Abstract
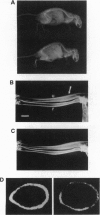
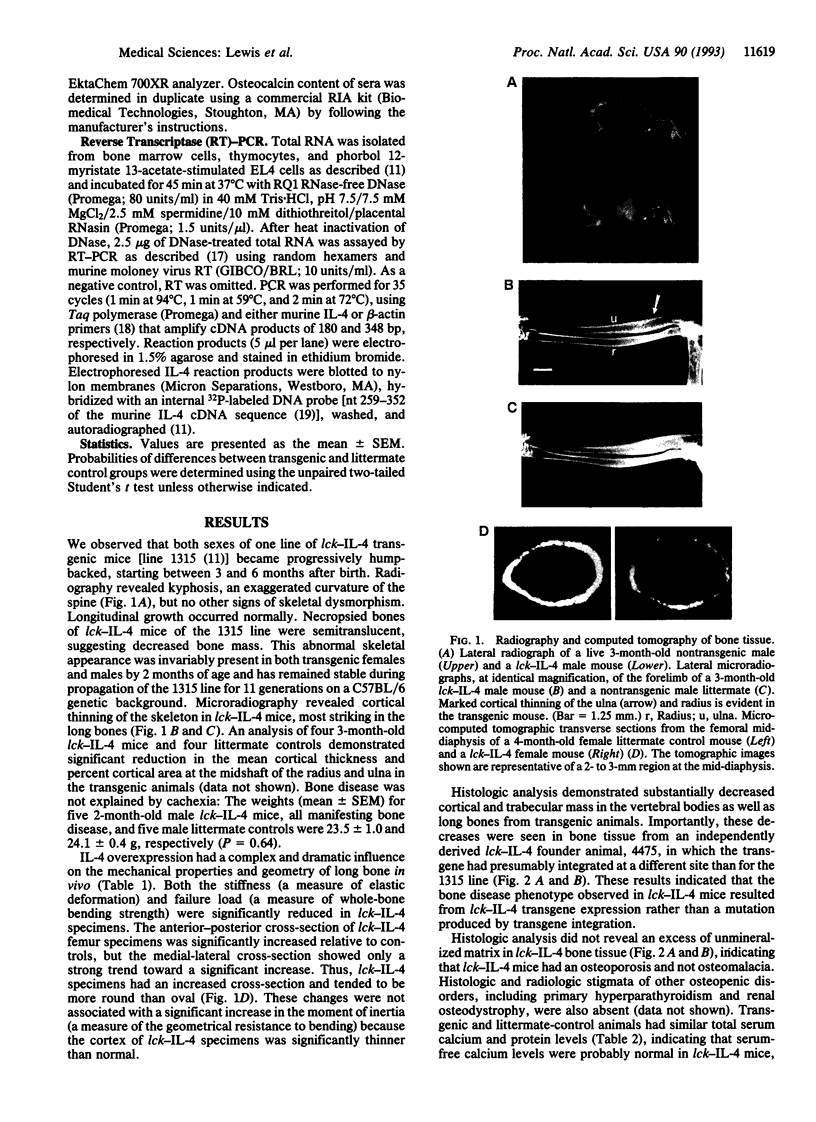
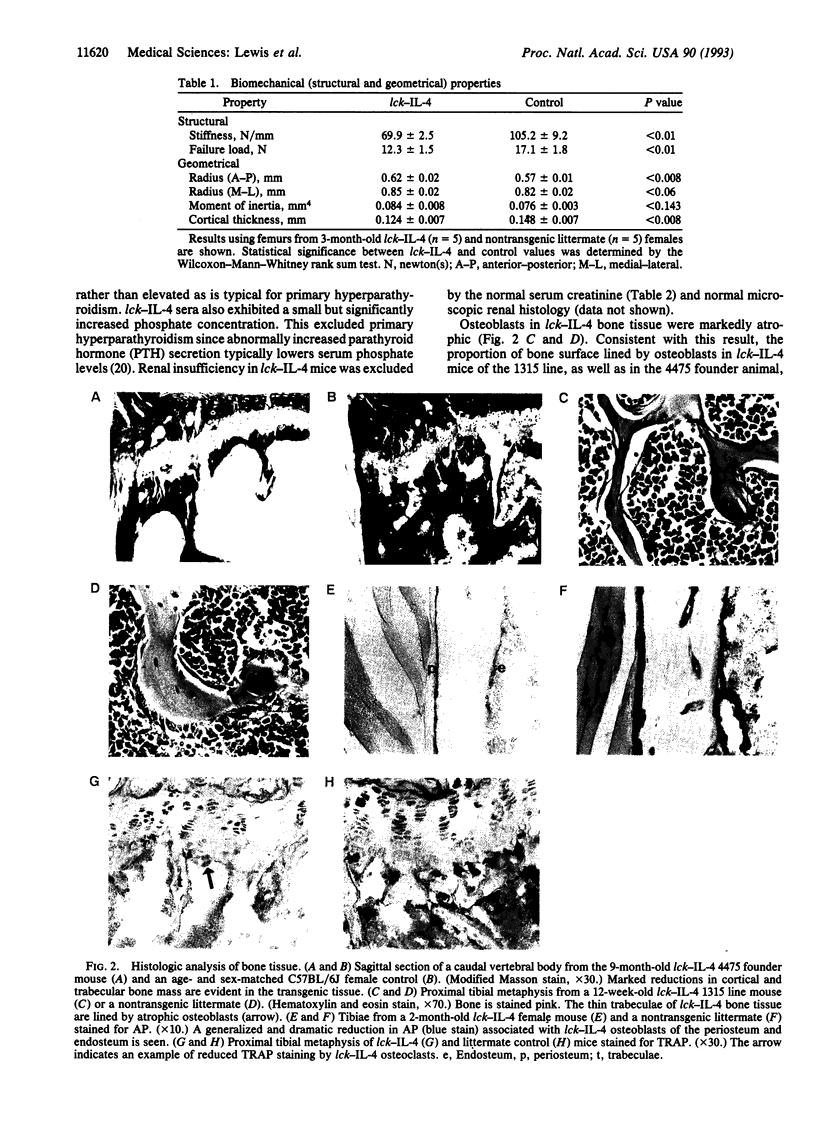
Osteoporosis is a common disease in which loss of bone mass results in skeletal fragility. The development of therapies for this disorder has been hampered by the lack of a convenient animal model. Here we describe a disorder in bone homeostasis in transgenic mice that inappropriately express the cytokine interleukin 4 (IL-4) under the direction of the lymphocyte-specific proximal promoter for the lck gene. Bone disease in lck-IL-4 mice appeared to result from markedly decreased bone formation by osteoblasts, features strikingly similar to those observed in cases of severe low-turnover human involutional osteoporosis. By 2 months of age, female and male lck-IL-4 mice invariably developed severe osteoporosis of both cortical and trabecular bone. Osteoporosis was observed in two independently derived founder animals, indicating that this phenotype was directly mediated by the IL-4 transgene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Jepsen K. J., Mansoura M. K., Jaenisch R., Kuhn J. L., Goldstein S. A. A murine skeletal adaptation that significantly increases cortical bone mechanical properties. Implications for human skeletal fragility. J Clin Invest. 1993 Oct;92(4):1697–1705. doi: 10.1172/JCI116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J., Saunders T. L., Tsai E., Goldstein S. A., Morris-Wiman J., Brinkley L., Dolan D. F., Altschuler R. A., Hawkins J. E., Jr, Bateman J. F. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7145–7149. doi: 10.1073/pnas.87.18.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Fallon M. D., Whyte M. P., Teitelbaum S. L. Systemic mastocytosis associated with generalized osteopenia. Histopathological characterization of the skeletal lesion using undecalcified bone from two patients. Hum Pathol. 1981 Sep;12(9):813–820. doi: 10.1016/s0046-8177(81)80084-6. [DOI] [PubMed] [Google Scholar]
- Garn S. M., Poznanski A. K., Nagy J. M. Bone measurement in the differential diagnosis of osteopenia and osteoporosis. Radiology. 1971 Sep;100(3):509–518. doi: 10.1148/100.3.509. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Ishimi Y., Miyaura C., Jin C. H., Akatsu T., Abe E., Nakamura Y., Yamaguchi A., Yoshiki S., Matsuda T., Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990 Nov 15;145(10):3297–3303. [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Geha R. S. Clinical and immunologic aspects of the hyperimmunoglobulin E syndrome. Hematol Oncol Clin North Am. 1988 Mar;2(1):81–100. [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Forbush K. A., Carpenter J., Sato T. A., Grossman A., Liggitt D. H., Perlmutter R. M. Interleukin 4 expressed in situ selectively alters thymocyte development. J Exp Med. 1991 Jan 1;173(1):89–100. doi: 10.1084/jem.173.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Sanghvi R., Burnell J. M., Howard G. A. Simultaneous demonstration of bone alkaline and acid phosphatase activities in plastic-embedded sections and differential inhibition of the activities. Histochemistry. 1987;86(6):559–565. doi: 10.1007/BF00489547. [DOI] [PubMed] [Google Scholar]
- Marie P. J., Hott M., Garba M. T. Contrasting effects of 1,25-dihydroxyvitamin D3 on bone matrix and mineral appositional rates in the mouse. Metabolism. 1985 Aug;34(8):777–783. doi: 10.1016/0026-0495(85)90030-7. [DOI] [PubMed] [Google Scholar]
- Martin R. B., Atkinson P. J. Age and sex-related changes in the structure and strength of the human femoral shaft. J Biomech. 1977;10(4):223–231. doi: 10.1016/0021-9290(77)90045-8. [DOI] [PubMed] [Google Scholar]
- Murray L. J., Lee R., Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1990 Jan;20(1):163–170. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Brown C., Puscheck E., Friedrich E., Slatopolsky E., Maggio D., McCracken R., Avioli L. V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., Teitelbaum S., Slatopolsky E., McCracken R., Bergfeld M., Lee W., Avioli L. V., Peck W. A. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A. M. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int. 1984;36 (Suppl 1):S37–S45. doi: 10.1007/BF02406132. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991 May 1;77(9):1859–1870. [PubMed] [Google Scholar]
- Price P. A., Parthemore J. G., Deftos L. J. New biochemical marker for bone metabolism. Measurement by radioimmunoassay of bone GLA protein in the plasma of normal subjects and patients with bone disease. J Clin Invest. 1980 Nov;66(5):878–883. doi: 10.1172/JCI109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J., Meunier P. J., Parsons J. A., Bernat M., Bijvoet O. L., Courpron P., Edouard C., Klenerman L., Neer R. M., Renier J. C. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. Br Med J. 1980 Jun 7;280(6228):1340–1344. doi: 10.1136/bmj.280.6228.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B. L., Melton L. J., 3rd The prevention and treatment of osteoporosis. N Engl J Med. 1992 Aug 27;327(9):620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- Shioi A., Teitelbaum S. L., Ross F. P., Welgus H. G., Suzuki H., Ohara J., Lacey D. L. Interleukin 4 inhibits murine osteoclast formation in vitro. J Cell Biochem. 1991 Nov;47(3):272–277. doi: 10.1002/jcb.240470313. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C. Coculture of human peripheral blood mononuclear cells with Trypanosoma cruzi leads to proliferation of lymphocytes and cytokine production. J Immunol. 1992 Jan 1;148(1):239–248. [PubMed] [Google Scholar]
- Whyte M. P., Bergfeld M. A., Murphy W. A., Avioli L. V., Teitelbaum S. L. Postmenopausal osteoporosis. A heterogeneous disorder as assessed by histomorphometric analysis of Iliac crest bone from untreated patients. Am J Med. 1982 Feb;72(2):193–202. doi: 10.1016/0002-9343(82)90810-5. [DOI] [PubMed] [Google Scholar]