Abstract
Attention can be conceptualized as comprising the functions of alerting, orienting, and executive control. Although the independence of these functions has been demonstrated, the neural mechanisms underlying their interactions remain unclear. Using the revised attention network test and functional magnetic resonance imaging, we examined cortical and subcortical activity related to these attentional functions and their interactions. Results showed that areas in the extended frontoparietal network (FPN), including dorsolateral prefrontal cortex, frontal eye fields (FEF), areas near and along the intraparietal sulcus, anterior cingulate and anterior insular cortices, basal ganglia, and thalamus were activated across multiple attentional functions. Specifically, the alerting function was associated with activation in the locus coeruleus (LC) in addition to regions in the FPN. The orienting functions were associated with activation in the superior colliculus (SC) and the FEF. The executive control function was mainly associated with activation of the FPN and cerebellum. The interaction effect of alerting by executive control was also associated with activation of the FPN, while the interaction effect of validity by executive control was mainly associated with the activation in the pulvinar. The current findings demonstrate that cortical and specific subcortical areas play a pivotal role in the implementation of attentional functions and underlie their dynamic interactions.
Keywords: Attentional networks, alerting, orienting, executive control, fMRI
Introduction
Attention refers to the activity of a set of brain networks that influence the priority of information processing for access to conscious awareness (Mackie et al., 2013; Posner and Fan, 2008). It can be conceptualized in specific functional and anatomical terms, with three separable networks of alerting, orienting, and executive control (Petersen and Posner, 2012; Posner and Fan, 2008; Posner and Petersen, 1990). The alerting network is responsible to achieve and maintain phasic and tonic states of readiness in order to process non-specific impending inputs and is associated with activation in the thalamus and a set of frontal and parietal regions, such as dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC) and anterior insular cortex (AI), and areas near or along the intraparietal sulcus (thereafter referred to as IPS) (Fan et al., 2005; Kinomura et al., 1996; Perin et al., 2010), which are part of the extended frontoparietal network (FPN) (Fan, 2014). The orienting network shifts the focus of attention to specific inputs within or among different sensory modalities, and is associated with activation in the frontal eye fields (FEF) and IPS (Corbetta et al., 2002; Corbetta and Shulman, 1998; Fan et al., 2005; Thompson et al., 2005). The executive control network detects and resolves conflict between competing mental processes (Fan et al., 2009; Fan et al., 2002) and is associated with activation in the ACC (Botvinick et al., 2001; Bush et al., 2000; Fan et al., 2005; MacDonald et al., 2000; Matsumoto and Tanaka, 2004), and other areas of the FPN (Fan, 2014). The synergy of the three attentional functions is needed to achieve cognitive control (Mackie et al., 2013), however, the neural substrates underlying the interactions of the attentional networks remains to be clarified.
Although the three attentional networks have been shown to act independently (Fan et al., 2002) and to be associated with distinct neural substrates (Fan et al., 2005), evidence suggests that the attentional networks also interact to influence performance (Callejas et al., 2004; Fan et al., 2009; Wen et al., 2013). Alerting has been shown to interact with executive control, resulting in an increase of the conflict effect (Fan et al., 2009). Orienting enhances the efficiency of executive control (Callejas et al., 2004; Fan et al., 2009; Spagna et al., 2015), and alerting has been shown to influence the behavioral effects of orienting (Callejas et al., 2004; Fuentes and Campoy, 2008; Spagna et al., 2014). However, neuroimaging studies have not yet systematically investigated brain regions and networks that support the interactions of attentional functions.
Much of the neuroimaging literature has focused on the cortical activity associated with the attentional functions. However, animal and human studies have also shown substantial evidence that subcortical regions play a critical role in attention (e.g., Fan et al., 2005; Karnath et al., 2002; Petersen et al., 1987; Rafal and Posner, 1987; Shipp, 2004). Alerting is influenced by the cortical distribution of the noradrenergic (NAergic) system that arises from the locus coeruleus (LC) (Beane and Marrocco, 2004; Marrocco and Davidson, 1998; Moruzzi and Magoun, 1949), a nucleus located in the dorsorostral pons which receives strong descending afferents from prefrontal brain regions such as the ACC (Aston-Jones and Cohen, 2005b). The presentation of a warning signal is often accompanied by activity in the LC (Petersen and Posner, 2012; Posner and Petersen, 1990). Orienting is modulated by cholinergic systems arising in the basal forebrain (Marrocco and Davidson, 1998). Subcortical activity related to the orienting function has been shown in the superior colliculus (SC) in the midbrain, as well as pulvinar and reticular nucleus in the thalamus (Ignashchenkova et al., 2004; Lee and Keller, 2006; Petersen et al., 1987; Salzmann, 1995; Shipp, 2004). Executive control relies on regions associated with the dopaminergic system (Marrocco and Davidson, 1998). The ventral tegmental area (VTA) projects to ACC and lateral prefrontal cortex, areas of the executive control network (Botvinick et al., 2004; Kerns et al., 2004; Raz and Buhle, 2006). Although subcortical regions have been shown to play a critical role in attention, the activation of these areas in attentional networks and their interactions remains to be thoroughly examined.
In this study, we used the revised attention network test (ANT-R) (Fan et al., 2012; Fan et al., 2009) together with functional magnetic resonance imaging (fMRI) to examine the neural substrates underlying the attentional functions and the interactions among them. We focused on identifying the activation of subcortical structures associated with the attentional networks and their interactions. We predicted that there would be substantial involvement of cortical and subcortical regions, such as LC, SC, VTA, and thalamus in the attentional functions and their interactions.
Materials and Methods
Participants
Twenty-four adult volunteers (11 females and 13 males; mean age = 26.3 years; range = 18–49 years) participated in this study. All participants were right-handed and had normal or corrected-to-normal vision with an average estimated intelligence quotient of 115 ± 17. The Institutional Review Board of Icahn School of Medicine at Mount Sinai approved the consent procedure, and written informed consent was obtained from each participant prior to the experimental procedures.
The revised attention network test
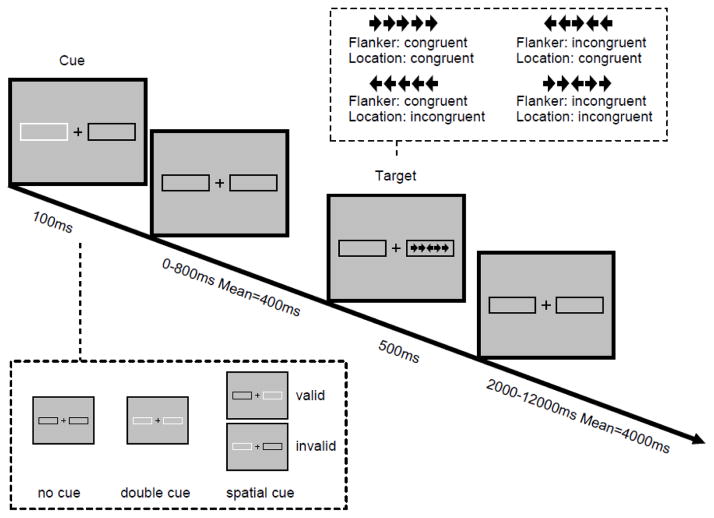
The ANT-R (Fan et al., 2009) was designed to magnify the interactions among the three attentional functions based upon the original task (Fan et al., 2002) by manipulating the validity of spatial cues in order to measure the orienting operations of disengaging and moving + engaging. The details of the ANT-R are illustrated in Figure 1. A central fixation cross and two boxes subtending 4.69° of the visual angle to the left and right of fixation remain visible on the screen throughout the duration of the task. In each trial, depending on the condition, either a transient cue (brightening of the box) is presented for 100 ms (the cued conditions) or the screen remains unchanged (the no cue condition). Three types of cues were used: (1) no cue (no brightening prior to target onset); (2) double cue (brightening of both boxes); and (3) spatial cue (one box brightening prior to target onset). The difference between the double cue and no cue conditions is that the former provides temporal information about the impending target, while in the latter condition no temporal information is provided because no cue is presented. The contrast between these two conditions gives a measure of how temporal information regarding the upcoming target benefits participants’ performance (the alerting effect). The spatial cue provides both temporal and spatial information about the target, and may be valid, indicating the exact position where the target will appear, or invalid, cueing the position opposite to where the target will appear. The contrast between these two conditions gives a measure of how valid spatial information about the upcoming target benefits participant’s performance, compared to a performance cost by invalid spatial information (validity effect). Within the validity effect, two components can be separated: disengaging (invalid cue minus double cue) and moving + engaging (double cue minus valid cue). After a variable duration (either 0, 400, or 800 ms, mean = 400 ms), the central target arrow and two flanker arrows on each side are presented at one of the two possible locations and remain visible for 500 ms. A single arrow subtends 0.58° of visual angle and the contours of adjacent arrows are separated by 0.06° of visual angle, so that the target + flanker array subtends a total of 3.27° of visual angle. Participants are instructed to respond to the direction of the central arrow as quickly and accurately as possible by pressing the left or right response buttons using the left or right index fingers respectively. There are two flanker conditions: the congruent condition with the target and the flankers pointing toward the same direction, and the incongruent condition with the target and the flankers pointing in opposite directions. The contrast between these two conditions (incongruent minus congruent) gives a measure of the cost of distracting stimuli on participants’ performance (the conflict effect). The duration between the offset of the target and the onset of the next trial is jittered systematically, approximating an exponential distribution ranging from 2000 to 12000 ms, with a mean of 4000 ms. The mean trial duration is 5000 ms. There are 12 trials for no cue, 12 trials for double cue, and 48 trials for spatial cue (75% valid and 25% invalid) conditions in each run, with 72 trials in each run. The run duration is 420 seconds. There are 4 runs in total. The total time to complete this task is approximately 30 minutes.
Figure 1.
Schematic of revised Attention Network Test (ANT-R). In each trial, depending on the cue condition (none, double, and valid or invalid cues), a cue box flashes for 100 ms. After a variable duration (0, 400, or 800 ms), the target (the center arrow) and two flanker arrows on both the left and right side (congruent or incongruent) are presented for 500 ms. Participants must indicate the target’s direction. Before the target appears, a cue in the form of a box flashing on one or both sides is displayed. The cue can be valid, which predicts the target position correctly, or invalid, which predicts the opposite position. There is also a double cue condition, in which both boxes flash, to provide temporal but not spatial information, while in the no cue condition no cue is presented. The post-target fixation period varies between 2000 and 12,000 ms. Note: The location congruency manipulation was not treated as a manipulation in data analysis in this study.
Behavioral data analysis
The three attentional networks and their interaction effects were operationally defined (see Table 1) as differences in performance between experimental conditions (Fan et al., 2009). Mean reaction time (RT) for each condition were calculated. Error trials (incorrect and missing responses) were excluded from the mean RT calculation. RT outliers, defined as responses beyond 1700 ms (due to either omission error or long RT), were excluded by the task program. The significance of the effects was tested using one-sample t-tests (one-tailed).
Table 1.
Operational definition of the attentional network effects and interactions for behavior performance
| Testing condition | minus | Reference condition | |
|---|---|---|---|
| Network effects | |||
| Alerting | No cue | Double cue | |
| Disengaging | Invalid cue | Double cue | |
| Moving + Engaging a | Double cue | Valid cue | |
| Validity b | Invalid cue | Valid cue | |
| Conflict | Incongruent | Congruent | |
| Interactions | |||
| Alerting by Flanker Conflict | No cue, incongruent | Double cue, incongruent | |
| minus | minus | ||
| No cue, congruent | Double cue, congruent | ||
| Validity by Flanker Conflict | Invalid cue, incongruent | Valid cue, incongruent | |
| minus | minus | ||
| Invalid cue, congruent | Valid cue, congruent | ||
The “Moving + Engaging” is equivalent to the “orienting” effect originally defined in (Fan et al., 2009). However, here we defined the orienting effect with the disengaging component included, which is the validity effect.
The validity effect = Disengaging + (Moving + Engaging).
Image acquisition
All MRI scans were acquired on a 3 T Siemens Allegra MRI system at the Icahn School of Medicine at Mount Sinai. Each scan run started with two dummy volumes before the onset of the task to allow for equilibration of T1 saturation effects, followed by 168 image volumes. All image volumes were acquired along axial planes parallel to the anterior commissure-posterior commissure (AC-PC) line. A high-resolution T2-weighted anatomical image volume of the whole brain was acquired on an axial plane parallel to the AC–PC line with a turbo spin-echo pulse sequence with the following parameters: 40 axial slices 4-mm thick, skip = 0 mm, repetition time (TR) = 4050 ms, echo time (TE) = 99 ms, flip angle = 170°, field of view (FOV) = 240 mm, matrix size = 448×512, voxel size = 0.47×0.47×4 mm. Four runs of T2*-weighted image volumes were acquired with a gradient echo-planar imaging sequence using the following parameters: 40 axial slices, 4-mm thick and skip = 0 mm, TR = 2500 ms, TE = 27 ms, flip angle = 82°, FOV = 240 mm, matrix size = 64×64, in-plane resolution = 3.75×3.75×4 mm.
Image analysis
Functional MRI preprocessing and the statistical modeling were conducted using the statistical parametric mapping package (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK). Image preprocessing was performed first for each participant: each image volume was realigned to the first volume, slice timing corrected, co-registered to the T2 image, and spatially normalized to the Montreal Neurological Institute (MNI) ICBM152 space based on normalization parameters of the T2 image, resampled to a voxel size of 2×2×2 mm3. To test the experimental effect on brainstem regions (such as LC, SC, and VTA) and thalamus, we also generated another set of normalized images using a 2-stage Automated Brainstem Co-registration (Napadow et al., 2006) to improve brainstem co-registration to the MNI ICBM 152 template. We created a mask of the MNI-152 brainstem (also including surrounding cerebral-spinal fluid voxels) across axial section from z = 13 to z = −57. Voxels inside this mask were set to 1, while other voxels were set to 0. The first stage involved global co-registration of the EPI images to MNI ICBM 152 space based on normalization parameters of the mean EPI image, also resampled to a voxel size of 2×2×2 mm3. The seconded stage involved co-registration of the normalized EPI images to both MNI ICBM 152 EPI template and the EPI template weighted by the brainstem mask. Finally all normalized images were spatially smoothed with an 8×8×8 mm full-width-at-half-maximum Gaussian kernel. To optimize the detection of the subcortical activation, we tested different kernel sizes of 2, 4, and 8 mm. The 8-mm kernel yielded the best power and therefore all results reported below include smoothing with the 8-mm kernel. This is possibly because the large variation in localization of subcortical regions due to individual indifference similar to cortical regions. This suggests that high-resolution scanning may not be the best solution to improve functional imaging of subcortical areas. Other studies have also used a similar kernel size for the detection of subcortical brain activation (Minzenberg et al., 2008; Riva et al., 2011; Tomasi and Volkow, 2014). For the activation in the cortical regions (and surface views in the figures), we reported results based on the whole-brain normalization method, while for the activation in the subcortical regions (and section views in the figures) results are based on the two-stage normalization method described above.
General linear modeling (GLM) was conducted for the functional scans from each participant by regressing the observed event-related blood oxygenation level-dependent (BOLD) signals on task-related regressors to identify the brain regions which show the hemodynamic response as a function of task events (Friston et al., 1994). The regressors were created by convolving a train of delta functions representing the sequence of individual events with the SPM basis function of hemodynamic response (HRF). The regressors included five cue-related hemodynamic responses: double cue, left valid cue, right valid cue, left invalid cue, right invalid cue. Regressors also included 16 target-related hemodynamic responses: four cue conditions (no cue, double cue, valid cue, invalid cue) × two flanker conditions (congruent and incongruent) × two target locations (left and right) (Fan et al., 2012). The six parameters generated during motion correction were entered as covariates. In addition, hemodynamic responses related to error response events for each condition were modeled separately to partial out the error related activity. The effects of the attentional functions were tested by applying linear contrasts to the regressors. The target responses under different cue-by-target conditions were equally weighted for the contrast between congruent and incongruent conditions. As in our previous study (Fan et al., 2012), the attentional network effects were defined differently from the behavioral effects for the contrasts. For the alerting effect, the contrast was defined as double cue compared to baseline. Moving + engaging was defined as valid cue minus double cue. The interaction of alerting by flanker conflict was defined as (double cue, flanker incongruent – double cue, flanker congruent) – (no cue, flanker incongruent – no cue, flanker congruent). The interaction of validity by flanker conflict was defined as (invalid cue, flanker incongruent – invalid cue, flanker congruent) – (valid cue, flanker incongruent – valid cue, flanker congruent).
The contrast images from all participants were entered into a second-level group analysis with random-effects statistical models. For multiple comparison correction, AlphaSim (http://afini.nimh.nih.gov/pub/dist/doc/manual/AphaSim.pdf) was used to determine the extent threshold for a given height threshold with a corrected p value of 0.05. An uncorrected p value of 0.01 for the height (intensity) threshold of each activated voxel and a threshold of extent cluster size k > 191 of 2×2×2 mm voxels were applied. This threshold is relatively liberal given that this study is more hypothesis-driven and based on a priori knowledge (rather than exploratory) regarding the brain regions involved in the attentional functions. Therefore, we believe this is a good balance in terms of minimizing Type I error, and having sufficient power to detect brain activity and connectivity. For the hypothesis-driven subcortical regions (such as LC and SC), which are much smaller than cortical regions (DuBois and Cohen, 2000; Keren et al., 2009), we used a more liberal height threshold of p < 0.01 uncorrected (without extent threshold) because the extent threshold estimated by AlphaSim was much larger than the volumes of these structures. The localization of the LC, SC, and VTA were referenced to previous MRI studies (D’Ardenne et al., 2008; Katyal et al., 2010; Keren et al., 2009; Minzenberg et al., 2008; Murphy et al., 2014). The conjunctions of the activation for the different attentional effects were also examined to reveal shared brain regions/networks between different attentional functions. An uncorrected p value of 0.01 for the conjunction was used with the same extent threshold as mentioned above.
Region of interest analysis
Based on the second-level analyses, three subcortical regions identified in the activation maps were chosen as the regions of interest (ROI): LC (2, −34, −20) for the alerting effect and SC for disengaging ([−8, −24, −4] for left SC and [8, −24, −4] for right SC) effects of orienting. The coordinates were close to the local maxima, with adjustments based on the references of previous studies. However, in the tables we listed the coordinates of local maxima of the clusters. The first eigenvariate of voxels in corresponding contrast images, which passed the height threshold inside the cluster and also inside the sphere around the activation peak, were extracted for each participant. That is, voxels included in the ROI satisfied two conditions in that they were: (1) located inside the sphere; and (2) nearby the activation peak of a specific brain structure. This method balances the Type I error with achieving sufficient power to detect activation in subcortical structures. The radiuses was 4-mm for the LC and 3-mm for the left and right SC, because left and right LC clusters were too close to be separated into two ROIs under current resolution. There were 14 voxels in the LC ROI for the alerting effect and 33 voxels in the SC ROI for the disengaging effect. Pearson’s correlation analyses were conducted to examine the relationship between behavioral effects and brain activation in the corresponding ROI.
Results
Behavioral results
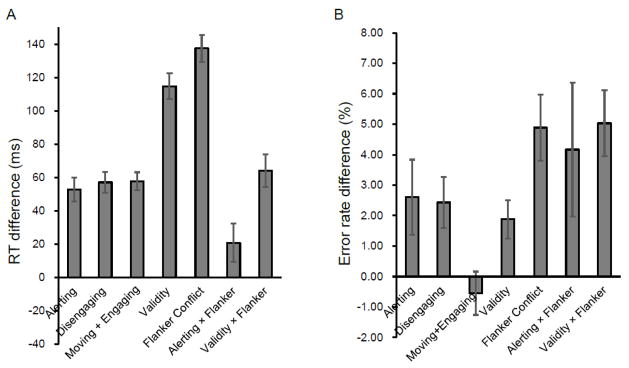
The overall mean RT was 710 ms (SD = 115 ms) and the overall error rate of the task performance was 4.41% (SD = 3.94%). Figure 2 shows the attentional network effects in RT and error rate. The alerting effect was significant for both RT (M ± SD = 53 ± 35 ms, t(23) = 7.39, p < 0.01) and error rate (2.60 ± 6.03 %), t(23) = 2.12, p < .05. The disengaging effect was significant for both RT (57 ± 30 ms, t(23) = 9.21, p < 0.01) and error rate (2.43 ± 4.11%, t(23) = 2.90, p < 0.01). The moving + engaging effect was significant for RT (58 ± 27 ms, t(23) = 10.62, p < 0.01) but not for error rate (−0.55 ± 3.50%, t(23) = −0.77, n.s.). The validity effect was significant for both RT (115 ± 38 ms, t(23) = 14.73, p < 0.01) and error rate (1.88 ± 3.08 %, t(23) = 2.99, p < 0.01). The flanker conflict effect was significant for both RT (138 ± 40 ms t(23) = 17.00, p < 0.01), and error rate (4.89 ± 5.30%, t(23) = 4.52, p < 0.01). The alerting by flanker conflict interaction effect was significant for RT (21 ± 56 ms, t(23) = 1.81, p < 0.05) and error rate (4.17 ± 10.78%, t(23) = 1.89, p < 0.05). The validity by flanker conflict interaction was significant for both RT (64 ± 48 ms, t(23) = 6.53, p < 0.01) and error rate (5.03 ± 5.32%, t(23) = 4.64, p < 0.01).
Figure 2.
Attentional effects and interactions in terms of (A) reaction time (RT in ms), and (B) error rate (%). Error bars represent the standard error of the mean.
fMRI results
Activation associated with the alerting effect
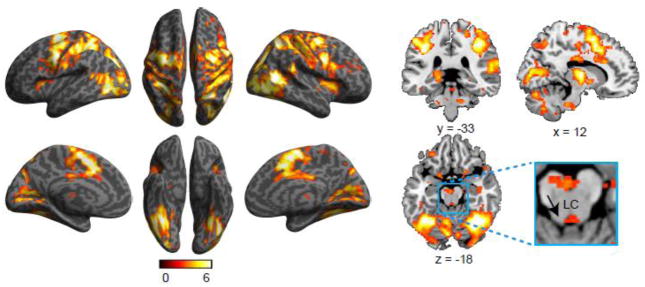
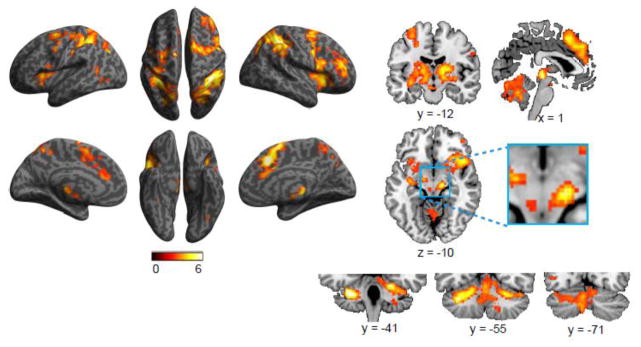
Figure 3 shows bilateral activation related to the alerting effect in ACC, AI, FEF, temporoparietal junction (TPJ), IPS, precentral and postcentral gyri, and other occipital regions. Activation of subcortical regions was also found in thalamus, putamen, LC, and cerebellum (see Table 2). The correlation between LC activation and the behavioral alerting effect was not significant (r = −0.24, p = 0.18, one-tailed). For fMRI of subcortical structures in attentional functions, caution is warranted due to the limitations in localizing these structures in fMRI. For example, the LC cluster we localized is more medial than the actual structure. Due to fMRI data acquisition distortion and signal loss, and most importantly individual differences in terms of localization, it is difficult to localize the activated voxels in the small anatomically defined ROIs.
Figure 3.
Brain regions showing increased activation associated with the alerting effect.
Table 2.
Activation associated with the alerting effect
| Region | L/R | BA | x | y | z | T | Z | K |
|---|---|---|---|---|---|---|---|---|
| Anterior cingulate cortex | L | 24 | 0 | −6 | 52 | 11.81 | 6.64 | 41914 |
| Inferior occipital gyrus | L | 19 | −40 | −66 | −6 | 9.21 | 5.90 | |
| Precentral gyrus | L | 6 | −58 | 4 | 32 | 9.09 | 5.87 | |
| Mid temporal gyrusa | R | 37 | 56 | −58 | 10 | 8.61 | 5.70 | |
| Fusiform gyrus | R | 37 | 34 | −52 | −16 | 8.50 | 5.66 | |
| Superior occipital gyrus | L | 18 | −22 | −70 | 28 | 8.11 | 5.52 | |
| Superior frontal gyrusb | L | 6 | −26 | −8 | 62 | 8.04 | 5.49 | |
| Precentral gyrus | R | 6 | 54 | 6 | 34 | 7.99 | 5.47 | |
| Inferior parietal lobule | L | 7 | −26 | −50 | 54 | 7.69 | 5.36 | |
| Precentral gyrusc | R | 6 | 30 | −8 | 54 | 7.63 | 5.34 | |
| Superior occipital gyrus | R | 19 | 26 | −74 | 26 | 7.39 | 5.24 | |
| Superior parietal lobule | R | 7 | 24 | −58 | 52 | 7.31 | 5.20 | |
| Anterior cingulate cortex | L | 32 | −8 | 12 | 38 | 7.27 | 5.19 | |
| Supramarginal gyrus | L | 40 | −38 | −38 | 36 | 7.14 | 5.13 | |
| Mid occipital gyrus | R | 19 | 34 | −82 | 2 | 6.91 | 5.03 | |
| Postcentral gyrus | L | 3 | −56 | −22 | 48 | 6.84 | 5.00 | |
| Postcentral gyrus | R | 3 | 46 | −26 | 46 | 6.55 | 4.87 | |
| Cerebellum | L | −24 | −50 | −28 | 6.40 | 4.80 | ||
| Cerebellum | L | −6 | −68 | −22 | 6.33 | 4.77 | ||
| Thalamus | R | 12 | −10 | 6 | 6.31 | 4.76 | ||
| Thalamus | L | −14 | −18 | 8 | 6.23 | 4.72 | ||
| Anterior insula | R | 34 | 12 | 0 | 5.51 | 4.35 | ||
| Precuneus | R | 17 | 20 | −54 | 6 | 5.47 | 4.33 | |
| Mid occipital gyrus | L | 18 | −26 | −92 | 0 | 5.39 | 4.29 | |
| Precuneus | L | 18 | −14 | −56 | 4 | 5.26 | 4.22 | |
| Calcarine cortex | R | 17 | 6 | −82 | 2 | 5.00 | 4.07 | |
| Pons | R | 10 | −20 | −44 | 5.00 | 4.07 | ||
| Putamen | L | −18 | 10 | −10 | 4.92 | 3.97 | ||
| Supramarginal gyrusd | R | 40 | 56 | −38 | 26 | 4.25 | 3.61 | |
| Rolandic operculum | R | 43 | 56 | −14 | 20 | 4.18 | 3.57 | |
| Cerebellum | R | 8 | −58 | −42 | 4.01 | 3.45 | ||
| Putamen | R | 22 | 12 | 2 | 4.00 | 3.45 | ||
| Cerebellum | R | 26 | −34 | −40 | 3.80 | 3.31 | ||
| Mid frontal gyrus | R | 24 | 36 | 24 | 3.62 | 3.18 | ||
| Precentral gyrus | L | 4 | −2 | −38 | 54 | 3.16 | 2.85 | |
| Anterior insula | L | −30 | 14 | 8 | 5.70 | 4.45 | 581 | |
| Locus coeruleus | 2 | −34 | −20 | 2.81 | 2.58 | 14 |
Note: Structures listed below the cluster with a k value were within the same cluster with different local maxima.
Extends to the temporal parietal junction;
Close to left frontal eye field;
Close to right frontal eye field;
Temporal parietal junction.
Activation associated with the orienting effects
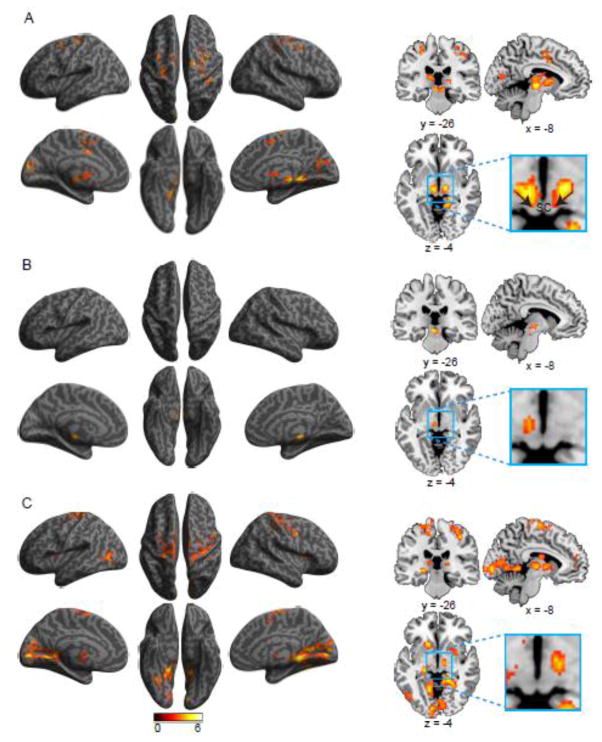
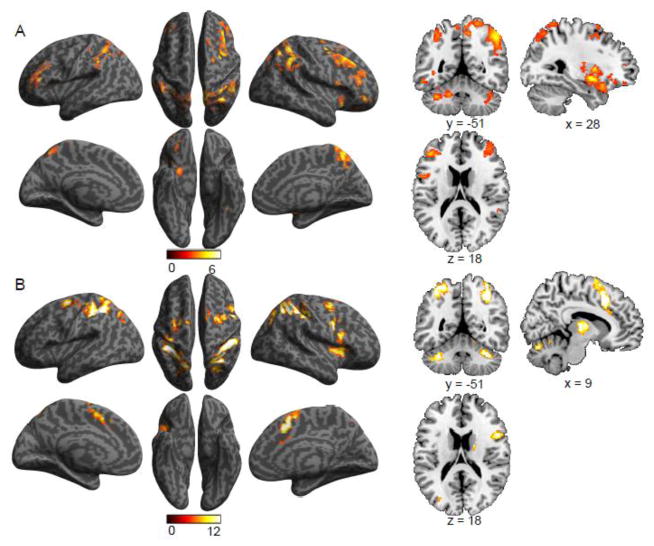
Figures 4A–C, and Table 3, show activation related to the disengaging, moving + engaging, and validity effects, respectively. The activation for disengaging was found in FEF bilaterally, left ACC, right superior frontal gyrus, left precentral gyrus, right precentral and postcentral gyri, right parahippocampal gyrus, left cuneus, right precuneus, right calcarine cortex, and subcortically in bilateral thalamus extending to SC and left caudate nucleus. Moving + engaging was only associated with left red nucleus. The validity effect was associated with activation in parahippocampal gyrus bilaterally, right lingual gyrus, right fusiform gyrus, and other frontal and parietal brain regions including both FEF, in addition to the thalamus bilaterally, right putamen, and left caudate nucleus. The correlation between SC activation and the behavioral disengaging effect was not significant (r = 0.05, p = 0.41, one-tailed). The localization of SC, as shown in the figure, is more toward the pretectal nucluei rather than the tectal nuclei. This may be due to inaccurate co-registration of some subjects resulting in cutting the activation in the tectal nuclei. It is also possible that the activation is in the pretectal nuclei that are related to gaze-shift and eye movement.
Figure 4.
Brain regions showing increased activation associated with the (A) disengaging, (B) moving + engaging, and (C) validity effects of orienting.
Table 3.
Activation associated with the orienting effects
| Region | L/R | BA | x | y | z | T | Z | K |
|---|---|---|---|---|---|---|---|---|
| Disengaging | ||||||||
| Parahippocampal gyrus | R | 27 | 14 | −38 | −4 | 6.10 | 4.66 | 209 |
| Superior colliculus | L | −10 | −20 | −2 | 6.02 | 4.62 | 1541 | |
| Thalamusa | L | −14 | −20 | 10 | 5.40 | 4.29 | ||
| Superior colliculus | R | 12 | −16 | −4 | 5.30 | 4.24 | ||
| Pulvinar | R | 16 | −20 | 8 | 4.90 | 4.01 | ||
| Caudate nucleus | L | −10 | 8 | 6 | 4.37 | 3.69 | ||
| Thalamus | R | 10 | −22 | 4 | 3.98 | 3.44 | ||
| Precentral gyrus | L | 6 | −26 | −26 | 64 | 5.05 | 4.10 | 295 |
| Anterior cingulate cortex | L | 24 | −6 | 2 | 38 | 4.77 | 3.94 | 715 |
| Precentral gyrus | R | 6 | 34 | −2 | 46 | 4.08 | 3.50 | |
| Mid frontal gyrusb | L | 6 | −30 | −2 | 54 | 3.90 | 3.38 | |
| Superior frontal gyrus | R | 6 | 18 | −12 | 64 | 4.67 | 3.88 | 541 |
| Postcentral gyrus | R | 3 | 40 | −32 | 48 | 4.33 | 3.66 | |
| Cuneus | L | 18 | −8 | −90 | 14 | 4.17 | 3.56 | 275 |
| Precuneus | R | 31 | 4 | −72 | 28 | 2.91 | 2.66 | |
| Calcarine cortex | R | 18 | 16 | −76 | 18 | 3.94 | 3.41 | 320 |
| Moving + Engaging | ||||||||
| Red nucleus | −2 | −24 | −12 | 6.00 | 4.61 | 284 | ||
| Validity | ||||||||
| Calcarine cortex | L | 17 | −8 | −86 | 0 | 6.97 | 5.06 | 5048 |
| Parahippocampal gyrus | R | 27 | 14 | −38 | −8 | 6.36 | 4.78 | |
| Calcarine cortex | R | 17 | 22 | −58 | 8 | 5.10 | 4.13 | |
| Parahippocampal gyrus | L | 27 | −10 | −42 | 0 | 5.08 | 4.12 | |
| Thalamus | R | 14 | −18 | 10 | 4.58 | 3.82 | ||
| Thalamus | L | −10 | −16 | 4 | 4.28 | 3.64 | ||
| Fusiform gyrus | R | 37 | 32 | −58 | −14 | 4.22 | 3.59 | |
| Cuneus | R | 19 | 12 | −84 | 22 | 3.71 | 3.25 | |
| Caudate nucleus | L | −10 | −6 | 20 | 3.77 | 3.29 | ||
| Putamen | L | −20 | 8 | 10 | 3.31 | 2.97 | ||
| Putamen | R | 28 | 18 | 8 | 2.90 | 2.65 | ||
| Lingual gyrus | R | 18 | 18 | −80 | −10 | 2.66 | 2.46 | |
| Postcentral gyrus | L | 3 | −26 | −30 | 70 | 4.62 | 3.85 | 1290 |
| Supplementary motor area | R | 6 | 8 | −8 | 68 | 4.15 | 3.55 | |
| Precentral gyrus | L | 6 | −26 | −14 | 52 | 3.14 | 2.84 | |
| Inferior occipital gyrus | L | 19 | −40 | −72 | 2 | 4.60 | 3.83 | 341 |
| Precentral gyrus | R | 4 | 52 | −2 | 38 | 4.46 | 3.75 | 916 |
| Postcentral gyrus | R | 3 | 36 | −32 | 50 | 3.99 | 3.44 | |
| Precentral gyrus | R | 4 | 20 | −30 | 68 | 3.44 | 3.06 | |
| Precentral gyrus | R | 6 | 30 | −8 | 60 | 3.44 | 3.05 | |
Note: Structures listed below the cluster with a k value were within the same cluster with different local maxima.
Extends to the pulvinar;
Close to frontal eye field.
Activation associated with the flanker conflict effect
Figure 5 shows FPN activation related to the executive control function, including ACC (peaked at right), AI, FEF, IPS, precentral gyrus bilaterally, and right middle and left inferior occipital cortex. Activation was also found in subcortical regions including bilateral thalamus (including pulvinar and extending to SC) and caudate nucleus, and regions in cerebellum including somatomotor regions of the cerebellum and the vermis (see the right bottom panel of Figure 5). We did not find activation specifically within the VTA, but in other nearby midbrain structures (see the enlarged section of the axial slice of Figure 5).
Figure 5.
Brain regions showing increased activation associated with the flanker conflict effect.
Activation associated with the interaction and conjunction of alerting and flanker conflict effect
Figure 6 and Table 5 show activity associated with the interaction and conjunction of the alerting and flanker conflict effects. The interaction of alerting by flanker conflict was related to the activation of bilateral inferior and middle frontal gyri, IPS bilaterally, right insula, and subcortical regions of right putamen and regions of the cerebellum (Figure 6A). Conjunction analysis revealed that alerting and flanker conflict shared activation in bilateral ACC, bilateral thalamus, right AI, bilateral FEF, bilateral IPS, and regions of the cerebellum (Figure 6B).
Figure 6.
Brain regions showing increased activation associated with (A) the alerting by flanker conflict interaction effect, and (B) the conjunction of alerting and flanker conflict effects.
Table 5.
Activation associated with the interaction alerting and flanker conflict effects and the conjunction of the alerting and flanker conflict effects
| Region | L/R | BA | x | y | z | T | Z | K |
|---|---|---|---|---|---|---|---|---|
| Interaction | ||||||||
| Inferior frontal gyrus | L | 45 | −40 | 28 | 10 | 5.81 | 4.51 | 707 |
| Mid frontal gyrus | L | 9 | −44 | 24 | 34 | 3.39 | 3.02 | |
| Putamen | R | 30 | 2 | −8 | 5.64 | 4.42 | 1058 | |
| Superior parietal lobule | R | 40 | 38 | −52 | 54 | 5.31 | 4.24 | 4304 |
| Precuneus | R | 7 | 8 | −54 | 54 | 5.20 | 4.19 | |
| Superior parietal lobule | L | 40 | −40 | −48 | 58 | 4.07 | 3.50 | |
| Inferior parietal lobule | R | 40 | 58 | −44 | 40 | 4.03 | 3.47 | |
| Supramarginal gyrus | L | 40 | −48 | −42 | 30 | 3.65 | 3.21 | |
| Mid occipital gyrus | R | 19 | 38 | −72 | 32 | 3.54 | 3.13 | |
| Superior parietal lobule | L | 7 | −20 | −66 | 52 | 3.22 | 2.89 | |
| Mid frontal gyrusa | R | 6 | 34 | 0 | 48 | 4.93 | 4.03 | 1966 |
| Mid frontal gyrus | R | 9 | 38 | 26 | 36 | 4.64 | 3.86 | |
| Inferior frontal gyrus | R | 45 | 44 | 36 | 12 | 3.87 | 3.36 | |
| Inferior frontal gyrus | R | 44 | 52 | 12 | 14 | 3.67 | 3.22 | |
| Superior frontal gyrus | R | 8 | 18 | 16 | 58 | 3.16 | 2.85 | |
| Cerebellum | L | −16 | −42 | −46 | 4.40 | 3.71 | 886 | |
| Cerebellum | L | −36 | −70 | −26 | 3.75 | 3.28 | ||
| Lateral orbital gyrus | R | 47 | 38 | 46 | −8 | 4.37 | 3.69 | 267 |
| Cerebellum | R | 28 | −70 | −24 | 4.25 | 3.61 | 191 | |
| Cerebellum | R | 36 | −48 | −26 | 4.11 | 3.52 | 211 | |
| Conjunction | ||||||||
| Superior parietal lobule | L | 40 | −36 | −44 | 56 | 33.60 | 5.35 | 4016 |
| Anterior cingulate cortex | L | 32 | 2 | 10 | 48 | 28.50 | 4.95 | |
| Superior frontal gyrusb | L | 6 | −20 | −6 | 58 | 25.48 | 4.69 | |
| Superior frontal gyrus | R | 6 | 12 | 0 | 70 | 12.32 | 3.24 | |
| Superior parietal lobule | L | 19 | −26 | −70 | 30 | 11.79 | 3.16 | |
| Anterior cingulate cortex | R | 32 | 10 | 22 | 26 | 10.94 | 3.04 | |
| Superior parietal lobulec | R | 40 | 40 | −38 | 48 | 32.23 | 5.25 | 2214 |
| Superior parietal lobuled | R | 7 | 28 | −58 | 54 | 24.43 | 4.59 | |
| Thalamus | L | −14 | −16 | 4 | 23.97 | 4.55 | 248 | |
| Precentral gyrus | R | 44 | 52 | 12 | 30 | 22.11 | 4.37 | 2380 |
| Thalamus | R | 18 | −18 | 10 | 22.09 | 4.37 | ||
| Mid frontal gyruse | R | 6 | 30 | 0 | 56 | 21.49 | 4.31 | |
| Insula | R | 34 | 16 | 0 | 21.47 | 4.31 | ||
| Cerebellum | L | −26 | −56 | −28 | 21.57 | 4.32 | 968 | |
| Vermis | L | 0 | −54 | −18 | 12.69 | 3.29 | ||
| Cerebellum | L | −6 | −72 | −34 | 11.05 | 3.05 | ||
| Cerebellum | R | 32 | −46 | −28 | 16.49 | 3.77 | 288 | |
Note: Structures listed below the cluster with a k value were within the same cluster with different local maxima.
Includes frontal eye fields;
Areas near or along the intraparietal sulcus.
Activation associated with the interaction and conjunction of validity and flanker conflict effect
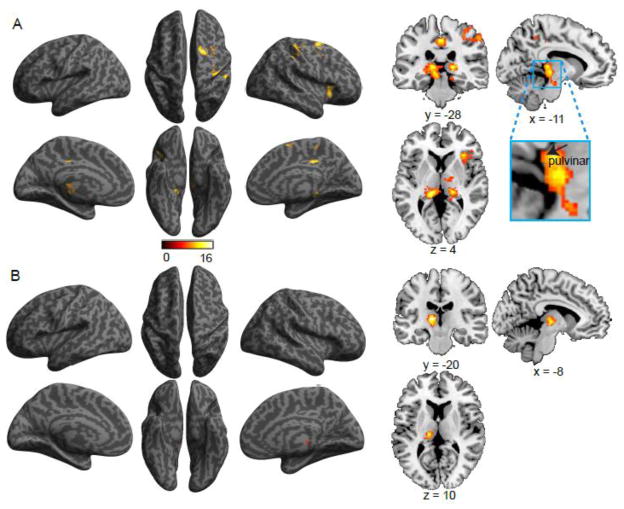
The interaction of validity by flanker conflict was related to the activation of the right AI, right superior frontal gyrus, right postcentral gyrus, and bilateral pulvinar (see Figure 7A and Table 6). The validity and flanker conflict conjunction was related to the activation of left thalamus (extending to pulvinar) (Figure 7B and Table 6).
Figure 7.
Brain regions showing increased activation associated with (A) the validity by flanker conflict interaction effect, and (B) the conjunction of validity and flanker conflict effects.
Table 6.
Interaction and conjunction of validity and flanker conflict effects
| Region | L/R | BA | x | y | z | F | Z | K |
|---|---|---|---|---|---|---|---|---|
| Interaction | ||||||||
| Pulvinar | L | −12 | −30 | 6 | 6.25 | 4.73 | 685 | |
| Posterior cingulate cortex | 23 | 0 | −26 | 42 | 5.24 | 4.21 | 268 | |
| Pulvinar | R | 18 | −28 | 4 | 5.03 | 4.09 | 342 | |
| Anterior insula | R | 32 | 22 | 6 | 4.95 | 4.04 | 499 | |
| Superior frontal gyrusa | R | 6 | 18 | 0 | 60 | 4.52 | 3.79 | 294 |
| Postcentral gyrus | R | 3 | 48 | −26 | 46 | 3.74 | 3.27 | 312 |
| Conjunction | ||||||||
| Thalamusb | L | −16 | −20 | 6 | 17.94 | 3.94 | 248 | |
Includes frontal eye fields;
extends to pulvinar.
Discussion
In this study, beyond associating cortical activation with the independent alerting, orienting, and executive control functions as in our previous study (Fan et al., 2005), we identified cortical and subcortical regions supporting the attentional functions and the interactions among them. These results expand upon previous knowledge about the brain networks involved in implementing attentional functions, and show that the recruitment of areas of the FPN together with subcortical brain regions underlies dynamic interactions of attentional functions to achieve cognitive/attentional control.
Cortical and subcortical contributions to the attentional functions
The alerting function
Consistent with previous theoretical (Petersen and Posner, 2012) and empirical work (Clerkin et al., 2009; Rajkowski et al., 2004; Ramos and Arnsten, 2007), we found that activation of the LC was related to the alerting effect (Aston-Jones and Cohen, 2005a; Petersen and Posner, 2012). Activation of the LC-noradrenergic system is thought to serve as a temporal “attentional filter” to facilitate goal-relevant information processing and response by modulating the responsiveness of cortical regions responsible for task performance (Aston-Jones and Cohen, 2005b; Aston-Jones et al., 2000; Morrison and Magistretti, 1983; Sara, 2009; Sara and Bouret, 2012). This increases the signal-to-noise ratio and consequently signal detection (Servan-Schreiber et al., 1990).
In addition to areas previously identified for the alerting network (Fan et al., 2005), such as thalamus and TPJ, activation was also observed in the ACC, AI, IPS, and other frontal and parietal sites of the FPN. The influence of ACC on alerting to modulate behavioral responsiveness has been previously suggested (Aston-Jones and Cohen, 2005b). These regions of the FPN are involved in attentional/cognitive control of information processing and are related to response anticipation (Fan, 2014; Fan et al., 2007a). The alerting cue carries the temporal information about the target onset, triggering the activation of FPN and other subcortical regions for the preparation of response. The AI, in addition to ACC, has a distinct functional role in monitoring baseline uncertainty (Fan et al., 2014). Also, in a recent study we found that TPJ is a necessary region in the interaction between bottom-up and top-down attentional control (Wu et al., 2015). Therefore, the alerting function is not only related to the arousal function of thalamus, but is implemented by a large brain network that supports timing, response preparation, and other functions of warning signals.
The orienting functions
Activation of the SC was associated with the disengaging component of orienting functions, consistent with its previously identified role as a critical structure in orienting of attention (Gitelman et al., 2002) and saccadic eye movements (Wurtz et al., 1982). The orienting functions are modulated by acetylcholine (Petersen and Posner, 2012; Posner and Petersen, 1990), and the SC is highly innervated by cholinergic inputs (Hall et al., 1989; Harting et al., 1991). SC activity can typically be modulated by gaze or covert visual shifts of attention (Krauzlis et al., 2013; Ngan et al., 2015). In this study, we were able to differentiate the activation associated with the orienting components by refining the operational definitions to include the disengaging and moving + engaging components of orienting. The SC has also been implicated in the disengaging component of orienting, most recently demonstrated at the neuronal level (Ngan et al., 2015). In addition to the ACC, the FEF was also involved in the disengaging component of orienting. For the moving + engaging component of orienting, we only found activation in the left red nucleus. Its role in orienting of attention is not clear, but may be related to the voluntary movement of attention towards the cued location and/or engaging to the cued location. For the validity effect, which is a combination of the two components of orienting, we also found the FEF involvement in addition to early visual areas and subcortical structures of thalamus and basal ganglia.
The executive control function
The dopaminergic system has been associated with the executive control of attention (Fan et al., 2003). The VTA is one of the two major dopamine sources in the brain, with wide projections to cortical and subcortical regions (Amalric and Koob, 1993; Chudasama and Robbins, 2004; Smith and Kieval, 2000; Tzschentke, 2001). However, we did not find activation in the VTA, possibly due to the scanner parameters that were not optimum to image such a small structure. Overall, our result is in line with our information theory account of cognitive control (Fan, 2014), which proposes that areas within the FPN, such as ACC, FEF, and IPS, dynamically interact to incorporate the functions of cognitive control.
Activation in the lobule VI bilaterally, and anterior and posterior lobules of the cerebellum were also associated with the flanker conflict effect, which is consistent with the previous studies showing cerebellar contribution to the inhibition of prepotent responses (Bellebaum and Daum, 2007) and in attention (Fan et al., 2003). Resting state functional connectivity has revealed that in addition to brain networks associated with motor function, the frontoparietal, ventral, and dorsal attention systems (among others) are also functionally connected to discrete cerebellar regions (Buckner et al., 2011). The task-related activation of the cerebellum for the executive control function found in this study confirms the involvement of cerebellum in attentional functions.
The involvement of thalamus and basal ganglia in attentional functions
While the recruitment of subcortical structures has been described in the context of individual attentional functions, this study identified the thalamus as a common structure that was involved in each of the attentional functions. The involvement of the intralaminar thalamic region and reticular nucleus for the alerting (Morrison and Magistretti, 1983), dorsal pulvinar, oculomotor thalamus and caudal intralaminar nuclei for the orienting (Murphy et al., 2014; Rafal and Posner, 1987), and more broadly of the thalamus for the executive control functions (Fan et al., 2005; Perin et al., 2010; Yanaka et al., 2010) has been previously demonstrated. The majority of input to cortical areas is routed through the thalamus (Scholey, 2002), which has also been increasingly appreciated as a critical structure in cognition, beyond its earlier simplified definition as a relay’ structure, with a role in attention posited several decades ago as the basis of the attentional searchlight’ (Crick, 1984).
The involvement of the basal ganglia in the orienting functions was demonstrated in the present study and is consistent with previous resting state connectivity evidence that the putamen is associated with the ventral attention system, while the caudate nucleus is associated with the FPN (Choi et al., 2012). These structures receive input from almost every brain region, and have been demonstrated to play an attentional role in both the enhancement of task-relevant information processing and the inhibition of task-irrelevant processing (van Schouwenburg et al., 2015).
Interactions of attentional networks
One of the important findings of this study is the involvement of FPN in attentional functions of alerting and executive control, although this is not surprising. Here the FPN is defined more broadly than in Petersen and Posner (2012) and includes the ACC, AI, and thalamus of the cingulo-opercular network (Dosenbach et al., 2008). Within the FPN, ACC and AI are involved in baseline uncertainty processing (Fan et al., 2014). The involvement of FPN in the alerting and executive control functions is supported by the identified brain regions of FPN (frontal and parietal regions), as well as occipital regions and putamen and cerebellum, associated with the interaction effect between these attentional functions. It is further supported by activation in bilateral thalamus, bilateral ACC, right insula, and parts of FPN found for the conjunction of alerting and flanker conflict effects, indicating a partial overlap in the neural substrate supporting these two functions.
We previously observed that behaviorally, alerting interacts with the executive control function indicated by an increase in conflict effect (Fan et al., 2009; Spagna et al., 2015). This may be explained by shared neural resources in FPN for these two functions. Both alerting and executive control functions are associated with an increase in information (warning cue vs. baseline for alerting and incongruent flankers vs. congruent flankers for executive control), supporting the case for involvement of the FPN in alerting. Therefore, the FPN is phasically activated for the general purpose of cognitive control in a task state with increased uncertainty. In our previous studies, we have argued that cognitive control is implemented by attentional functions (Mackie et al., 2013), and demonstrated that the activation of the regions of FPN is a linear function of cognitive control load, estimated in units of information entropy (Fan et al., 2014).
The validity by flanker conflict interaction effect was associated with activation in pulvinar bilaterally, right AI, right FEF and PCC. The pulvinar is an association thalamus nucleus that receives its major inputs from the visual cortex, and ascending SC projections relay through dorsal and ventral pulvinar to the FEF and other frontal areas (Guillery, 1995; Shipp, 2004). It is often activated in studies of the orienting network (LaBerge and Buchsbaum, 1990; Petersen et al., 1987). The pulvinar has been proposed to be involved in synchronizing information transfer according to the allocation of spatial attention (Saalmann et al., 2012) and in response anticipation (Fan et al., 2007b). Lesion studies showed that the pulvinar plays a key role in modulating attentional selection mechanisms by integrating frontoparietal attentional control signals within visual processing areas (Snow et al., 2009). The increased involvement of the pulvinar in the validity by flanker conflict interaction may suggest this structure is recruited when there is a need to disengage attention from one location and move and engage to another location during conflict processing.
In summary, this study revealed that attentional control is implemented via complex corticosubcortical relationships underlying alerting, orienting, and executive control and their interactions. Attention is a dynamic mental operation that is implemented by distinct yet interactive brain networks. Each function is associated with cortical and subcortical regions to produce the attentional effects, and some specific brain regions are activated for multiple attentional functions, depending on functional requirements. Not only do the attentional functions interact to achieve cognitive control, but also involve common and functionally specific regions.
Table 4.
Activation associated with the flanker conflict effect
| Region | L/R | BA | x | y | z | T | Z | K |
|---|---|---|---|---|---|---|---|---|
| Superior frontal gyrusa | L | 6 | −14 | −4 | 58 | 7.27 | 5.19 | 19137 |
| Supplementary motor area | R | 6 | 8 | 6 | 62 | 7.15 | 5.14 | |
| Anterior cingulate cortex | R | 32 | 10 | 20 | 40 | 6.81 | 4.99 | |
| Superior parietal lobule | L | 40 | −32 | −42 | 48 | 6.12 | 4.67 | |
| Thalamusb | L | −18 | −20 | 8 | 6.45 | 4.82 | ||
| Thalamusc | R | 16 | −4 | 10 | 6.26 | 4.74 | ||
| Superior colliculus | 4 | −24 | −6 | 5.81 | 4.51 | |||
| Precentral gyrus | R | 6 | 50 | 6 | 40 | 5.43 | 4.31 | |
| Anterior insula | R | 42 | 18 | −6 | 5.35 | 4.27 | ||
| Mid frontal gyrus | R | 45 | 38 | 46 | 16 | 5.21 | 4.19 | |
| Anterior insula | L | −30 | 16 | 8 | 4.80 | 3.95 | ||
| Precentral gyrus | R | 6 | 32 | −4 | 58 | 4.61 | 3.84 | |
| Inferior parietal lobule | R | 40 | −44 | −28 | 30 | 4.59 | 3.83 | |
| Anterior cingulate cortex | R | 24 | 4 | 36 | 20 | 3.96 | 3.42 | |
| Precentral gyrus | L | 6 | −52 | 6 | 16 | 3.37 | 3.01 | |
| Mid frontal gyrus | R | 47 | 40 | 48 | −10 | 3.19 | 2.87 | |
| Cerebellum | L | −22 | −60 | −28 | 6.89 | 5.03 | 4446 | |
| Cerebellum | R | 26 | −52 | −28 | 6.08 | 4.65 | ||
| Vermis | 0 | −54 | −20 | 3.93 | 3.40 | |||
| Supramarginal gyrus | R | 40 | 44 | −36 | 50 | 6.36 | 4.78 | 4424 |
| Superior parietal lobule | R | 7 | 26 | −54 | 54 | 5.68 | 4.44 | |
| Middle occipital gyrus | R | 19 | 30 | −68 | 32 | 4.84 | 3.98 | |
| Supramarginal gyrus | R | 40 | 64 | −42 | 36 | 4.00 | 3.45 | |
| Precentral gyrus | L | 6 | −50 | −2 | 48 | 4.25 | 3.61 | 203 |
| Inferior occipital gyrus | L | 37 | −44 | −66 | −2 | 4.20 | 3.58 | 212 |
Note: Structures listed below the cluster with a k value were within the same cluster with different local maxima.
Close to left frontal eye field;
Includes left thalamus and caudate nucleus;
Includes right thalamus and caudate nucleus.
Acknowledgments
Funding
This work was supported by the National Institute of Mental Health of the National Institutes of Health (NIH) under Award R01 MH094305 and by NIH Grant M01 RR000071 from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NCRR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. B. X. is supported by the grant from National Natural Science Foundation of China 31171076.
We thank Dr. Michael I. Posner for comments on the study design and the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amalric M, Koob GF. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res. 1993;99:209–226. doi: 10.1016/s0079-6123(08)61348-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005a;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005b;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Beane M, Marrocco RT. Norepinephrine and acetylcholine mediation of the components of reflexive attention: implications for attention deficit disorders. Prog Neurobiol. 2004;74:167–181. doi: 10.1016/j.pneurobio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Callejas A, Lupianez J, Tudela P. The three attentional networks: on their independence and interactions. Brain Cogn. 2004;54:225–227. doi: 10.1016/j.bandc.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–1636. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Halperin JM, Newcorn JH, Ivanov I, Tang CY, Fan J. Guanfacine potentiates the activation of prefrontal cortex evoked by warning signals. Biol Psychiatry. 2009;66:307–312. doi: 10.1016/j.biopsych.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353:1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Dosenbach, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois RM, Cohen MS. Spatiotopic organization in human superior colliculus observed with fMRI. Neuroimage. 2000;12:63–70. doi: 10.1006/nimg.2000.0590. [DOI] [PubMed] [Google Scholar]
- Fan J. An information theory account of cognitive control. Front Hum Neurosci. 2014;8:680. doi: 10.3389/fnhum.2014.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Bernardi S, Van Dam NT, Anagnostou E, Gu X, Martin L, Park Y, Liu X, Kolevzon A, Soorya L, Grodberg D, Hollander E, Hof PR. Functional deficits of the attentional networks in autism. Brain Behav. 2012;2:647–660. doi: 10.1002/brb3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI. The relation of brain oscillations to attentional networks. Journal of Neuroscience. 2007a;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci U S A. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Gu XS, Guise KG, Liu X, Fossella J, Wang HB, Posner MI. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009;70:209–220. doi: 10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD. Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J Neurosci. 2007b;27:2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, Van Dam NT, Gu XS, Liu X, Wang HB, Tang CY, Hof PR. Quantitative characterization of functional anatomical contributions to cognitive control under uncertainty. J Cogn Neurosci. 2014;26:1490–1506. doi: 10.1162/jocn_a_00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley KJ, Poline J, Frackowiak RS. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- Fuentes LJ, Campoy G. The time course of alerting effect over orienting in the attention network test. Exp Brain Res. 2008;185:667–672. doi: 10.1007/s00221-007-1193-8. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Parrish TB, Friston KJ, Mesulam MM. Functional anatomy of visual search: regional segregations within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage. 2002;15:970–982. doi: 10.1006/nimg.2001.1006. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187:583–592. [PMC free article] [PubMed] [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol. 1989;287:495–514. doi: 10.1002/cne.902870408. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T, van Lieshout DP. Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J Comp Neurol. 1991;304:275–306. doi: 10.1002/cne.903040210. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Katyal S, Zughni S, Greene C, Ress D. Topography of covert visual attention in human superior colliculus. J Neurophysiol. 2010;104:3074–3083. doi: 10.1152/jn.00283.2010. [DOI] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47:1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Keller EL. Symbolic cue-driven activity in superior colliculus neurons in a peripheral visual choice task. J Neurophysiol. 2006;95:3585–3595. doi: 10.1152/jn.01071.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mackie MA, Van Dam NT, Fan J. Cognitive control and attentional functions. Brain Cogn. 2013;82:301–312. doi: 10.1016/j.bandc.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco RT, Davidson MC. Neurochemistry of attention. In: Parasuraman R, editor. The attentive brain. The MIT Press; Cambridge, MA, US: 1998. pp. 35–50. [Google Scholar]
- Matsumoto K, Tanaka K. Neuroscience. Conflict and cognitive control. Science. 2004;303:969–970. doi: 10.1126/science.1094733. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322:1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Magistretti PJ. Monoamines and peptides in cerebral cortex — contrasting principles of cortical organization. Trends Neurosci. 1983;6:146–151. [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp. 2014;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Kennedy D, Hui KK, Makris N. Automated brainstem co-registration (ABC) for MRI. Neuroimage. 2006;32:1113–1119. doi: 10.1016/j.neuroimage.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Ngan NH, Matsumoto J, Takamura Y, Tran AH, Ono T, Nishijo H. Neuronal correlates of attention and its disengagement in the superior colliculus of rat. Front Integr Neurosci. 2015;9:9. doi: 10.3389/fnint.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin B, Godefroy O, Fall S, de Marco G. Alertness in young healthy subjects: an fMRI study of brain region interactivity enhanced by a warning signal. Brain Cogn. 2010;72:271–281. doi: 10.1016/j.bandc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Morris JD. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Posner M, Fan J. Attention as an organ system. In: Pomerantz JR, editor. Topics in integrative neuroscience: From cells to cognition. Cambridge University Press; 2008. pp. 31–61. [Google Scholar]
- Posner M, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci U S A. 1987;84:7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 2004;92:361–371. doi: 10.1152/jn.00673.2003. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Riva D, Bulgheroni S, Aquino D, Di Salle F, Savoiardo M, Erbetta A. Basal forebrain involvement in low-functioning autistic children: a voxel-based morphometry study. Am J Neuroradiol. 2011;32:1430–1435. doi: 10.3174/ajnr.A2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann E. Attention and memory trials during neuronal recording from the primate pulvinar and posterior parietal cortex (area PG) Behav Brain Res. 1995;67:241–253. doi: 10.1016/0166-4328(94)00153-7. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Scholey A. Attention. In: Perry EK, Ashton H, Young AH, editors. Neurochemistry of consciousness: neurotransmitters in mind. John Benjamins Publishing Company; 2002. pp. 43–64. [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000;23:S28–33. doi: 10.1016/s1471-1931(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Snow JC, Allen HA, Rafal RD, Humphreys GW. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc Natl Acad Sci U S A. 2009;106:4054–4059. doi: 10.1073/pnas.0810086106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagna A, Mackie MA, Fan J. Supramodal executive control of attention. Front Psychol. 2015;6:65. doi: 10.3389/fpsyg.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagna A, Martella D, Sebastiani M, Maccari L, Marotta A, Casagrande M. Efficiency and interactions of alerting, orienting and executive networks: the impact of imperative stimulus type. Acta Psychol. 2014;148:209–215. doi: 10.1016/j.actpsy.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb Cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HE, Cools R. Selective attentional enhancement and inhibition of fronto-posterior connectivity by the basal ganglia during attention switching. Cereb Cortex. 2015;25:1527–1534. doi: 10.1093/cercor/bht345. [DOI] [PubMed] [Google Scholar]
- Wen X, Liu Y, Yao L, Ding M. Top-down regulation of default mode activity in spatial visual attention. J Neurosci. 2013;33:6444–6453. doi: 10.1523/JNEUROSCI.4939-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Chang CF, Xi S, Huang IW, Liu Z, Juan CH, Wu Y, Fan J. A critical role of temporoparietal junction in the integration of top-down and bottom-up attentional control. Hum Brain Mapp. 2015;36:4317–4333. doi: 10.1002/hbm.22919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME, Robinson DL. Brain mechanisms of visual attention. Sci Am. 1982;246:124–135. doi: 10.1038/scientificamerican0682-124. [DOI] [PubMed] [Google Scholar]
- Yanaka HT, Saito DN, Uchiyama Y, Sadato N. Neural substrates of phasic alertness: a functional magnetic resonance imaging study. Neurosci Res. 2010;68:51–58. doi: 10.1016/j.neures.2010.05.005. [DOI] [PubMed] [Google Scholar]