Abstract
Background
Self-sampling is a convenient, feasible and acceptable way of collecting genital specimens, but the veracity of reported self-collection is difficult to verify. We investigated whether a host gene, β-globin, can be used to confirm adequacy of self-collected mucosal and skin genital specimens in studies of genital HSV infection.
Methods
HSV-2 seropositive adults self-collected daily anogenital and oral swabs. Mucosal samples were tested for HSV DNA using a real-time quantitative PCR assay. A real time Taqman PCR detecting the β-globin gene was used to quantify host cells.
Results
One hundred twelve participants collected 5559 genital and 2002 oral swabs. Sixty (54%) were women, 65% were HSV-2 seropositive, and 35% were HSV-1 & HSV-2 seropositive by Western blot. β-globin DNA was detected in 99% and 93% of swabs obtained from women and men, respectively. The quantity of β-globin DNA detected was significantly higher when HSV was present in genital swabs in women (0.1 log10 copies/mL; p=0.001) and in men (0.6 log10 copies/mL; p<0.001), but not in oral swabs in women (0.2 log10 copies/mL; p=0.08) or men (0.0 log10 copies/mL; p=0.70).
Conclusions
Human β-globin DNA detection rate was high, and the quantity obtained significantly increased with genital, but not oral HSV shedding. The high rate of β-globin DNA detection is consistent with high adherence to study procedures in longitudinal studies of genital herpes shedding.
Keywords: Self-sampling, HSV, β-globin, DNA, PCR
Introduction
Herpes simplex virus type 2 (HSV-2) is the most frequent cause of genital ulcer disease worldwide (1). HSV-2 reactivates frequently, with genital viral shedding occurring in up to 30% of days in infected persons (2, 3). Repeated sampling of genital and oral mucosa provides precise estimation of HSV DNA detection frequency, a measure which varies from person-to-person and is indicative of both clinical disease severity and transmission risk (4). Self-collected swabs are a convenient and acceptable sampling method, with equivalent sensitivity and specificity for detection of Chlamydia trachomatis, Neisseria gonorrhea and Human papilloma virus infection as clinician-collected specimens (5, 6). Vaginal swabs are the preferred specimen for screening women for sexually transmitted infections, and self-sampling increases rates of participation in screening and research programs in a variety of settings (7-10). Self-sampling by study participants at home is a convenient, feasible and acceptable way of collecting genital specimens in natural history studies of HSV shedding as well as in clinical trials evaluating new antivirals (11, 12). While studies have shown that the likelihood of HSV detection is at least as great in home-as in clinic-collected swabs (13), veracity of home self-collection is difficult to prove. In addition, quantity of pathogen is difficult to interpret in secretions, as the amount of secretions may vary widely. For example, cervical secretions vary both in amount and consistency throughout the menstrual cycle, and it is unclear that either standardization to the host cell copy number or to protein level is appropriate, although both have been reported in literature (14, 15). Furthermore, in women, the swabs are collected from mucosa while in men the swabs are collected from keratinized skin on the penile shaft. To better understand the relationship between host cell number and viral copy number, we investigated whether a host cell gene, β-globin, can be used to test the adequacy of self-collected genital specimens, and to determine correlates of β-globin detection.
Materials and Methods
Subjects and procedures
Healthy, HSV-2 seropositive adults enrolled in studies of genital herpes at the University of Washington Virology Research Clinic (VRC) provided mucosal swabs (11, 16). Participants were instructed to self-collect daily swabs of the anogenital area during the course of the study. Women collected the mixed genital sample by inserting the swab into the vagina, followed by rubbing the swab across the vulva, perineum, and perianal areas. Men swabbed the penile skin initially, then the perineum, and ended with the perianal area. Men swabbed the penile skin initially, then the perineum, and ended with the perianal area. Swabs were placed into vials containing 1 mL of digestion buffer, and stored at 4°C until processed in the laboratory as previously reported (17, 18). The participants with both HSV-2 and HSV-1 antibodies also collected daily swabs of oral mucosa. On days with oral or genital lesions, a separate swab was collected from the lesion site. Participants returned to clinic every 2 weeks for collection of swabs. The University of Washington Human Subjects Review Committee approved all protocols, and all participants signed a written consent form.
Laboratory Methods
Serum samples were tested for HSV-1 and HSV-2 antibodies using Western blot (19). Mucosal samples from each participant were tested for HSV DNA using a real-time quantitative PCR assay at the University of Washington, as previously described, without typing to distinguish HSV-1 from HSV-2 (20). A real time TaqMan PCR assay (Applied Biosystems, Foster City, CA) detecting β-globin gene was used to measure the number of host DNA copies in the samples: 150 copies/mL or greater determined positivity for human β-globin and HSV DNA (21, 22). QIAmp 96 DNA blood kit was used to extract DNA from 200μl of digestion buffer and DNA was eluted into 100μl of AE buffer (QIAGEN, CA). The 30μL PCR reaction consisted of 830nM of each primer, 100nM of probe, 15μL of QuantiTect 2× multiplex PCR master mix (QIAGEN, CA), and 10μL of extracted DNA. Each reaction was also spiked with EXO internal control to monitor PCR reaction efficiency and inhibition. To prevent PCR product contamination, UNG was added in the PCR mix. Cycling conditions were as follows: 50°C for 2 minutes, 95°C for 15 minutes, then 45 cycles of 94°C for 1 minute and 60°C for 1 minute.
Statistical Analysis
Quantities of HSV and β-globin DNA (copies/mL) were log10 transformed for analysis. Shedding rates were computed as number of positive swabs out of swabs collected. Shedding rates for HSV, and β-globin were calculated separately by compartment (genital or oral); these included swabs collected by the participant or clinicians and by type (either mixed genital/oral or lesional). Quantity of virus detected was summarized and evaluated on positive days only. Mixed (i.e. non-lesion) genital and oral swabs were included in analyses evaluating the association between β-globin quantity and HSV detection if they were collected on days without lesions present. Linear mixed models accounting for both fixed and random effects were used to evaluate differences in β-globin collected on days with versus without HSV shedding. Data were analyzed using SAS 9.3 (Cary, NC, USA). Two-sided p-values ≤0.05 were considered statistically significant.
Results
A total of 112 participants were included in the analysis; 54% were women and most (65%) were HSV-2 seropositive/HSV-1 seronegative (77% of women and 52% of men). Overall, 7651 swabs were collected over 6879 days, and all were assayed for both β-globin and HSV. Most swabs (95%) were self-collected at home. One hundred and one participants provided 5559 anogenital swabs, and 47 provided 2002 oral swabs. Of these, 328 swabs from genital lesions were obtained on 308 days and 59 swabs from oral lesions on 48 days. Genital lesion sites included vulvar (45%), penile (25%), buttock (15%), perianal (14%), and thigh (1%). The median number of days with swabs per person was 57 (range, 15 to 91) for genital swabs and 44 (range, 15 to 73) for oral swabs, and the median number of swabs per person was 61 (range, 16 to 151).
β-globin DNA was detected in 99% of genital swabs from women and 90% of genital swabs from men (Table 1). The median quantity of β-globin DNA detected from genital swabs was 6.2 log10 copies/mL for women and 3.5 log10 copies/mL for men (p<0.001) (Table 1). The oral β-globin detection rate was 99-100% for both genders, and the median quantity of β-globin DNA detected from oral swabs did not differ by sex (5.7 vs. 5.8 log10 copies/mL for women and men, respectively; p=0.94).
Table 1. Swabbing Characteristics.
| Men (n=52) % (n/N), or median (range) per-swab | Women (n=60) % (n/N), or median (range) per-swab | |||||
|---|---|---|---|---|---|---|
| Genital swabs | During lesions | Non lesional mixed | All swabs | During lesions | Non lesional mixed | All swabs |
| β-globin detected | 96% (85/89) | 90% (2039/2276) | 90% (2124/2365) | 97% (233/239) | 99% (2933/2955) | 99% (3166/3194) |
| HSV detected | 80% (71/89) | 9% (198/2276) | 11% (269/2365) | 70% (168/239) | 22% (660/2955) | 26% (828/3194) |
| Log10 c/mL β-globin in positive swabs | 4.7 (2.3, 6.4) | 3.5 (2.2, 6.6) | 3.6 (2.2, 6.6) | 5.1 (2.2, 7.4) | 6.2 (2.2, 7.6) | 6.2 (2.2, 7.6) |
| Log10 c/mL HSV in positive swabs | 5.9 (2.4, 8.5) | 4.5 (2.2, 8.2) | 4.8 (2.2, 8.5) | 6.4 (2.4, 9.1) | 5.5 (2.2, 8.7) | 5.6 (2.2, 9.1) |
| Oral Swabs | During lesions | Non lesional mixed | All swabs | During lesions | Non lesional mixed | All swabs |
| β-globin detected | 100% (49/49) | 99% (1293/1305) | 99% (1343/1354) | 100% (10/10) | 100% (637/638) | 100% (647/648) |
| HSV detected | 37% (18/49) | 5% (67/1305) | 6% (85/1354) | 30% (3/10) | 3% (19/638) | 3% (22/648) |
| Log10 c/mL β-globin in positive swabs | 4.9 (3.6, 6.9) | 5.8 (2.9, 7.0) | 5.8 (2.9, 7.0) | 5.4 (4.5, 5.9) | 5.7 (3.0, 7.1) | 5.7 (3.0,7.1) |
| Log10 c/mL HSV in positive swabs | 6.4 (3.1, 8.6) | 4.5 (2.2, 7.0) | 4.7 (2.2 8.6) | 7.7 (6.5, 8.5) | 4.6 (2.3, 6.6) | 5.2 (2.3, 8.5) |
Overall, HSV DNA was detected in 26% of genital and 3% of oral swabs from women and 11% of genital and 6% of oral swabs from men. While oral HSV shedding was less common than genital, both oral and genital shedding of HSV were more common and in greater amount when lesions were present versus not present (Table 1); for example, in men HSV was detected orally in 37% of lesion swabs and only in 5% of non-lesion swabs. Median HSV DNA levels detected in genital lesion swabs were 6.4 log10 copies/mL from women and 5.9 log10 copies/mL from men, and in mixed genital swabs were 5.5 log10 copies/mL in women and 4.5 log10 copies/mL in men. The median quantity of HSV DNA detected from oral lesion swabs was 7.7 log10 copies/mL in women and 6.4 log10 copies/mL in men. In mixed oral swabs from women and men, the median quantity of HSV DNA detected was 4.6 and 4.5 log10 copies/mL, respectively. In sensitivity analysis, when we included only swabs with detectable β-globin, there was little change in the overall HSV detection rate, which increased from 15.9% to 16.4%.
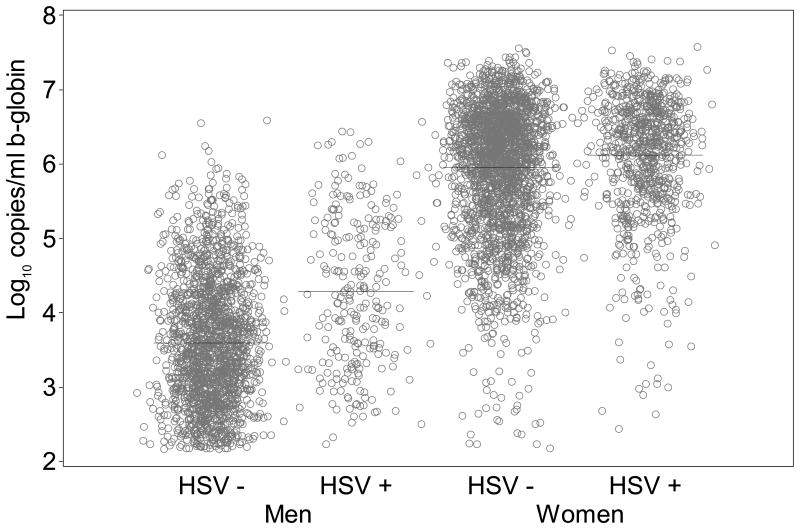
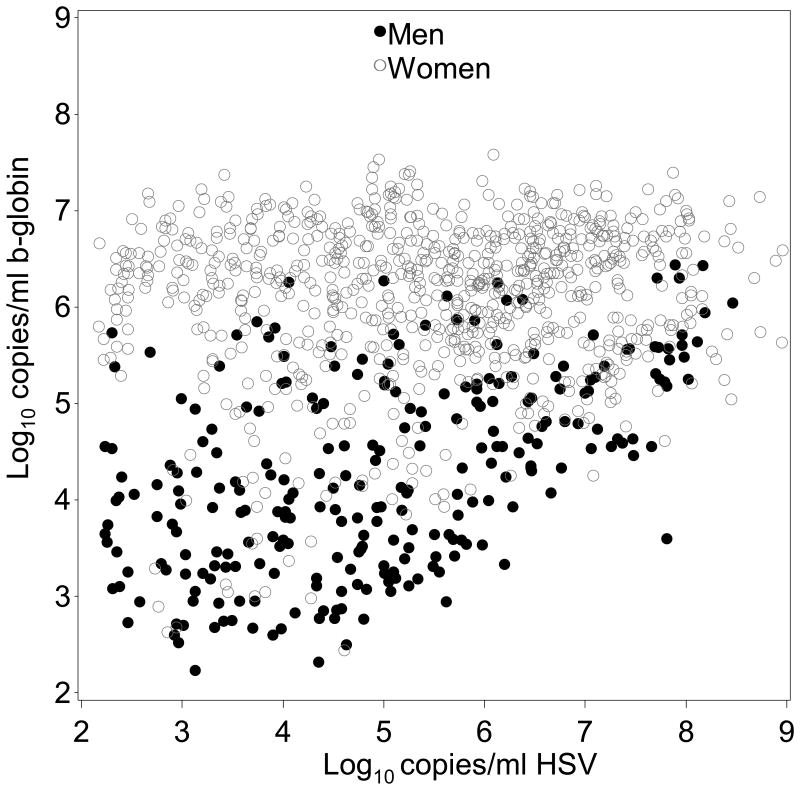
β-globin DNA levels in samples positive for HSV were slightly higher than samples negative for HSV (0.1 log10 copies/mL higher, 95% CI: 0.1 to 0.2; p=0.001) in mixed genital swabs from women on days without lesions. Similarly, in mixed genital swabs from men, β-globin DNA was 0.6 log10 copies/mL (95% CI: 0.5 to 0.7; p<0.001) higher with HSV present versus not present (Figure 1a). The copy number of β-globin was also higher when HSV was detected versus not in genital lesion swabs from women (0.7 log10 copies/mL; 95% CI: 0.4 to 0.9; p<0.001) and genital lesion swabs from men (0.8 log10 copies/mL; 95% CI: 0.3 to 1.2; p=0.001) (Table 2). Subsequently, we graphically examined swabs in which both β-globin and HSV DNA were detected. Overall, the quantity of β-globin DNA detected in HSV positive mixed genital swabs was greater in samples from women than men, and in men the quantity increased with log copies HSV DNA detected (Figure 2a).
Figure 1a. Quantity of β-globin DNA detected on genital swabs by whether HSV DNA was detected genitally (lines show mean shedding quantity).
Table 2. Effect of HSV shedding on quantity of β-globin DNA detected.
| Women | Men | |||
|---|---|---|---|---|
| Site | # swabs | Difference in β-globin when shedding HSV (log10 copies/mL) | # swabs | Difference in β-globin when shedding HSV (log10 copies/mL) |
| Genital lesion | 233 | 0.7 (0.4, 0.9), p<0.001 | 85 | 0.8 (0.3, 1.2), p=0.001 |
| Mixed genital, non-lesion days | 2700 | 0.1 (0.1, 0.2), p=0.001 | 1952 | 0.6 (0.5, 0.7), p<0.001 |
| Oral lesion | 10 | 0.3 (-1.1, 1.7), p=0.65 | 49 | 0.3 (-0.1, 0.8), p=0.13 |
| Mixed oral, non-lesion days | 627 | 0.2 (0.0, 0.4), p=0.083 | 1249 | 0.0 (-0.2, 0.1), p=0.49 |
Figure 2a. β-globin DNA quantification in β-globin-positive and HSV-positive genital swabs.
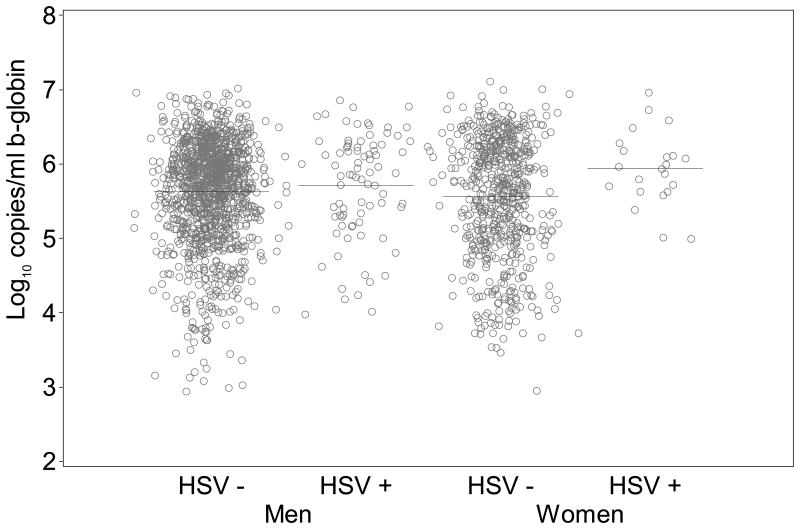
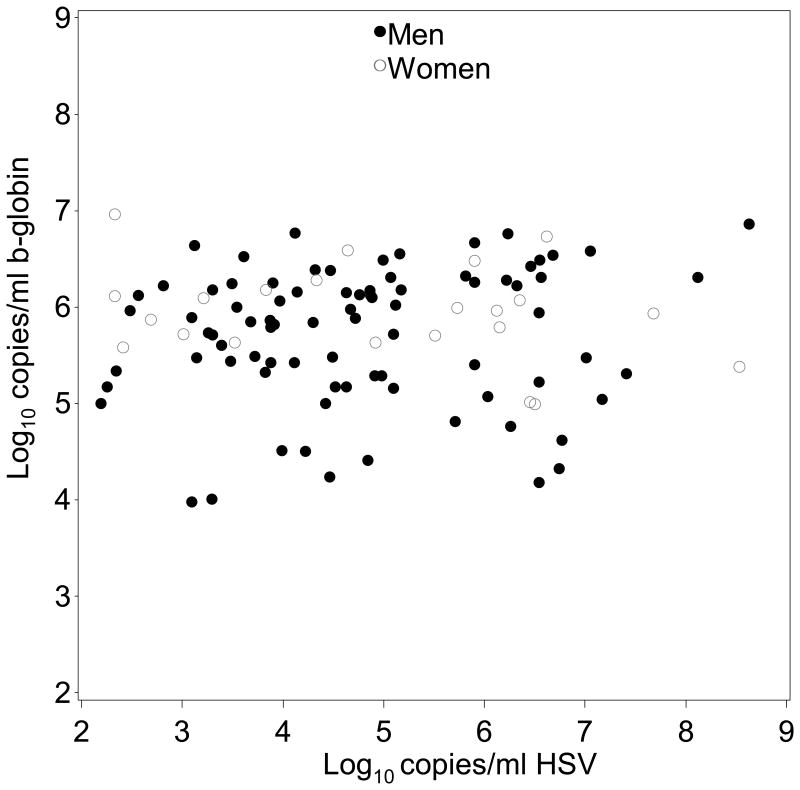
In mixed oral swabs we found a somewhat higher quantity of β-globin DNA detected during HSV shedding versus HSV negative on days without oral lesions from women (0.2 log10 copies/mL; 95% CI: 0.0 to 0.4; p=0.083), but not from men (0.0 log10 copies/mL; 95% CI: -0.2 to 0.1; p=0.49) (Figure 1b). On days when oral lesions were present, no significant differences in quantity of β-globin DNA were detected when HSV was also present in women (0.3 log10 copies/mL; 95% CI: -1.1 to 1.7; p=0.65), or men (0.3 log10 copies/mL; 95% CI: -0.1 to 0.8; p=0.13). The quantity of β-globin DNA detected in HSV positive mixed oral swabs was similar in women and men (Figure 2b).
Figure 1b. Quantity of β-globin DNA detected by whether HSV DNA was detected orally.
Figure 2b. β-globin DNA quantification in β-globin-positive and HSV-positive oral swabs.
Twelve swabs from 7 subjects (6 men) were HSV positive but β-globin negative. Of these, 10 were genital swabs and 2 were oral swabs. Five of these swabs from 4 subjects (all genital) tested positive for β-globin but the quantity of β-globin DNA detected was below the lower limit of quantification (<150 copies/mL).
Discussion
Our study shows that the rate of human β-globin DNA detection in genital and oral samples was very high, suggesting the participants adhered closely to the protocols and collected adequate samples. Using a sensitive Taqman PCR assay, we found β-globin DNA in more than 96% of swabs sampled. The amount of β-globin DNA detected from the genital area was higher in women than in men, and higher when genital (but not oral) HSV DNA was also detected. HSV shedding, as measured by DNA detection in genital and oral swabs, was frequent and differed by anatomic site.
Our results show that genital HSV shedding significantly increased the quantity of β-globin DNA detected. The higher amount of β-globin when HSV was detected in women suggests that injury to the epithelium may occur even in the absence of lesions. Not surprisingly, the amount detected was even higher when lesions were observed. The amount of β-globin DNA detected was higher in women than men likely because obtaining cellular material from the mucosal epithelium of the vagina and vulva is easier than from the keratinized penile shaft. Only 12 swabs (0.2%) had HSV but no β-globin DNA. Given this high level of β-globin detection, there is little reason to routinely test HSV negative swabs for β-globin.
Our findings suggest that self-obtained specimens can reliably be used for the detection of HSV DNA since high β-globin detection rates imply thorough swabbing. Prior studies have studied the utility of self-collected specimens for the diagnosis of sexually transmitted infections (STIs) in clinical and research settings. In cervical cancer screening programs, self-sampling is equivalent to clinician sampling for the detection of human papilloma virus (23). Self-sampling is also comparable to clinic collected samples for the diagnosis of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis (24), and has been used for the diagnosis of Mycoplasma genitalium (25), Human papilloma virus (6), and organisms associated with bacterial vaginosis (26). Importantly, self-sampling facilitates STI testing in a variety of settings where conventional specimen collection is not feasible or acceptable (24, 27). In addition, self-swabbing protocols allow for cost-effective studies of natural history and trials of investigational therapeutics for HSV; in these contexts, daily visits would not be feasible.
The strengths of our study include the large number (>7500) of self-collected samples evaluated for the detection of human β-globin, inclusion of both men and women, and the use of validated, sensitive PCR assays. A limitation of our study is that we evaluated self-sampling in a motivated cohort of research participants that may not reflect the general population. Nevertheless, our results indicate that self-collection of genital specimens is feasible in a clinic-based research studies, and is particularly useful for natural history studies of herpes simplex virus, which reactivates frequently and for brief periods, and thus requires prolonged follow up to assess.
In summary, the high frequency of β-globin detection confirms that participants obtained swabs from the skin and mucosa in the clinical research protocols. The high prevalence of β-globin suggests that continued confirmation of this concept by measuring β-globin in each sample is not necessary. Quantitative measurement of HSV DNA in swabs offers the most cost effective approach to assessing HSV mucosal reactivation.
Acknowledgments
This study was supported through research grants from the National Institutes of Health/NIAID P01 AI 030731 (MLH, SS, LS and AW), K24 AI 071113 (AW) and Fogarty International Center D43 TW000007 (AM).
Footnotes
Conflict of Interest: None declared.
References
- 1.Corey L, Wald A, Celum CL, Quinn TC. The Effects of Herpes Simplex Virus-2 on HIV-1 Acquisition and Transmission: A Review of Two Overlapping Epidemics. J Acquir Immune Defic Syndr. 2004;35(5):435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198(12):1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs MM, van der Pol B, Totten P, et al. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sexually transmitted diseases. 2008;35(1):8–13. doi: 10.1097/OLQ.0b013e31815d968d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magaret AS, Johnston C, Wald A. Use of the designation “shedder” in mucosal detection of herpes simplex virus DNA involving repeated sampling. Sexually transmitted infections. 2009;85(4):270–5. doi: 10.1136/sti.2008.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lunny C, Taylor D, Hoang L, et al. Self-Collected versus Clinician-Collected Sampling for Chlamydia and Gonorrhea Screening: A Systemic Review and Meta-Analysis. PLoS One. 2015;10(7):e0132776. doi: 10.1371/journal.pone.0132776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–83. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 7.Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(Rr-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360(14):1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 9.Lack N, West B, Jeffries D, et al. Comparison of non-invasive sampling methods for detection of HPV in rural African women. Sexually transmitted infections. 2005;81(3):239–41. doi: 10.1136/sti.2004.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haguenoer K, Sengchanh S, Gaudy-Graffin C, et al. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: a randomised trial. Br J Cancer. 2014;111(11):2187–96. doi: 10.1038/bjc.2014.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic HSV-2 seropositive persons. The New England Journal of Medicine. 2000;342:844–50. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 12.Wald A, Corey L, Timmler B, et al. Helicase-primase inhibitor pritelivir for HSV-2 infection. N Engl J Med. 2014;370(3):201–10. doi: 10.1056/NEJMoa1301150. [DOI] [PubMed] [Google Scholar]
- 13.Garland SM, Tabrizi SN. Diagnosis of sexually transmitted infections (STI) using self-collected non-invasive specimens. Sex Health. 2004;1(2):121–6. doi: 10.1071/sh03014. [DOI] [PubMed] [Google Scholar]
- 14.Huggins GR, Preti G. Vaginal odors and secretions. Clin Obstet Gynecol. 1981;24(2):355–77. doi: 10.1097/00003081-198106000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Zegels G, Van Raemdonck GA, Tjalma WA, Van Ostade XW. Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci. 2010;8:63. doi: 10.1186/1477-5956-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Annals of Internal Medicine. 1996;124:8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Wald A, Krantz E, et al. Valacyclovir and Acyclovir for suppression of shedding of Herpes Simplex Virus in the genital tract. J Infect Dis. 2004;190(8):1374–81. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 18.Wald A, Zeh JE, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996;124(1 Pt 1):8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (Immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. Journal of clinical microbiology. 2007;45(5):1618–20. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45(5):1618–20. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. Journal of clinical microbiology. 2002;40(7):2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper DM, Noll WW, Belloni DR, Cole BF. Randomized clinical trial of PCR-determined human papillomavirus detection methods: self-sampling versus clinician-directed--biologic concordance and women's preferences. American Journal of Obstetrics and Gynecology. 2002;186(3):365–73. doi: 10.1067/mob.2002.121076. [DOI] [PubMed] [Google Scholar]
- 24.Knox J, Tabrizi SN, Miller P, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29(11):647–54. doi: 10.1097/00007435-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Wroblewski JK, Manhart LE, Dickey KA, Hudspeth MK, Totten PA. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. Journal of Clinical Microbiology. 2006;44(9):3306–12. doi: 10.1128/JCM.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss RA, Eucker B, Savitz DA, Thorp JM., Jr Diagnosis of bacterial vaginosis from self-obtained vaginal swabs. Infectious Diseases in Obstetrics and Gynecology. 2005;13(1):31–5. doi: 10.1080/10647440400025611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs MM, van der Pol B, Totten P, et al. From the NIH: proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis. 2008;35(1):8–13. doi: 10.1097/OLQ.0b013e31815d968d. [DOI] [PMC free article] [PubMed] [Google Scholar]