Abstract
Parastomal hernias represent a clinically significant problem for many patients after radical cystectomy and ileal conduit diversion. The prevalence may be as high as 60% and in some series, up to 30% of patients require surgical intervention due to the complications of pain, poor fit of an ostomy appliance, leakage, urinary obstruction, and bowel obstruction or strangulation. Due to the potential morbidity associated with PH repair, there have been efforts to prevent PH development at the time of the index surgery. Four randomized trials of prophylactic mesh placement at the time of colostomy and ileostomy stoma formation have demonstrated significant reductions in PH rates with acceptably low complication rates. In this review, we describe the clinical and radiographic definitions of PH, the clinical impact and risk factors behind its development, and the rationale behind prophylactic mesh placement for patients undergoing ileal conduit urinary diversion. Additionally, we report our experience with prophylactic mesh placed at radical cystectomy at our institution.
Keywords: parastomal hernia, ileal conduit, radical cystectomy
Introduction
A parastomal hernia (PH) is defined as an “incisional hernia related to an abdominal wall stoma”. 1 The prevalence of PH may be as high as 60% but the quoted incidence varies widely depending on the definition used, the length of follow up, and whether the diagnosis is made clinically or radiographically. 2 While many patients are asymptomatic, PH has been shown to have a significantly negative impact on QOL after radical cystectomy.3 Up to 30% of patients can require surgical intervention, most commonly due to discomfort, poor fit of the ostomy appliance, and rarely due to the devastating consequences of obstruction, bowel perforation, or strangulation. 4-6
Due to the potential morbidity associated with PH repair and the high recurrence rates associated with some approaches, surgeons have attempted maneuvers at the time of stoma formation to reduce the incidence of PH development. Randomized trials of prophylactic mesh placement at the time of colostomy or ileostomy creation have demonstrated significant reduction in PH rates without increases in the rates of complications. 7-10 There have been no randomized, controlled trials of mesh placement in patients undergoing urinary diversion and published experience is limited to single institution series of select patients.11
The purpose of this review is to describe the rationale behind the placement of prophylactic mesh at the time of radical cystectomy and ileal conduit formation and our experience with patients at high-risk for PH development.
Clinical and Radiographic Definitions of Parastomal Hernia
Ileal conduit (IC) remains the most common type of urinary diversion after radical cystectomy12 due to its relative ease of construction and track record of safety. Despite over 60 years of experience with this form of diversion, stomal complications remain a significant problem with some series reporting an incidence of up to 60%.13 Parastomal hernia (PH) is one of the most frequent complications following stoma formation and has been shown to have a significant negative impact on quality of life after radical cystectomy.3 While PH have been reported as late as 27 years after surgery14, the majority will occur within the first two years after stoma formation.15,16
The quoted incidence rates for parastomal hernias vary widely between 5- 65% 4, 13, 17-21 depending on the definition used, the length of follow up, and whether the diagnosis is made clinically or radiographically. Many historical reports fail to state the definition used for parastomal hernia in their studies and standardized criteria were lacking. Over the past decade, several studies have used similar definitions for PH and reported rates of approximately XX-50% after 12 months of follow up.22(second citation) Commonly accepted clinical criteria for PH are any palpable defect or bulge adjacent to the stoma when the patient is supine with legs elevated or with Valsalva maneuver when upright. If cross-sectional imaging is added to the clinical exam, PH can be defined as any intra-abdominal content protruding along the ostomy. 23 A classification system for radiographic evidence of PH has been proposed by Moreno-Matias 24 and successfully applied in a randomized setting 8 as well as retrospective analysis 17 to assess trial outcomes and the natural history of PH. A Type 1 parastomal hernia is defined as a hernia sac that contains the prolapsed bowel forming the stoma, while a Type 2 PH contains abdominal fat or omentum herniating through the abdominal wall defect created by the stoma. A Type 3 hernia contains herniated loops of bowel other than that forming the stoma.
How a parastomal hernia is both defined and diagnosed can significantly impact the reported incidence rates. Most studies have depended upon a clinical diagnosis of PH, which can vary depending on whether the data is collected prospectively or retrospectively and whether it is self reported or documented by the operating surgeon or wound-ostomy nurse. Most clinical definitions of a PH are based upon the finding of a protrusion in the vicinity of the stoma, but studies differ greatly in terms of how the examinations were performed, i.e., supine or upright with or without Valsalva maneuvers. Radiographic diagnosis of PH is typically more sensitive and generally associated with higher reported rates, leading to a concern that clinically insignificant hernias are being identified. Standardized radiographic classification systems for reporting PH are relatively new and though the experience with them is limited, they appear to have good concordance between identifying clinically symptomatic and radiographically evident hernias. In these studies, radiographic Type 3 hernias were universally identified on physical exam, while Grade 2 hernias had a concordance rate of 60-80%, suggesting radiographic identification of PH can be a relevant means for reporting incidence rates, especially in the retrospective setting. 17, 2425, 26 Radiographic, rather than clinical criteria have the advantage of being morphologic, objective, reproducible, less impacted by physical factors such as body habitus, and less subject to bias when used for retrospective review of clinical data. Additionally, radiographic classification allows for objective measures of the size of the hernia and any changes that occur over time.
Natural History of Radiographic Parastomal Hernia Progression
In our prior report on the risk factors for developing a PH after IC, we noted that progression to a higher grade of hernia on subsequent CT scans occurred in over a third of patients when followed longitudinally. 17 Radiographic evidence of a parastomal hernia was identified in 136 patients of 386 patients (36%) at a median of 299 days after radical cystectomy. First radiographic evidence of Type 1, 2, and 3 parastomal hernias developed in 5 (4%), 90 (66%), and 41 (30%) patients, respectively. Progression to a higher grade of hernia occurred in 34 patients of 95 patients (37%). Four of 5 initial Type I hernias progressed to Type 3 a median of 288 days (range 191- 441 days) after initial radiographic diagnosis, while 30 of 90 initial Type 2 hernias progressed to Type 3 also at a median of 288 days (range 72-1260 days) after being identified on cross-sectional imaging. These data are consistent with the clinical observations that the natural history of PH is for progression and enlargement over time. 14
Clinical Impact of Parastomal Hernias
While many patients with PH are asymptomatic, up to 30% of patients can require surgical correction due to symptoms such as pain or discomfort, issues related to the appliance, or rarely secondary to bowel obstruction, perforation, or strangulation. 16 Ripoche et al reported on 782 ostomy patients, 202 (25.6%) of whom developed clinical evidence of PH over a median follow up of 10.5 years. They noted stomal prolapse in 18% and at least one episode of obstruction in 15%. Only 24% of patients with PH denied the presence of symptoms; in the remaining, 46% reported pain, 37% stomal appliance problems, 36% leakage, 29% skin irritation, and 20% described psychologic and aesthetic concerns secondary to the PH. 14 Liu et al reported a PH rate of 29% at a median follow-up of 29 months, 45% of whom underwent surgical repair for abdominal pain (58%), acute strangulation or bowel obstruction (15%), partial small bowel obstruction (15%), or due to elective reasons (12%). 18 In our prior series, the clinical PH rate was 24% (93 of 386 patients), which is consistent with clinically detectable PH rates reported elsewhere. 4, 27,28,29,30 Of the 93 patients with a clinically evident PH, 32 (34%) were radiographically a Type 2 PH and 61 (66%) were Type 3. Symptoms related to the PH, such as pain, discomfort, bowel incarceration, appliance difficulties, or leakage attributable to the PH were present in 37 of 93 patients (40%). Of the 93 patients with a clinically evident PH, an abdominal hernia belt or binder was prescribed for 75 patients (81%) and 16 (17%) were referred to general surgery for possible repair. Only 8 patients (9%) of those with clinically evident PH underwent surgical repair. Two PH repairs were performed emergently due to bowel incarceration and ultimately 3 of the 8 repairs developed a PH recurrence a median of 13 months (range 10-22 months) later. Nearly half of the patients (n=45; 48%) with clinically evident hernias had advanced or metastatic disease and no patients with advanced or metastatic disease underwent hernia repair or consultation for hernia repair. The low rates of referral may be reflective of the need to balance the competing issues of advanced disease and short life expectancy with the high recurrence rates and potential morbidity associated with PH repair. This may also account for why 80% were managed conservatively with abdominal binders/hernia belts. 17
Risk Factors for Development of Parastomal Hernias after Ileal Conduit
The development of parastomal hernias may be influenced by both clinical and surgical factors. Patient-related issues, such as obesity, malnutrition, increasing age, history of radiation exposure, and increased intra-abdominal pressure from chronic coughing, constipation, or ascites, have been cited in the literature as potential risk factors for the development of a PH. 4, 27, 28, 31, 32 Technical factors, such as the type of stoma created, the size and location of the abdominal wall defect through which the stoma is formed, and preoperative marking of the stoma site by a wound-ostomy nurse may also impact the risk of PH formation. 25, 29, 33, 34
Increasing body mass index (BMI) has been reported in multiple case series of urinary diversions, as well as in series of colostomies and ileostomies, as a significant risk factor for PH development. 4, 17, 18 Other risk factors reported to be associated with the development of PH after stoma formation are increased waist circumference (>100cm), high intra-abdominal pressure, malnutrition, advanced age, smoking, chronic respiratory disease, use of corticosteroids, and prior radiation therapy, all of which are common risk factors associated with ventral or incisional hernias. 4, 14, 24, 28, 31,32
We previously described risk factors for the development of PH in a cohort of 386 consecutive patients undergoing open radical cystectomy and ileal conduit at our institution. On multivariable analysis, patients who were female (HR 2.25; 95% CI 1.58, 3.21; p<0.0001), had a higher body mass index (HR 1.08 per unit increase; 95% CI 1.05, 1.12; p<0.0001) and lower preoperative albumin (HR 0.43 per gm/dl; 95% CI 0.25, 0.75; p=0.003) were more likely to develop a PH after adjusting for age, diabetes, smoking history, COPD, estimated blood loss, prior abdominal surgery, preoperative radiation therapy, neoadjuvant chemotherapy and stoma type (end-stoma vs. Turnbull technique). BMI was analyzed as a continuous variable, which did not allow for a specific BMI cut-point that would place a patient at increased risk for PH. It is notable that in the cohort of 396 patients, 75 had a BMI ≥30 and ultimately 66 of these patients developed a parastomal hernia. 17 Liu et al found increasing BMI and prior abdominal surgery as significant risk factors for PH after ileal conduit formation. 18
The type of stoma formed has been evaluated in multiple retrospective studies. Typically the greatest differences in parastomal hernia rates exist between the use of colon or ileum for the conduit limb with end-colon stomas having the highest reported rates of PH and loop ileostomies having the lowest. It is important to recognize that the bulk of this literature comes from general surgery patients where loop ileostomies are frequently done on an elective basis with the intention of reversal at a later date. The follow up for many loop ileostomy series may not be long enough to identify significant rates of PH. The use of end versus loop stomas for ileal conduit diversions has been evaluated in a few case series with equivalent complication rates reported with both techniques. Chechile et al reported on 458 patients almost evenly divided between end and loop stoma diversion (44% and 56% respectively) and found equivalent parastomal hernia rates of 4% and 4.3%. Similarly, we did not identify an association between the type of stoma (end stoma versus Turnbull loop stoma) and the risk of developing a PH after ileal conduit. 17
Surgical Repair of Parastomal Hernias
Surgical repair of parastomal hernias has been associated with significant recurrence rates depending on the type of approach. Local repair using native tissues has been associated with unacceptably high recurrence rates as high as 76% 2, 4-6, 14, 35-37 and relocation of the stoma to another quadrant of the abdomen still requires closure of the original stoma defect, placing both sites at risk for the development of hernias in up to 60% of patients. 37 Greater familiarity in other hernia types led to the development of a mesh repair for parastomal hernias. Varying techniques, such as intraperitoneal versus preperitoneal placement and laparoscopic versus open surgery, have all demonstrated roughly equal improvements over local tissue repairs with recurrence rates of approximately 10% in small, non-randomized reports with relatively short follow-up. 37
There are four potential techniques for mesh repair of PH: onlay – mesh is placed on the anterior fascial aponeurosis; inlay – mesh is cut to the size of the defect and sutured to the wound edge at the margins of the stomal defect; sublay – mesh is placed dorsal to the rectus muscle, anterior to the posterior rectus sheath; and intraperitoneal onlay – mesh is placed intraperitoneally on the peritoneum. The intraperitoneal onlay technique has been used in open and laparoscopic PH repairs, but there are concerns with this approach. Meshes that induce an inflammatory response cannot be placed in contact with abdominal contents without a high risk of fistula formation, adhesions, and septic complications. Because of these concerns, mesh constructed of two layers is typically used for this technique. The non-absorbable surface of the mesh is oriented toward the abdominal wall to allow for integration; the absorbable, non-reactive side of the mesh is oriented toward the abdominal contents. These concepts are particularly important for those considering prophylactic mesh placement at the time of minimally invasive, intracorporeal diversion.
The onlay, sublay, and intraperitoneal onlay techniques all require the mesh to be placed with considerable overlap in all directions extending 5-10cm beyond the edge of the defect. The inlay technique has largely been abandoned in incisional hernia repairs due to the high recurrence rates. The sublay technique has produced good results and has been proposed as the most advantageous technique for mesh repairs of PH. The anatomic planes are often preserved and the intra-abdominal pressure does not easily allow the mesh to be displaced. The onlay technique for PH repair tacks the mesh to the anterior rectus aponeurosis and requires extensive mobilization of the subcutaneous fat to achieve an adequate border. Only a few non-randomized reports describing between 3-9 patients each have used this technique and the follow up for each was short.
Prophyactic Mesh to Prevent Parastomal Hernia Development
Despite improved success with these newer mesh-based approaches, with mortality rates as high as 7% and complication rates of 60% in some series,38 PH repairs are often performed only when compelling indications dictate. The high prevalence of parastomal hernias, the negatively associated quality of life issues, morbidity of surgical repair, and high recurrences rates have prompted surgeons to attempt to prevent their appearance from the time of the index operation. Prospective, randomized trials of prospective mesh placement have demonstrated over 50% reductions in the rates of parastomal hernias without postoperative complications or long-term morbidity.7,8,9,10
There have been four prospective, randomized studies where mesh was placed at the time of stoma formation in an attempt to prevent parastomal hernias. (Table 1) Three studies used partially absorbable mesh and the fourth was a phase I trial of a biologic mesh in patients undergoing loop ileostomy with a planned reversal six months later. The most comprehensive series comes from Janes et al who reported short and long term results from their randomized trial. After 1 and 12 month follow up, they reported no mesh infections, fistula formation, or strictures in patients who had a partially absorbable mesh placed at the time of ostomy formation. In patients who underwent standard surgery, 13 of 27 patients (48%) had PH at 12 months compared to 1 of 27 patients (4%) who had prophylactic mesh placed. 7 Due to the significant difference in outcomes between the two groups of patients, it was deemed unethical to continue the trial and it was terminated early.
Table 1.
Randomized Controlled Trials of Prophylactic Mesh Placement
| Author | Type of Stoma |
Type of Mesh | Number of Patients |
Median Follow Up | Primary Endpoint | PH Rate | Mesh-related Complications |
|---|---|---|---|---|---|---|---|
| Janes | Permanent End Colostomy |
Partially absorbable (Vypro) |
Mesh = 27 Control = 27 |
14 months (95% CI 12-17 months) |
Clinical PH | Mesh: 0/16 (0%) Control: 8/18 (44.4%) |
None reported |
| Serra-Aracil | Permanent End Colostomy |
Partially absorbable (Ultrapro) |
Mesh = 27 Control = 27 | 29 months (range 13-49) |
Clinical PH Radiographic PH |
Clinical PH:
Mesh: 4/27 (14.8%) Control: 11/27 (40.7%) Radiographic PH: Mesh: 6/27 (22.2%) Control: 12/27 (44.4%) |
None reported |
| Hammond | Loop Stoma | Xenogenic Collagen |
Implant = 10 Control = 10 |
6.5 months | Clinical PH | Implant: 0/10 (0%) Control: 3/10 (33.3%) |
None reported |
| Lambrecht | Permanent End Colostomy |
Polypropylene | Mesh = 32 Control = 26 |
40 months (range 3-87) |
Clinical PH | Clinical PH: Mesh: 2/32 (6.3%) Control: 12/26 (46.2%) |
None reported |
These same investigators reported 5 year follow up data on 54 patients randomized to receive either conventional surgery or mesh at the time of colostomy. After 5 years, 21 of 27 patients who underwent standard surgery were alive and 17 patients (81%) had parastomal hernias. At total of 12 patients in the study arm died of malignancy by the time of analysis. In the remaining 15 patients who had mesh placed at the time of initial surgery, PH was present in 2 (13%), which represented a statistically significant reduction over standard surgery. No fistulas, strictures, and mesh infections were noted. No mesh was removed during the study period. Of note, patients who were available at the 5-year follow up were re-examined at 6-12 months intervals. No additional PH were detected in 15 patients at a mean of 72 months after the index operation. 39
The experience gained from their randomized trial led these authors to alter their practice pattern and place prophylactic mesh at every end ostomy surgery. They subsequently reported the outcomes of 93 consecutive ostomies where there was an intent to place prophylactic mesh. Prophylactic mesh was placed in 75 of 93 patients. In 9 patients, it was not technically feasible to place the mesh in the sublay position due to abdominal wall scarring; the remaining 9 did not have mesh placed at the discretion of the surgeon due to reasons such as short life expectancy after surgery and planned reversal of the ostomy. Almost one third of stomas were created at an emergent operation with severe contamination. Mesh was placed in 19 of 29 patients with pus and fecal contents present in the abdominal cavity. With mesh, 6 of 73 patients (8%) developed a surgical site infection compared with 4 of 15 (27%) patients who did not have mesh placed. The rate of PH in patients with mesh was 13% (8 of 61) and without mesh it was 67% (8 of 12). 22
These authors reported an interesting clinical observation regarding restoring bowel continuity in patients with prophylactic mesh. There is, contrary to the situation without a mesh, no parastomal hernia sac present at all. The bowel must be sharply dissected through all layers of the abdomen without the help of entering a hernia sac. Additionally, the abdominal wall defect through which the stoma passes is never larger than the circle originally cut in the mesh and it is easy to close with a running suture. Without mesh, the abdominal wall defect is often much larger and must be treated like an incisional hernia. 22
Surgical Technique
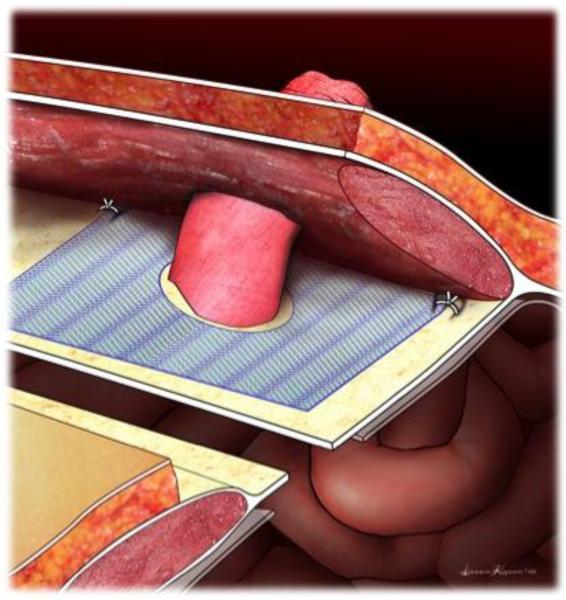
At the time of IC formation, the partially absorbable mesh is placed in the sublay position dorsal to the rectus muscle and anterior to the posterior rectus sheath. (Figure 1) If the stoma position is below the arcuate line, the mesh is placed anterior to the peritoneum. Care is taken to leave a margin of peritoneum/posterior rectus fascia along the medial aspect of the dissection to allow for closure with running or interrupted sutures once the mesh is positioned.
Figure 1.
Placement of mesh in the sublay position dorsal to the rectus muscle, anterior to the posterior rectus sheath. A 2-3cm circle is cut in the center of the mesh just large enough to allow the mesh to pass through. An overlap of 3-5cm of mesh beyond the center cut is needed. Two sutures of absorbable suture are placed in the lateral corners to minimize movement of the mesh.
The ileal conduit is brought through a circle or cross cut in the center of the mesh. ULTRAPO mesh (Ethicon, Somerville, NJ) is available in a 6” × 6” mesh that is tailored by roughly a third on two sides to make it approximately 10 × 10 cm. The circle or crosscut should be just large enough to let the bowel through. The mesh is cut along its periphery to allow for a 5cm overlay circumferentially around the conduit. The mesh is anchored to the posterior rectus sheath with 2 absorbable sutures placed in the lateral corners. (Figure 1)
The ileal conduit is secured to the anterior rectus fascia and the stoma is matured to the abdominal wall skin according to surgeon preference. Drains placed for purposes of monitoring the intestinal or uretero-intestinal anastomoses are positioned in quadrants remote from the mesh and stoma.
When possible, the anterior peritoneum/posterior rectus sheath is closed primarily in the midline to prevent mesh from coming in contact with the abdominal contents. Standard perioperative antibiotics are administered according to established postoperative pathways.
Prophylactic Mesh after Open Radical Cystectomy
To date, there are no randomized, controlled trials of prophylactic mesh placement in patients undergoing urinary diversion. Styrke reported a single institution, 10-year consecutive series of 114 patients having prophylactic mesh placed at RC/IC. At a median follow-up of 35 months, they reported a clinical PH rate of 14% and no mesh-related complications. 11
Recognizing PH represents a clinically significant problem for patients and based upon randomized evidence demonstrating significantly reduced rates of PH with acceptably low complications, we changed our practice pattern in September 2013 to offer placement of prophylactic mesh in patients at highest risk for PH formation after RC. Based upon our previous report of risk factors associated with PH development 17, mesh placement was offered to all female patients and men with a BMI > 30.
Of 40 patients having mesh placed, 33 have a minimum of 90 days follow-up. The 16 women and 17 men had a median BMI of 31.3 (IQR 27.5, 34.3). The rates and types of immediate (< 30 days postoperatively) and early (> 30 and < 90 days postoperatively) complications for patients having prophylactic mesh placed were similar to previously reported series. 40 Wound related complications were the most commonly seen, with nearly a third of patients experiencing superficial wound infections and seromas, which is not unexpected considering the higher BMI of this select cohort. Notably, one patient developed an enterocutaneous fistula not involving the mesh and was managed conservatively with bowel rest, percutaneous drainage, and subsequently healed with no further intervention; four patients had percutaneous management of pelvic abscesses; three patients had urine leaks managed with percutaneous drainage and uretero-intestinal stents. No patient experienced complications attributable to the mesh, such as erosion, urinary fistula, stricture, or infection in the first 90 days after surgery. Over the short period of follow-up (median = 297 days), no mesh-related complications have been identified.
The median follow-up and non-randomized nature of our study cohorts limit our ability to speculate on the efficacy of mesh placement.2 What is notable in our short period of follow-up is the discrepancy between the radiographic and clinical rates of hernias in our cohorts. We have seen six (18%) patients in the prophylactic mesh arm develop radiographic PH, only one of which was clinically apparent. Longer follow-up is needed to assess the efficacy of this approach in these high-risk patients.
Conclusions
Parastomal hernias represent a clinically significant issue for patients undergoing conduit urinary diversion. Experience from the colorectal literature has demonstrated beneficial results from prophylactic mesh placement in a series of randomized trials. Urinary diversion presents a different set of potential complications unique to the surgery due to the presence of both bowel and uretero-intestinal anastomoses. Early experience with this technique suggests that placement of prophylactic, partially absorbable mesh in ileal conduit patients at high risk for PH formation appears feasible and safe. The degree to which placement of prophylactic mesh at the time of conduit formation reduces the rate of PH should be established in the setting of a randomized, controlled trial.
Contributor Information
Timothy F. Donahue, John P. Murtha Cancer Center, Walter Reed National Military Medical Center, Bethesda, MD 20889, Phone: 301 319 2900, Fax: 301 319 2900, timothy.f.donahue@gmail.com
Eugene K. Cha, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY 10065, Phone: 646 422 4387, Fax: 212 988 0759, chae@mskcc.org
Bernard H. Bochner, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY 10065, Phone: 646 422 4387, Fax: 212 988 0759, bochnerb@mskcc.org
References
- 1.Pearl RK. Parastomal hernias. World J Surg. 1989;13:569. doi: 10.1007/BF01658872. [DOI] [PubMed] [Google Scholar]
- 2.Israelsson LA. Parastomal hernias. Surg Clin North Am. 2008;88:113. doi: 10.1016/j.suc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Gerharz EW, Mansson A, Hunt S, et al. Quality of life after cystectomy and urinary diversion: an evidence based analysis. J Urol. 2005;174:1729. doi: 10.1097/01.ju.0000176463.40530.05. [DOI] [PubMed] [Google Scholar]
- 4.Kouba E, Sands M, Lentz A, et al. Incidence and risk factors of stomal complications in patients undergoing cystectomy with ileal conduit urinary diversion for bladder cancer. J Urol. 2007;178:950. doi: 10.1016/j.juro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Goligher JC, Lloyd-Davies OV, Robertson CT. Small-gut obstructions following combined excision of the rectum with special reference to strangulation round the colostomy. Br J Surg. 1951;38:467. doi: 10.1002/bjs.18003815208. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson AM, Collins JP. Strangulated para-ileostomy hernia. Aust N Z J Surg. 1977;47:86. doi: 10.1111/j.1445-2197.1977.tb03941.x. [DOI] [PubMed] [Google Scholar]
- 7.Janes A, Cengiz Y, Israelsson LA. Randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surg. 2004;91:280. doi: 10.1002/bjs.4417. [DOI] [PubMed] [Google Scholar]
- 8.Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J, et al. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg. 2009;249:583. doi: 10.1097/SLA.0b013e31819ec809. [DOI] [PubMed] [Google Scholar]
- 9.Hammond TM, Huang A, Prosser K, et al. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia. 2008;12:475. doi: 10.1007/s10029-008-0383-z. [DOI] [PubMed] [Google Scholar]
- 10.Lambrecht RJ, Larsen GS, Reiertsen O, et al. Prophylactic mesh at end-colostomy construction reduces parastomal hernia rate: a randomised trial. Colorectal Dis. 2015 doi: 10.1111/codi.13065. [DOI] [PubMed] [Google Scholar]
- 11.Styrke J, Johansson M, Granasen G, et al. Parastomal hernia after ileal conduit with a prophylactic mesh: a 10 year consecutive case series. Scand J Urol. 2015:1. doi: 10.3109/21681805.2015.1005664. (This is the only published series of prophylactic mesh placement in a cohort of urinary diversion patients.) [DOI] [PubMed] [Google Scholar]
- 12.Hautmann RE, Abol-Enein H, Lee CT, et al. Urinary diversion: how experts divert. Urology. 2015;85:233. doi: 10.1016/j.urology.2014.06.075. [DOI] [PubMed] [Google Scholar]
- 13.Farnham SB, Cookson MS. Surgical complications of urinary diversion. World J Urol. 2004;22:157. doi: 10.1007/s00345-004-0429-5. [DOI] [PubMed] [Google Scholar]
- 14.Ripoche J, Basurko C, Fabbro-Perray P, et al. Parastomal hernia. A study of the French federation of ostomy patients. J Visc Surg. 2011;148:e435. doi: 10.1016/j.jviscsurg.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Martin L, Foster G. Parastomal hernia. Ann R Coll Surg Engl. 1996;78:81. [PMC free article] [PubMed] [Google Scholar]
- 16.Marimuthu K, Vijayasekar C, Ghosh D, et al. Prevention of parastomal hernia using preperitoneal mesh: a prospective observational study. Colorectal Dis. 2006;8:672. doi: 10.1111/j.1463-1318.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 17.Donahue TF, Bochner BH, Sfakianos JP, et al. Risk factors for the development of parastomal hernia after radical cystectomy. J Urol. 2014;191:1708. doi: 10.1016/j.juro.2013.12.041. (The largest series to date analyzing risk factors for radiographic PH development in patients undergoing ileal conduit diversion.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu NW, Hackney JT, Gellhaus PT, et al. Incidence and risk factors of parastomal hernia in patients undergoing radical cystectomy and ileal conduit diversion. J Urol. 2014;191:1313. doi: 10.1016/j.juro.2013.11.104. [DOI] [PubMed] [Google Scholar]
- 19.Wood DN, Allen SE, Hussain M, et al. Stomal complications of ileal conduits are significantly higher when formed in women with intractable urinary incontinence. J Urol. 2004;172:2300. doi: 10.1097/01.ju.0000141140.56022.7a. [DOI] [PubMed] [Google Scholar]
- 20.Bloom DA, Grossman HB, Konnak JW. Stomal construction and reconstruction. Urol Clin North Am. 1986;13:275. [PubMed] [Google Scholar]
- 21.Fontaine E, Barthelemy Y, Houlgatte A, et al. Twenty-year experience with jejunal conduits. Urology. 1997;50:207. doi: 10.1016/S0090-4295(97)00210-0. [DOI] [PubMed] [Google Scholar]
- 22.Janes A, Cengiz Y, Israelsson LA. Experiences with a prophylactic mesh in 93 consecutive ostomies. World J Surg. 2010;34:1637. doi: 10.1007/s00268-010-0492-6. (This follow-on paper after their randomized trial demonstrates the applicability and safety of the technique in both the elective and emergent, contaminated cases) [DOI] [PubMed] [Google Scholar]
- 23.Janes A, Weisby L, Israelsson LA. Parastomal hernia: clinical and radiological definitions. Hernia. 2011;15:189. doi: 10.1007/s10029-010-0769-6. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Matias J, Serra-Aracil X, Darnell-Martin A, et al. The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis. 2009;11:173. doi: 10.1111/j.1463-1318.2008.01564.x. [DOI] [PubMed] [Google Scholar]
- 25.Seo SH, Kim HJ, Oh SY, et al. Computed tomography classification for parastomal hernia. J Korean Surg Soc. 2011;81:111. doi: 10.4174/jkss.2011.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SY, Oh SY, Lee JH, et al. Risk factors for parastomal hernia: based on radiological definition. J Korean Surg Soc. 2013;84:43. doi: 10.4174/jkss.2013.84.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parmar KL, Zammit M, Smith A, et al. A prospective audit of early stoma complications in colorectal cancer treatment throughout the Greater Manchester and Cheshire colorectal cancer network. Colorectal Dis. 2011;13:935. doi: 10.1111/j.1463-1318.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 28.Nastro P, Knowles CH, McGrath A, et al. Complications of intestinal stomas. Br J Surg. 2010;97:1885. doi: 10.1002/bjs.7259. [DOI] [PubMed] [Google Scholar]
- 29.Pilgrim CH, McIntyre R, Bailey M. Prospective audit of parastomal hernia: prevalence and associated comorbidities. Dis Colon Rectum. 2010;53:71. doi: 10.1007/DCR.0b013e3181bdee8c. [DOI] [PubMed] [Google Scholar]
- 30.Caricato M, Borzomati D, Ausania F, et al. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. Eur J Surg Oncol. 2006;32:126. doi: 10.1016/j.ejso.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.De Raet J, Delvaux G, Haentjens P, et al. Waist circumference is an independent risk factor for the development of parastomal hernia after permanent colostomy. Dis Colon Rectum. 2008;51:1806. doi: 10.1007/s10350-008-9366-5. [DOI] [PubMed] [Google Scholar]
- 32.Arumugam PJ, Bevan L, Macdonald L, et al. A prospective audit of stomas--analysis of risk factors and complications and their management. Colorectal Dis. 2003;5:49. doi: 10.1046/j.1463-1318.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 33.Emmott D, Noble MJ, Mebust WK. A comparison of end versus loop stomas for ileal conduit urinary diversion. J Urol. 1985;133:588. doi: 10.1016/s0022-5347(17)49099-9. [DOI] [PubMed] [Google Scholar]
- 34.McGrath A, Porrett T, Heyman B. Parastomal hernia: an exploration of the risk factors and the implications. Br J Nurs. 2006;15:317. doi: 10.12968/bjon.2006.15.6.20679. [DOI] [PubMed] [Google Scholar]
- 35.Rubin MS, Schoetz DJ, Jr., Matthews JB. Parastomal hernia. Is stoma relocation superior to fascial repair? Arch Surg. 1994;129:413. doi: 10.1001/archsurg.1994.01420280091011. [DOI] [PubMed] [Google Scholar]
- 36.Horgan K, Hughes LE. Para-ileostomy hernia: failure of a local repair technique. Br J Surg. 1986;73:439. doi: 10.1002/bjs.1800730607. [DOI] [PubMed] [Google Scholar]
- 37.Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. Br J Surg. 2003;90:784. doi: 10.1002/bjs.4220. [DOI] [PubMed] [Google Scholar]
- 38.Helgstrand F, Rosenberg J, Kehlet H, et al. Risk of morbidity, mortality, and recurrence after parastomal hernia repair: a nationwide study. Dis Colon Rectum. 2013;56:1265. doi: 10.1097/DCR.0b013e3182a0e6e2. [DOI] [PubMed] [Google Scholar]
- 39.Janes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh: a 5-year follow-up of a randomized study. World J Surg. 2009;33:118. doi: 10.1007/s00268-008-9785-4. (The authors' randomized trial was terminated early due to the overwhelming superiority of prophylactic mesh placement in reducing the rates of clinical parastomal hernia formation. This 5-year follow up of the cohort from the RCT provides intermediate term data on the efficacy of prophylactic mesh placement.) [DOI] [PubMed] [Google Scholar]
- 40.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]