Studies reported in this paper were supported by a grant to R.H. and J.P. (NIH/NIDA 1R21DA016435-01A1) and by the help of Hope for Depression Research Foundation to J.P.
References
- Abramson CI, Stone SM, Ortez RA, Luccardi A, Vann KL, Hanig KD, Rice J. The development of an ethanol model using social insects I: behavior studies of the honey bee (Apis mellifera L.) Alcohol Clin Exp Res. 2000;24(8):1153–1166. [PubMed] [Google Scholar]
- Abramson CI, Kandolf A, Sheridan A, Donohue D, Bozic J, Meyers JE, Benbassat D. Development of an ethanol model using social insects: III. Preferences for ethanol solutions. Psychol Rep. 2004;94(1):227–239. doi: 10.2466/pr0.94.1.227-239. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56(2):283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. doi: 10.1126/science.285.5430.1066. [DOI] [PubMed] [Google Scholar]
- Andretic R, Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci USA. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LTY, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Balaban P. Behavioral neurobiology of learning in terrestrial snails. Prog Neurobiol. 1993;41:1–19. doi: 10.1016/0301-0082(93)90038-t. [DOI] [PubMed] [Google Scholar]
- Balaban PM, Chase R. Interrelationships of the emotionally positive and negative regions of the brain of the edible snail. Neurosci Behav Physiol. 1991;21(2):172–180. doi: 10.1007/BF01182895. [DOI] [PubMed] [Google Scholar]
- Balaban PM, Maksimova OA. Positive and negative brain zones in the snail. Eur J Neurosci. 1993;5:768–774. doi: 10.1111/j.1460-9568.1993.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Bartoletti M, Gaiardi M, Gubellini C, Bacchi A, Babbini M. Previous treatment with morphine and sensitization to the excitatory actions of opiates: dose–effect relationship. Neuropharmacology. 1987;26(2–3):115–119. doi: 10.1016/0028-3908(87)90197-3. [DOI] [PubMed] [Google Scholar]
- Bellen HJ. The fruit fly: a model organism to study the genetics of alcohol abuse and addiction? Cell. 1998;93:909–912. doi: 10.1016/s0092-8674(00)81195-2. [DOI] [PubMed] [Google Scholar]
- Beltz BS. Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc Res Tech. 1999;44:105–120. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<105::AID-JEMT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–368. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci. 2001;98(16):8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin (2C) receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 2008;1:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy. Wiley; New York: 1996. [Google Scholar]
- Cadieu N, Cadieu JC, El Ghadraoui L, Grimal A, Lamboeuf Y. Conditioning to ethanol in the fruit fly—a study using and inhibitor of ADH. J Insect Physiol. 1999;45:579–586. doi: 10.1016/s0022-1910(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28(6):1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Corey JL, Quick MW, Davidson N, Lester HA, Guastella J. A cocaine-sensitive Drosophila serotonin transporter: cloning, expression, and electrophysiological characterization. Proc Natl Acad Sci. 1994;91:1188–1192. doi: 10.1073/pnas.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournil I, Casasnovas B, Helluy SM, Beltz BS. Dopamine in the lobster Homarus gammarus: II. Dopamine-immunoreactive neurons and development of the nervous system. J Comp Neurol. 1995;362:1–16. doi: 10.1002/cne.903620102. [DOI] [PubMed] [Google Scholar]
- Cournil I, Helluy SM, Beltz BS. Dopamine in the lobster Homarus gammarus. I Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the nervous system of the juvenile. J Comp Neurol. 1994;344:455–469. doi: 10.1002/cne.903440308. [DOI] [PubMed] [Google Scholar]
- Davis W, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976;11(4):222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- Demchyshyn LL, Pristupa ZB, Sugamori KS, Barker EL, Blakely RD, Wolfgang WJ, Forte MA, Niznik HB. Cloning, expression, and localization of a chloride-facilitated, davcocaine-sensitive serotonin transporter from Drosophila melanogastor. Proc Natl Acad Sci. 1994;91:5158–5162. doi: 10.1073/pnas.91.11.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden DA, Davis BA. Determination of regional distributions of phenylethylamine and meta- and para-tyramine in rat brain regions and presence in human and dog plasma by an ultra sensitive negative chemical ion gas chromatography–mass spectrometric (NCIGC–MS) method. Neurochem Res. 1993;18:995–1002. doi: 10.1007/BF00966759. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Issa FI, Herberholz J. The neural basis ofdominance hierarchy formation in crayfish. Microsc Res Tech. 2003;60:369–373. doi: 10.1002/jemt.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elofsson R, Kauri T, Nielsen SO, Stromberg JO. Localization of monoaminergic neurons in the central nervous system of Astacus astacus Linne (Crustacea) Z Zellforsch. 1966;74:464–473. doi: 10.1007/BF00496839. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36(2–3):129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, et al. Endocannabinoid system and opioid addiction: behavioral aspects. Pharmacol Biochem Behav. 2005;81(2):349–359. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127(3):621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ, Potter DD. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959;145:289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R. Evolution and function in serotonergic systems. Integr Comp Biol. 2006;46:838–846. doi: 10.1093/icb/icl024. [DOI] [PubMed] [Google Scholar]
- Hen R. Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci. 1992;13:160–165. doi: 10.1016/0165-6147(92)90054-a. [DOI] [PubMed] [Google Scholar]
- Hen R. Structural and functional conservation of serotonin receptors throughout evolution. EXS. 1993;63:266–278. doi: 10.1007/978-3-0348-7265-2_14. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson CA, Shpppenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Heinrich R, Cromarty SI, Horner M, Edwards DH, Kravitz EA. Autoinhibition of serotonin cells: an intrinsic regulatory mechanism sensitive to the pattern of usage of cells. Proc Natl Acad Sci. 2002;96:2473–2478. doi: 10.1073/pnas.96.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Delago A. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: the motivational concept revisited. J Comp Physiol A. 1998;182:573–583. [Google Scholar]
- Ikemoto S, Panksepp J. The role of the nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL, Bonese K. Self-administration of psychomotor stimulant drugs: the effects of unlimited access. Pharmacol Biochem Behav. 1976;4:45–51. doi: 10.1016/0091-3057(76)90174-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neural basis of sensitization to cocaine. In: Hammer RP, editor. The Neurobiology of Cocaine: Cellular and Molecular Mechanisms. CRC Press; 1995. pp. 81–98. [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40(1):45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Neural integrative activities of nucleus accumbens subregions in relation to learning and motivation. Psychobiology. 1999;27:198–213. [Google Scholar]
- Kravitz EA. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J Comp Physiol A. 2000;186:221–238. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Glusman S, Harris-Warrick RM, Livingstone MS, Schwarz T, Goy MF. Amines and a peptide as neurohormones in lobsters: actions on neuromuscular preparations and preliminary behavioural studies. J Exp Biol. 1980;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11(113):2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- Li H, Chaney S, Forte M, Hirsh J. Ecotopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogastor. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Schaeffer SF, Kravitz EA. Biochemistry and ultrastructure of serotonergic nerve endings in the lobster: serotonin and octopamine are contained in different nerve endings. J Neurobiol. 1981;12(1):24–53. doi: 10.1002/neu.480120104. [DOI] [PubMed] [Google Scholar]
- Lu L, Xu NJ, Ge X, Yue W, Su WJ, Pei G, et al. Reactivation of morphine conditioned place preference by drug priming: role of environmental cues and sensitization. Psychopharmacology (Berl) 2002;159(2):125–132. doi: 10.1007/s002130100885. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence of the motivational salience of drug cues in opiate addiction. Psychol Med. 2007;37(8):1203–1209. doi: 10.1017/S0033291707009932. [DOI] [PubMed] [Google Scholar]
- Ma PM, Beltz BS, Kravitz EA. Serotonin-containing neurons in lobsters: their role as gain-setters in postural control mechanisms. J Neurophysiol. 1992;68(1):36–56. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Ragozzimo ME, Gold PE. Stimulus and response factors affecting the development of behavioral sensitization to apormorphine. Psychopharmacology. 1997;130:109–116. doi: 10.1007/s002130050217. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypical behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 1998;8(2):109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol. 1999;9:853–860. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- McMahon A, Blair WP, Macmillan DW. Exploration in a T-Maze by the crayfish Cherax destructor suggests bilateral comparison of antennal tactile information. Biol Bull. 2005;208:183–188. doi: 10.2307/3593150. [DOI] [PubMed] [Google Scholar]
- Mellon D., Jr Convergence of multimodal sensory input onto higher-level neurons of the crayfish olfactory pathway. J Neurophysiol. 2000;84(6):3043–3055. doi: 10.1152/jn.2000.84.6.3043. [DOI] [PubMed] [Google Scholar]
- Morgan PG, Sedensky MM. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol Clin Exp Res. 1995;19(6):1423–1429. doi: 10.1111/j.1530-0277.1995.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Morozova TV, Anholt RR, Mackay TF. Transcriptional response to alcohol exposure in Drosophila melanogaster. Genome Biol. 2006;7(10):R95. doi: 10.1186/gb-2006-7-10-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullonev B. During fictive locomotion, graded synaptic currents drive bursts of impulses in swimmeret motor neurons. J Neurosci. 2003;23:5953–5962. doi: 10.1523/JNEUROSCI.23-13-05953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel TI, Panksepp J, Huber R. Drug-seeking behavior in an invertebrate system: evidence of morphine-induce reward, extinction an reinstatement in crayfish. Behav Brain Res. 2009;1:331–338. doi: 10.1016/j.bbr.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathaniel TI, Panksepp J, Huber R. Effects of a single and repeated morphine treatment on conditioned and unconditioned behavioral sensitization in Crayfish. Behav Brain Res. 2010;207:310–320. doi: 10.1016/j.bbr.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112(3):677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriay T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59(4):894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug-, food- and sexual-reward: interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta VA. Pharmacological study of cocaine activity in planaria. Comp Biochem Physiol. 1996;115C(1):41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford University Press; New York: 1998. [Google Scholar]
- Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res. 2000;115:25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of emotional brain systems in addictions: a neuro-evolutionary perspective. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: a neuroethological perspective. In: Bevins RA, Bardo MT, editors. 50th Nebraska Symposium on Motivation: Motivational Factors in the Etiology of Drug Abuse. Nebraska, Lincoln: 2004a. pp. 85–126. [PubMed] [Google Scholar]
- Panksepp JB, Huber R. Chronic alterations in serotonin function: dynamic neurochemical properties in agonistic behavior of the crayfish, Orconectes rusticus. J Neurobiol. 2002;50(4):276–290. doi: 10.1002/neu.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Huber R. Ethological analyses of crayfish behavior: a new invertebrate system for measuring the rewarding properties of psychostimulants. Behav Brain Res. 2004;153:171–180. doi: 10.1016/j.bbr.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Panksepp JB. Seven sins of evolutionary psychology. Evol Cogn. 2000;6(2):108–131. [Google Scholar]
- Panksepp J. Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn. 2005;14(1):30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: a neuroethological perspective. In: Bevins RA, Bardo MT, editors. Motivational Factors in the Etiology of Drug Abuse. Univ Nebraska Press; Lincoln, NE, USA: 2004b. pp. 85–126. [PubMed] [Google Scholar]
- Parsons PA. Larval responses to environmental ethanol in Drosophila melanogaster: variation within and among populations. Behav Genet. 1979;10:183–191. doi: 10.1007/BF01066268. [DOI] [PubMed] [Google Scholar]
- Patullo BW, Macmillan DL. Corners and bubble wrap: the structure and texture of surfaces influence crayfish exploratory behaviour. J Exp Biol. 2006;209(Pt 3):567–575. doi: 10.1242/jeb.02020. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Howell TA. The molecular evolution of G protein-coupled receptors: focus on 5-hydroxytryptamine receptors. Neuropharmacology. 1994;33:319–324. doi: 10.1016/0028-3908(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Poerzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol Pharmacol. 2001;59(1):83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Stagliano GW, Tallarida RJ. Subadditive withdrawal from cocaine/kappa-opioid agonist combinations in Planaria. Brain Res. 2006;1114(1):31–35. doi: 10.1016/j.brainres.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Sandeman DC. Serotonin-like immunoreactivity of giant olfactory interneurons in the crayfish brain. Brain Res. 1987;403(2):371–374. doi: 10.1016/0006-8993(87)90078-3. [DOI] [PubMed] [Google Scholar]
- Sandeman RE, Watson AH, Sandeman DC. Ultrastructure of the synaptic terminals of the dorsalgiant serotonin-IR neuron and deutocerebral commissure interneurons in the accessory and olfactory lobes of the crayfish. J Comp Neurol. 1995;361(4):617–632. doi: 10.1002/cne.903610406. [DOI] [PubMed] [Google Scholar]
- Schafer WR. Addiction research in a simple animal model: the nematode Caenorhabditis elegans. Neuropharmacology. 2004;47(S1):123–131. doi: 10.1016/j.neuropharm.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Distribution of centrifugal neurons targeting the soma clusters of the olfactory midbrain among decapod crustaceans. Brain Res. 1997;752(1–2):15–25. doi: 10.1016/s0006-8993(96)01441-2. [DOI] [PubMed] [Google Scholar]
- Scholz H. Influence of the biogenic amine tyramine on ethanol-induced behaviors in Drosophila. J Neurobiol. 2005;63(3):199–214. doi: 10.1002/neu.20127. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rodaros M, Stewart J. Reinstatement of heroin-reinforced behavior following a long-term extinction: implications for the treatment of relapse to drug-taking. Behav Pharmacol. 1994;5:360–364. doi: 10.1097/00008877-199406000-00015. [DOI] [PubMed] [Google Scholar]
- Singaravelan N, Nee'man G, Inbar M, Izhaki I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J Chem Ecol. 2005;31(12):2791–2804. doi: 10.1007/s10886-005-8394-z. [DOI] [PubMed] [Google Scholar]
- Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res. 2000;24(8):1127–1136. [PubMed] [Google Scholar]
- Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated release, transporter rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- Smith JB. Effects of single and repeated daily injections of morphine, clonidine and l-nantradol on responding of squirrel monkeys under escape titration. J Pharmacol Exp Ther. 1985;234(1):94–99. [PubMed] [Google Scholar]
- Smith JB. Situational specificity of tolerance to decreased operant responding by morphine and l-nantradol. Psychopharmacology (Berl) 1991;103(1):115–120. doi: 10.1007/BF02244085. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Srivastava HK, Singh D. Honeybees foraging response in genetically diversified opium poppy. Bioresour Technol. 2006;97(13):1578–1581. doi: 10.1016/j.biortech.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Integration and segregation of inputs to higher-order neuropils of the crayfish brain. J Comp Neurol. 2005;481(1):118–126. doi: 10.1002/cne.20346. [DOI] [PubMed] [Google Scholar]
- Tao PL, Liang KW, Sung WY, Wu YT, Huang EY. Nalbuphine is effective in decreasing the rewarding effect induced by morphine in rats. Drug Alcohol Depend. 2006;84(2):175–181. doi: 10.1016/j.drugalcdep.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Tierney AJ. Structure and function of invertebrate 5-HT receptors a review. Comp Biochem Physiol A. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Godleski MS, Rattananont P. Serotonin-like immunoreactivity in the stomatogastric nervous system of crayfishes. Cell Tissue Res. 1999;295:537–551. doi: 10.1007/s004410051259. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Kim T, Abrams R. Dopamine in crayfish and other crustaceans: distribution in the central nervous system and physiological functions. Microsc Res Tech. 2003;60:325–335. doi: 10.1002/jemt.10271. [DOI] [PubMed] [Google Scholar]
- Timára J, Gyarmatia Z, Fürsta Z. The development of tolerance to locomotor effects of morphine and the effect of various opioid receptor antagonists in rats chronically treated with morphine. Brain Res Bull. 2005;64(5):417–424. doi: 10.1016/j.brainresbull.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Torres G, Horowitz JM. Activating properties of cocaine and cocaethylene in a behavioral preparation of Drosophila melanogastor. Synapse. 1998;29:148–161. doi: 10.1002/(SICI)1098-2396(199806)29:2<148::AID-SYN6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vanderschueren LJ, Tjon GH, Nestby P, Mulder AH, Schoffelmeer AN, De Viries TJ. Morphine-induced long-term sensitization to the locomotor effects of morphine and amphetamine depends on the temporal pattern of the pretreatment regime. Psychopharmacology (Berl) 1997;131:115–122. doi: 10.1007/s002130050273. [DOI] [PubMed] [Google Scholar]
- Vanderschueren LJ, Schmidt ED, De Viries TJ, Van Moorsel CA, Tilders FJ, Schoffelmeer AN. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschueren MJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151(2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vernier P, Cardinaud B, Valdenair O, Philippe H, Vincent JD. An evolutionary view of drug receptor interaction: the bioamine receptor family. Trends Pharm Sci. 1995;16:375–381. doi: 10.1016/s0165-6147(00)89078-1. [DOI] [PubMed] [Google Scholar]
- Vernier P, Cardinaud B, Philippe H, Vincent JD. The classification of bioamine receptors. How helpful are molecular phylogenies? Ann NY Acad Sci. 1997:812. doi: 10.1111/j.1749-6632.1997.tb48154.x. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Vincent JD, Cardinaud B, Vernier P. The evolution of the receptors of monoamines and the origin of motivational and emotional systems in vertebrates. Bull Acad Natl Med. 1998;182:1505–1516. [PubMed] [Google Scholar]
- Walker RJ, Brooks HL, Holdendye L. Evolution and overview of classical transmitter molecules and their receptors. Parasitology. 1996;113:S3–S33. doi: 10.1017/s0031182000077878. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci. 1982;5:39–87. [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- White FJ. Neurophysiological alterations in the mesocorticolimbic dopamine system with repeated cocaine administration. In: RP HJ, editor. The neurobiology of cocaine. CRC; Boca Raton, FL: 1995. pp. 99–119. [Google Scholar]
- Wolf ME. Cocaine addiction: clues from Drosophila on drugs. Curr Biol. 1999;9:R770–R772. doi: 10.1016/S0960-9822(00)80009-3. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. J Neurobiol. 2003;54(1):161–178. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Ebrahimi-Ghirir M, Rostami P, Rezayof A. Repeated pre-exposure to morphine into the ventral pallidum enhances morphine-induced place preference: involvement of dopaminergic and opioidergic mechanisms. Behav Brain Res. 2007;181(1):35–41. doi: 10.1016/j.bbr.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Zhao W, Gong Z, Liang J. A new buprenorphine analogy, thenorphine, inhibits morphine-induced behavioral sensitization in mice. Acta Pharmacol. 2004;25(11):1413–1418. [PubMed] [Google Scholar]


