Abstract
Objective
This study’s objective was to evaluate the effect of two common components of meditation (mindfulness and slow breathing) on potential mechanistic pathways.
Methods
102 combat veterans with posttraumatic stress disorder (PTSD) were randomized to: 1) the body scan mindfulness meditation (MM), 2) slow breathing (SB) with a biofeedback device, 3) mindful awareness of the breath with an intention to slow the breath (MM+SB), or 4) sitting quietly (SQ). Participants had six weekly one-on-one sessions with 20 minutes of daily home practice. The mechanistic pathways and measures were: 1) Autonomic Nervous System: hyperarousal symptoms, heart-rate (HR), heart-rate variability (HRV); 2) Frontal Cortex Activity: Attentional Network Task (ANT) conflict effect and event-related negativity, and intrusive thoughts; and 3) Hypothalamic-pituitary-adrenal axis: awakening cortisol. PTSD measures were also evaluated.
Results
Meditation participants had significant but modest within-group improvement in PTSD and related symptoms although there were no between-group effects. Perceived impression of PTSD symptom improvement was greater in the meditation arms compared to controls. Resting respiration decreased in the meditation arms compared to SQ. For the mechanistic pathways 1) Subjective hyperarousal symptoms improved within-group (but not between-group) for MM, MM+SB, and SQ while HR and HRV did not; 2) Intrusive thoughts decreased in MM compared to MM+SB and SB while the ANT measures did not change; and 3) MM had lower awakening cortisol within-group but not between-group.
Conclusion
Treatment effects were mostly specific to self-report rather than physiological measures. Continued research is needed to further evaluate mindfulness meditation’s mechanism in people with PTSD.
PTSD is a very serious public health concern. Veterans are especially vulnerable to acquiring PTSD and veterans with chronic PTSD are a growing population. The National Vietnam Veterans Readjustment Survey reported a 30.9% lifetime prevalence of PTSD in Vietnam combat veterans (Kulka et al., 1990). Prevalence of PTSD in 11,441 Gulf War veterans was estimated to be 10.1% (Kang, Natelson, Mahan, Lee, & Murphy, 2003) and among 2,863 Operation Iraqi Freedom soldiers, 16.6% met screening criteria for PTSD (Hoge, Terhakopian, Castro, Messer, & Engel, 2007). Veteran rates of PTSD are higher than Americans in the general population, reported at 3.5% prevalence in a given year and 6.8% over a lifetime (Kessler, Chiu, Demler, Merikangas, & Walters, 2005). One study about military personnel deployed to Iraq and Afghanistan put the economic impact of PTSD, including medical care, productivity and suicides, at $4–6 billion over two years (Tanielian et al., 2008). Clearly, the economic, societal and personal costs of PTSD are high.
The 2010 VA/DOD Clinical Practice Guidelines for the Management of PTSD recommend Cognitive Processing Therapy, Prolonged Exposure Therapy, Eye Movement Desensitization and Reprocessing, and Stress Inoculation Therapy as evidence-based treatment (Department of Veteran Affairs & Department of Defense, 2010). However, these trauma-focused therapies may not be appropriate or tolerated in all cases (Grunert, Weis, Smucker, & Christianson, 2007) and thus there is a need for other therapeutic options.
Mindfulness meditation (MM) may help patients observe and regulate strong emotions that can arise in trauma-focused therapies (Vujanovic, Niles, Pietrefesa, Potter, & Schmertz, 2014) and seem feasible and potentially beneficial for people with PTSD (Kearney, McDermott, Malte, Martinez, & Simpson, 2012; Wahbeh, 2014). A systematic review of complementary and alternative medicine for PTSD found positive evidence for meditation (Wahbeh, Senders, Neuendorf, & Cayton, 2014). Since that review, a randomized controlled trial of 47 veterans with PTSD found clinically meaningful change in mental health-related quality of life and PTSD symptoms compared to a control group at a 4-month follow-up (Kearney, McDermott, Malte, Martinez, & Simpson, 2013). Another study randomized 116 veterans with PTSD to Mindfulness-Based Stress Reduction (MBSR) or a time and attention matched active control and found greater reductions in PTSD symptoms in the mindfulness group during treatment and also two months post-treatment (Polusny et al., 2015).
Even though the evidence for MM’s use for people with PTSD is growing, the mechanism by which MM works to improve clinical symptoms is still not clear. Some have attempted to evaluate its mechanism by studying formal structured programs like MBSR (Kabat-Zinn, 1990) and Mindfulness-Based Cognitive Therapy (MBCT)(Segal, Williams, & Teasdale, 2002) using electroencephalography, functional magnetic resonance imaging, and other physiological measures (Cahn & Polich, 2006; Chiesa, Brambilla, & Serretti, 2011; Hölzel et al., 2011). However, most MM programs are multi-dimensional often including group support, physical movement in yoga poses, psychoeducation, and guided meditations, so it is difficult to assess which components generate the positive clinical effects.
This study’s primary goal was to explore MM’s mechanism of action in combat veterans with PTSD by separately examining two common components of structured MM programs, slowed breathing and mindfulness concepts. We proposed three pathways by which these components may potentially improve clinical outcomes, understanding that they do interact with each other:1) Autonomic nervous system via slowed breathing; 2) Mental state and frontal cortex activity through the mindfulness training; and 3) Hypothalamic-pituitary-adrenal (HPA) axis, which may be affected by both slowed breathing and MM.
Potential Pathway # 1: Autonomic Nervous System
Slowed breathing may happen inadvertently during MM even though there is explicit direction to simply observe and not change one’s breathing rate. Experienced and novice meditators are reported to have slower respiration rates compared to controls at rest and during MM (Ahani et al., 2014). Importantly, slowed breathing increases parasympathetic activation (Ducla-Soares et al., 2007; Jerath, Edry, Barnes, & Jerath, 2006). People with PTSD are often in a state of sympathetic dominance with increased heart rate and blood pressure, slowed digestive functioning, decreased blood flow to the extremities, and increased stress hormones preparing the body to fight or flight. Parasympathetic activation would decrease heart rate and blood pressure, speed up digestive functioning, return blood flow to the extremities, and normalize stress hormones (Benson, Beary, & Carol, 1974). Thus, slowed breathing during meditation activates the parasympathetic nervous system and may improve PTSD symptoms, regardless of whether it is slowed consciously or unconsciously.
Potential Pathway # 2: Mental state and frontal cortex activity
The mental state of MM may produce clinical changes through increasing frontal cortex activity, an area of the brain which regulates emotion processing. Any training that improves frontal cortex activity and thus emotion regulation could alleviate PTSD symptoms. Meditation studies demonstrate improvements in emotion processing (Creswell, Way, Eisenberger, & Lieberman, 2007). Remarkably, meditation studies also show improvements in frontal lobe function and changes in structure (Cahn & Polich, 2006; Holzel et al., 2007; Travis & Arenander, 2004). A systematic review of 21 neuroimaging meditation studies (n~300 meditators) found eight brain regions associated with self- and emotion-regulation that were consistently altered in cross-sectional studies of experienced meditators, including areas key to meta-awareness (frontopolar cortex/BA 10), exteroceptive and interoceptive body awareness (sensory cortices and insula), memory consolidation and reconsolidation (hippocampus), self and emotion regulation (anterior and mid cingulate; orbitofrontal cortex), and intra- and interhemispheric communication (superior longitudinal fasciculus; corpus callosum) (Fox et al., 2014). All these studies have been cross-sectional. Only one treatment study has examined frontal cortex activity as a mediator for mental state change and found that relative left frontal alpha activation was associated with greater positive mood in the meditation arm compared to controls (Barnhofer et al., 2007). More research is needed in this area.
Potential Pathway #3: The HPA axis
The HPA axis is one of the major mediators of the stress response. Of note, cortisol levels do not correspond directly to stress level in part because of “burnout” (Pruessner, Hellhammer, & Kirschbaum, 1999) and people with PTSD are reported to have lower cortisol values (Morris, Compas, & Garber, 2012; Wahbeh & Oken, 2013c). Meditation studies have shown improvements in cortisol values in many populations including veterans with PTSD (Bergen-Cico, Possemato, & Pigeon, 2014). Meditation may normalize HPA axis function resulting in an increase or decrease in cortisol depending on the abnormality. How meditation does this is unclear.
These three simple pathways were chosen with the understanding that they also interact with each other in complex ways. Psychoneuroimmunology has long studied the interactions between these systems which are clearly at play in PTSD (Furtado & Katzman, 2015) and are affected by mind-body interventions including meditation (Bower & Irwin, 2015). For example, increasing evidence supports interactions between the pre-frontal cortex and HPA axis (Liu et al., 2012). The flow of information between systems is complex and not completely understood. Clinical improvements could be mediated from the top-down or bottom-up, where the “top” refers to mental state and frontal cortex and “bottom” refers to involuntary physiological processes, such as autonomic nervous system and the HPA axis. Researchers question whether a reduction in mental stress reactivity happens before normalization in cortisol values (top-down) or cortisol values normalize and then stress reactivity lessens (bottom-up). We postulate that improvements are a downstream effect from the mental state of MM or through a physiological relaxation response from slowed breathing. It could also be some other mechanism that we are not examining in this study. The interactions between pathways will also be informally assessed with a correlation analysis of the pathway outcomes.
Veterans with PTSD were sampled in this study because they are a growing population who may potentially benefit from MM. Veterans without PTSD have increased mindfulness, especially mindful non-judging, compared to veterans with PTSD (Wahbeh & Oken, 2011). Also, a meditation study in veterans with PTSD found that mindful attention gains mediated reductions in PTSD and depression symptoms and improvements in psychological well-being (Bormann, Oman, Walter, & Johnson, 2014). Veterans with PTSD were also chosen because their pathophysiology allows for a greater potential range of change in the assessed measures. When healthy participants are used in mind-body studies, the ability to observe true effects is undermined and often results in erroneously negative studies.
The current study is a controlled trial where 102 combat veterans with PTSD were randomized to one of four treatment arms: mindfulness meditation (MM), slow breathing (SB), mindfulness meditation plus slow breathing (MM+SB), and sitting quietly (SQ). Participants received a one-on-one training session weekly for six weeks and practiced for 20 minutes per day between sessions. Outcomes for each potential mechanistic pathway were collected before and after the training period. We hypothesized that the slow breathing arms would improve autonomic nervous system outcomes, the MM arms would improve frontal cortex outcomes, and all arms would improve HPA axis outcomes.
METHOD
Participants
Combat veterans with PTSD were recruited from the Portland Metropolitan Area through flyer postings, advertisements and local talks. The study was approved by the Oregon Health & Science University and VA Portland Health Care System Institutional Review Boards. All volunteers signed consent forms prior to study activities. Approximately twenty-five combat veterans were allocated to each arm based on power calculations from preliminary data for each pathway’s primary outcomei (Sample Power 1.2, SPSS, Inc. 1997; See Supplemental Data for details of power calculations). Inclusion and exclusion criteria were chosen to allow for generalizability and to avoid excessive heterogeneity. Inclusion criteria were: 1) Combat veteran (defined by a score of ≥ 7 on the Combat Exposure Scale (Keane et al., 1989)); 2) Chronic PTSD diagnosis confirmed through clinician interview; 3) Age (25–65 years); 4) Either gender; 5) Good general medical health; 6) Stable dose of medications and therapy for duration of the study; and 7) Willing and able to provide informed consent. Exclusion criteria were: 1) Significant chronic medical illness where symptoms and/or treatment precluded participation; 2) Psychiatric or behavioral illness such as schizophrenia, schizoaffective disorder, bipolar disorder, psychotic disorder (not including transient dissociative states or flashbacks associated with PTSD re-experiencing symptoms), any DSM-IV cognitive disorder, current delirium, psychiatric instability or situational life crises, including evidence of being actively suicidal or homicidal, or any behavior which poses an immediate danger to the participant or others; 3) Substance dependence disorder within 3 months of the study or current substance use other than marijuana and alcohol (no more than 2 drinks/day by self-report); 4) Sexual assault as primary PTSD event(s) (to reduce heterogeneity from traumatic event); 5) Planning to move from the area in the next year; or 6) Prior or current meditation practice defined as more than 5 minutes per day for 30 days over the last 6 months. PTSD diagnosis confirmation was made through the Clinician-Administered PTSD Scale for DSM-IV (CAPS) (Blake et al., 1995) and other mental disorders were assessed through the Structured Clinical Interview for DSM-IV (SCID-IV) (First, Spitzer, Gibbon, & Williams, 2002). Other inclusion and exclusion criteria such as medication use and health conditions were determined through self-report during the screening process.
Procedure
All participants had a Telephone Screening, Screening Visit, Baseline Visit, six Training Visits, and an Endpoint Visit. All visits occurred in the OHSU Hatfield Research Center. After a Telephone Screening, interested and potentially eligible veterans attended a Screening Visit where the consenting procedure, CAPS and SCID were performed, and age at PTSD symptoms onset, time of initial PTSD diagnosis, duration of disease, lifestyle factors and psychiatric co-morbidities were recorded. The visit was conducted by HW, a clinician with appropriate training on the CAPS and SCID who was blinded to randomization assignments. Participants were asked not to start new exercise regimens and to report any significant changes in activity level. At the end of the visit, participants received a questionnaire packet with self-report measures and a saliva collection kit. The kit had six tubes for two days of at-home collection at: 1) waking before getting out of bed, 2) 30 minutes post-waking, and 3) bedtime.
Participants were reminded not to use recreational drugs or alcohol 24 hours prior to the Baseline Visit, where electrophysiological-monitoring devices were attached to record electroencephalography (EEG), electrocardiography (ECG), blood pressure and respiration, as previously described (Wahbeh & Oken, 2013a). Several cognitive tasks, including the Attention Network Task, were administered (Fan, McCandliss, Sommer, Raz, & Posner, 2002). The Endpoint Visit replicated the Baseline Visit.
Randomization
Allocation was determined with a covariate adaptive randomization approach to ensure arms were well matched on important baseline characteristics and to reduce selection bias (Cai, Xia, Xu, Gao, & Yan, 2006; Pocock & Simon, 1975). Baseline characteristics used were: age (3 levels: 25–38, 39–52, 53–65), depressive symptoms (2 levels: ≥17 high, ≤16 low (Beck, Steer, & Brown, 1996)) and gender. Participants were informed of their assignment by the research assistant (RA) at the first Training Visit.
Trainings
Participants were trained one-on-one by an unblinded and trained RA once a week for six weeks. The training sessions included discussion with the RA, respiration data collection set-up and clean-up, and the 20-minute intervention. Each intervention was structured to maintain equipoise and reduce performance bias. For the mindfulness arms, the RA read a one-page script defining mindfulness as “paying attention in a particular way, on purpose, in the present moment and non-judgmentally” (Kabat-Zinn, 1990). Each aspect of the definition was explained and examples given for how to deepen the skills and their importance to mindfulness practice. For example, the “in a particular way” section talks about the attitude that one brings to the act of paying attention. The goal of having an attitude of open curiosity, compassion, and kindness for oneself during the practice is emphasized rather than a cold, critical or judgmental attitude. Participants were able to ask questions about the concepts. There were no instructional scripts for the SB or SQ arms.
Both MM arms used an audio recording. MM participants practiced the Body Scan, which is the first formal mindfulness technique introduced and practiced intensively in the MBSR and MBCT programs. Body scanning involves directing one’s attention to different regions of the body, starting with the toes of the left foot and moving slowly upwards to the top of the head. Scanning was performed in silence, stillness and sitting upright. Participants were trained to gently redirect wandering attention back to the body part that was the focus of awareness. Nowhere in the guided meditation was attention directed to the breath or breathing process. When the Body Scan instructions reached the abdominal and chest area, body parts were noted without describing their movement. MM+SB participants sat upright and attempted to focus their attention on the breath as it passed the opening of the nostrils and created movement in the abdomen or chest and naturally slow their breath as they consciously observed their breathing. Whenever attention wandered from the breath, the participant was directed to simply notice the distracting thought and return their attention to the breath.
SB participants used a breathing device designed to reduce respiratory rate (RESPeRATE, Intercure, Inc; Montclair, NJ) (Schein et al., 2001; Wahbeh & Oken, 2013b). Resperate has no known contraindications for its use including in people with low or normal blood pressure.
SQ participants sat quietly and listened to a neutral-content audiobook in the laboratory sessions. Participants choose a book at their first training. Audiobooks were chosen to allow participants to complete the book by the end of their sixth session.
Home Practice and Adherence
All participants were asked to practice at home for 20 minutes per day between sessions. MM and MM+SB participants listened to the same guided meditations used in the laboratory. The guided meditations were administered using iPod Touch devices (Apple, Inc; Cupertino, CA) and adherence was objectively monitored with iMindr (Wahbeh, Zwickey, & Oken, 2011). SB participants used the RESPeRATE device at home. SQ participants were asked to sit quietly by themselves and engage in an activity of their choice—anything other than watching TV or browsing the Internet. All participants subjectively recorded home practice on paper logs that they submitted at the weekly sessions. All participants received the guided meditations on CD’s at the end of the study.
Blinding
As with most mind-body intervention trials, double-blinding in the traditional sense was not feasible. The PI was blinded to participant allocation and conducted all data analyses. The staff conducting biological sample analyses was blinded to reduce evaluation bias. The RA conducting the Baseline, Training and Endpoint Visits was unblinded. Effects of non-specific factors that may potentially confound outcomes due to participant and RA non-blinding were evaluated through an expectancy and instructor evaluation. Expectancy was assessed before randomization and credibility of the instructor was assessed after the intervention.
Measures
All psychological assessments administered are validated instruments widely used for clinical outcome trials. Several instruments were used during screening to determine eligibility. The CAPS had to be positive for at least one Re-experiencing symptom, three Avoidance/Numbing symptoms, and two Hyper-arousal symptoms to meet DSM-IV diagnosis for PTSD. A positive symptom was defined as a Frequency score ≥1 and Intensity score ≥2 (Blake et al., 1995). The Life Events Checklist (LEC) was also administered to assess lifetime trauma as part of the CAPS interview (Gray, Litz, Hsu, & Lombardo, 2004). The SCID-IV ruled out exclusions (First et al., 2002). The Combat Exposure Scale (CES) evaluated the degree of combat exposure (Keane et al., 1989).
The following measures were used as pre-post evaluative measures: PTSD Checklist (PCL) (Weathers, Litz, Herman, Huska, & Keane, 1993), Intrusive Thoughts Scale (IT) (Weiss & Marmar, 1997), Perceived Stress Scale (PSS) (Cohen, 1988), Beck Depression Inventory-2 (BDI) (Beck et al., 1996), Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988), General Perceived Self-Efficacy Scale (GPSE) (Schwarzer & Jerusalem, 1995), Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), and the non-judging and awareness subscales of the Five-Factor Mindfulness Questionnaire (MQ) (Baer et al., 2008). The Attention Network Task (ANT) is a cognitive task that yields reliable estimates of executive function and thus frontal cortex activity (Fan et al., 2002).
The Credibility/Expectancy Questionnaire (CEQ) assessed perceived credibility and intervention expectancy and was only administered at the Baseline Visit (Devilly & Borkovec, 2000). The Global Impression of Change (GIC) measured perceived clinical symptom improvement (Fischer, Stewart, Lorig, & Holman, 1995) and the Instructor Credibility Questionnaire measured instructor equipoise at study end.
EEG, ECG, respiration, and salivary cortisol were collected at Baseline and Endpoint Visits and respiration was collected during the six training sessions. EEG data were collected using a 32-channel system (Biosemi; Amsterdam; The Netherlands) and analyzed with BrainVision Analyzer (Version 2.0.1.3931, Professional Edition, BrainVision, LLC, Morrisville, NJ). Mean activity was exported for all event related negativity statistical analyses (Fan et al., 2007). ECG data were extracted using BrainVision Analyzer 2.0. Heart rate and heart rate variability was analyzed with Kubios HRV 2.0 (University of Kuopio, Kuopio, Finland). Respiration (average breaths per minute) was calculated to ensure breathing rate changed appropriately (Matlab, r2007a; The Mathworks, Inc. USA). The Cortisol Awakening Response (CAR) was calculated by taking the 30-minute post-waking value and subtracting the waking value as previously described (Clow, Thorn, Evans, & Hucklebridge, 2004).
Please see Supplemental Data for details on questionnaire psychometric properties, physiologic data processing and cognitive testing.
Analytic Strategy
In general, the four arms were assessed for differences with and without adjustment for covariates. Means, medians, and standard deviations were calculated for each arm. All data were examined with histograms to look for outlying data points and to determine the appropriateness of the normality assumption. Data were transformed if necessary or non-parametric tests were used. A completer rather than intention-to-treat analysis was conducted due to the mechanistic study aims.
Participant baseline characteristics were assessed with the χ2 test for discrete variables, by an analysis of variance for normally distributed variables and by using the two-sample Kruskall-Wallis test for non-normally distributed data. Each measure was subjected to linear mixed model analysis. The mixed model approach to repeated measures provides valid results in the presence of missing data assuming that the data are missing at random (Little & Rubin, 2002). For each dependent measure, the random variable was participants, the repeated variable was Visit, the fixed factor was intervention arm, and the covariates were age, gender, combat exposure (CES score), PTSD duration (years), baseline PTSD severity (CAPS score), baseline co-morbid depression (BDI score) and adherence (Total Minutes home practice). Covariates with greater than 0.10 p-value were removed from the model. Post-hoc examinations included paired-arm comparisons and pre-post changes within arms. This study was powered to examine between-group differences in the three primary outcomes: PCL-Hyperarousal score, ANT conflict effect score, and awakening cortisol. Analyses on other measures and post-hoc comparisons were not powered to detect differences and were exploratory. A p-value of 0.05 was considered significant in this context. A false discovery rate (FDR) correction for multiple comparisons was used for other measures (Benjamini & Hochberg, 1995). The number of hypotheses tested for each FDR correction is listed after the analysis description (arm, characteristics at baseline (FDR-14), PTSD and related symptom changes (FDR-8), Mindfulness (FDR-2), Autonomic Nervous System pathway (FDR-3), and Frontal Lobe Activity pathway (FDR-5)). Additionally, correlations were calculated for the primary outcomes of all participants between Baseline and Endpoint values and between change values (Endpoint minus Baseline scores) to evaluate relationships between the pathways. Analyses were conducted in SPSS 21.0 (IBM, Armonk, NY) and Stata 12.0 (StataCorp, LP, College Station, TX).
Results
Sample characteristics
One hundred and two combat veterans completed the study (Figure 1). Participants were mostly college-educated male combat veterans from the Vietnam Era. There were no significant differences on important demographic variables (Table 1). Intrusive thoughts and other self-rated PTSD symptoms, including hyper-arousal, heart rate and respiration were the same across arms at baseline (all p’s >0.10). Perceptions of credibility and expectancy of the interventions were the same across arms (all p’s >0.60). Expectancy of intervention effects were the same before randomization and the instructor evaluations were the same between arms (all p’s >0.05). Participants positively endorsed the instructor being confident about the intervention, comfortable working with them and enthusiastic about the intervention (MM - 4.78 ± 0.05, SB - 4.80 ± 0.13, MM+SB - 4.49 ± 0.05, SQ - 4.88 ± 0.13; p >0.05; 5 = Strongly Agree.)
Figure 1.
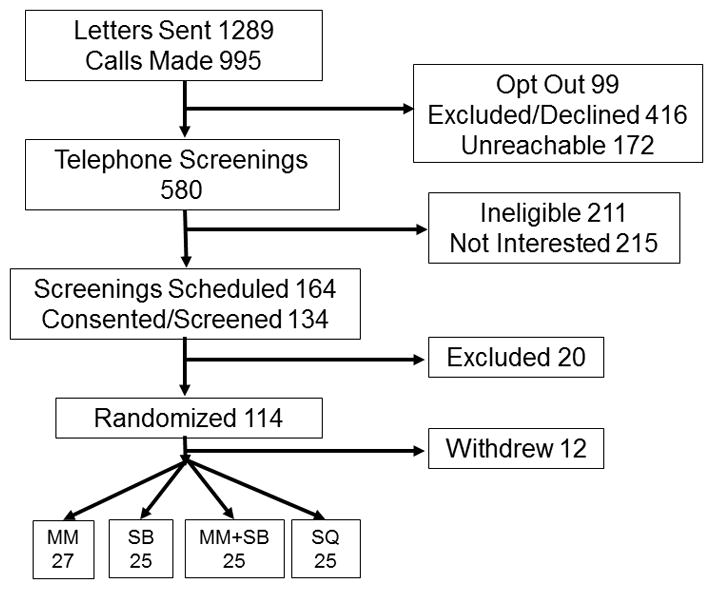
Table 1.
No significant differences between arms on important baseline characteristics.
| MM n=27 |
SB n=25 |
MM + SB n=25 |
SQ n=25 |
Stats | |
|---|---|---|---|---|---|
| Male (%) | 93 | 92 | 96 | 96 | X2(3)=0.64; p=0.88 |
| Era (n) | |||||
| Vietnam | 16 | 12 | 12 | 15 | X2(6)=3.8; p=0.70 |
| OEF/OIF | 9 | 8 | 11 | 7 | |
| Other combat | 2 | 5 | 2 | 3 | |
| Married (%) | 63 | 64 | 64 | 72 | X2(6)= 4.2; p=0.70 |
| Race (% Caucasian) | 85 | 88 | 88 | 84 | X2(15)= 11.9; p=0.70 |
| Age | 53.3 (12.6) | 52.2 (12.5) | 50.0 (12.8) | 53. (11.8) | F(3,101) = 0.39; p=0.76 |
| Education (n) | |||||
| <12 years | 0 | 1 | 0 | 1 | X2(9)= 11.9; p=0.90 |
| 12–14 years | 15 | 10 | 10 | 13 | |
| >14 years | 12 | 14 | 15 | 11 | |
| Combat Exposure | 24.1 (9.4) | 22.5 (8.5) | 26.6 (10.3) | 25.8 (9.8) | F(3,101) = 0.92; p=0.43 |
| PTSD (clinician interview) | 70.7 (14.8) | 67.3 (15.7) | 66.6 (15.7) | 70.6 (13.8) | F(3,101) = 0.52; p=0.67 |
| PTSD (self-rated) | 56.3 (9.8) | 54.5(11.7) | 52.0 (10.8) | 55.3 (9.7) | F(3,101) = 0.77; p=0.52 |
| Hyper-arousal | 17.7 (2.8) | 17.4 (4.4) | 17.2 (4.2) | 18.5 (4.2) | F(3,101) = 0.51; p=0.68 |
| Time Since Event | 30.1 (16.4) | 29.2 (15.1) | 25.3 (17.1) | 29.2 (17.1) | F(3,100) = 0.44; p=0.73 |
| Lifetime Trauma | 32.2 (7.8) | 33.7 (5.7) | 32.7 (6.3) | 31.3 (8.1) | F(3,101) = 0.51; p=0.68 |
| Depression | 21 (9.06) | 23.52 (9.9) | 21.2 (10.1) | 22.5 (11.4) | F(3,101) = 0.33; p=0.81 |
| Intrusive Thoughts | 26.1 (5.8) | 24.6 (5.8) | 21.9 (6.2) | 24.16 (0.32) | F(3,101) = 1.92; p=0.13 |
Note: OEF-Operation Enduring Freedom, OIF-Operation Iraqi Freedom, MM-Mindfulness Meditation, SB-Slow Breathing, MM + SB-Mindfulness Meditation plus Slow Breathing, SQ-Sitting Quietly. Lifetime Trauma score calculated from Life Events Checklist.
PTSD and related symptoms
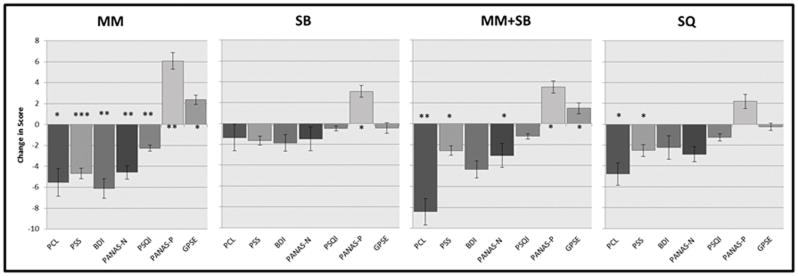
PTSD symptoms, perceived stress, depression, positive and negative emotions, self-efficacy and sleep quality were not different between-group overall. The global impression of change scores and adherence were markedly different between-group (Table 2). Post-hoc between-group comparisons showed multiple differences. MM was different than SB on BDI (p=0.03), PSQI (p=0.009), GIC (p<0.00005), and adherence (p=<0.00005) and different than SQ on adherence (p=<0.00005). MM+SB was different than SB on PANAS-N (p=0.008) and GIC (p=0.004) and different than SQ on PSS (p=0.05), GPSE (p=0.05) and adherence (p=<0.00005). Many PTSD and related symptoms showed significant within-group improvement (Figure 2).
Table 2.
PTSD and related symptom changes.
| MM | SB | MM+SB | SQ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Statistic | Pre | Post | Statistic | Pre | Post | Statistic | Pre | Post | Statistic | Mixed Model Statistics Significant covariates |
|
| PCL | 56.3 (9.7) | 50.7 (3.9) |
t(23)=2.1 p=0.05* |
54.5 (11.7) | 54.2 (12.1) |
t(21)=0.54 p=0.59 |
52.0 (10.8) | 46.9 (11.0) |
t(20)=3.06 p=0.006* |
55.3 (9.7) | 51.5 (12.1) |
t(21)=2.22 p=0.04* |
F(3,95)=1.06, p=0.37 CAPS, BDI |
| PSS | 23.4 (4.7) | 18.7 (5.7) |
t(26)=4.66 p=0.0001** |
22.0 (5.8) | 20.4 (6.8) |
t(22)=1.91 p=0.07 |
21.4 (7.9) | 18.9 a* (6.7) |
t(24)=2.06 p=0.05* |
24.76 (5.6) | 22.2 a* (5.1) |
t(24)=2.28 p=0.03* |
F(3,96)=1.6, p=0.20 CAPS, BDI |
| BDI | 21.0 (9.1) | 14.3a* (10.2) |
t(23)=3.29 p=0.003* |
23.4 (9.8) | 22.5a* (12.2) |
t(21)=1.15 p=0.26 |
21.2 (10.1) | 17.5 (11.3) |
t(21)=1.89 p=0.07 |
22.5 (11.4) | 20.9 (13.6) |
t(21)=0.98 p=0.34 |
F(3,96)=1.66, p=0.18 CAPS, Gender |
| PANAS-N | 27.4 (6.0) | 25.5 (8.0) |
t(25)=3.56 p=0.002* |
28.6 (8.4) | 27.1a* (12.7) |
t(24)=0.65 p=0.52 |
23.7 (8.1) | 20.3 a* (7.5) |
t(21)=2.08 p=0.05* |
27.7 (8.4) | 24.8* (8.0) |
t(21)=2.02 p=0.06 |
F(3,96)=2.95, p=0.06 CAPS, BDI |
| PANAS-P | 25.6 (8.0) | 31.5 (6.6) |
t(24)= −3.84 p=0.0008** |
25.6 (6.4) | 29.7 (7.9) |
t(24)= −2.66 p=0.01* |
27.8 | 31.7 |
t(22)= −2.18 p=0.04* |
24.6 (6.5) | 26.8 (6.8) |
t(24)= −1.62 p=0.12 |
F(3,98)=1.54, p=0.21 CAPS |
| GPSE | 27.9 (6.2) | 30.3 (4.1) |
t(26)= −2.63 p=0.01* |
28.7 (4.8) | 28.2 (5.9) |
t(24)=0.43 p=0.67 |
30.4 (5.3) | 31.7a* (4.9) |
t(22)= −2.44 p=0.02* |
28.6 (4.2) | 28.3a* (5.7) |
t(24)=0.34 p=0.74 |
F(3,97)=1.6, p=0.22 CES, BDI |
| PSQI | 10.7 (4.0) | 8.4a* (4.3) |
t(26)=4.04 p=0.0004** |
11.8 (3.9) | 11.3a* (4.0) |
t(24)=0.99 p=0.33 |
10.1 (3.7) | 9.5 (3.5) |
t(21)=0.80 p=0.43 |
12.2 (5.2) | 11.0 (3.9) |
t(24)=1.78 p=0.09 |
F(3,96)=2.43, p=0.07 CAPS, Adherence |
| GIC | 1.1 a** (0.8) | 0.52 a**b* (0.8) | 1.0b* (0.8) | 0.64 (0.6) |
F(3,96)=3.71p<0.00005** Age, time since event, adherence |
||||||||
| Adherence (Total Min over study) | 663a*b*c** (184) | 584a*d** (457) | 540b*e** (224) | 1044c**d**e** (343) |
F(3,95)=20.8 p<0.00005** Age, CES, and GIC |
||||||||
Note: Age, gender, CES, PTSD duration, CAPS, baseline BDI, adherence, GIC included as covariates for mixed model. MM-Mindfulness Meditation, SB-Slow Breathing, MM + SB-Mindfulness Meditation plus Slow Breathing, SQ-Sitting Quietly. PCL-PTSD Checklist; PSS-Perceived Stress Scale; BDI-Beck Depression Inventory-2; PANAS-Positive and Negative Affect Scale; GPSE-General Perceived Self-Efficacy Scale; PSQI-Pittsburgh Sleep Quality Index; GIC-Global Impression of Change. Covariates removed from model if p>0.10. F statistics reported are from between group mixed model with significant covariates.
p<0.05,
p<0.001.
Pre-post differences are reported for each measure for each group. These post-hoc comparison significances are denoted by a,b,c, or d for each pair.
Figure 2.
MM arm – PCL, PSS, BDI, PANAS-N, PANAS-P, GPSE, PSQI;
MM+SB arm – PCL, PSS, PANAS-N, PANAS-P, GPSE;
SB arm – PANAS-P;
SQ arm – PCL, PSS.
Mindfulness
Acting with awareness and mindful non-judging increased within-group for the combined MM arms but was not different than the no-MM arms (Table 3).
Table 3.
Mindfulness changes before and after intervention.
| Mindfulness (MM; MM+SB) | No Mindfulness (SB; SQ) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pre | Post | Statistic | Pre | Post | Statistic | Mixed Model Statistics | |
| Mindful Nonjudging | 24.9 (7.6) | 27.1 (7.9) |
t(49)= −2.17 p=0.04* |
24.4 (7.1) | 25.2 (7.7) |
t(48)= −0.84 p=0.41 |
F(1,99)=0.85, p=0.36 |
| Mindful Awareness | 23.6 (6.2) | 25.4 (7.0) |
t(49)= −2.04 p=0.05* |
22.4 (5.3) | 23.5 (7.1) |
t(48)= −1.10 p=0.28 |
F(1,99)=1.86, p=0.18 |
Note: Age, gender, CES, PTSD duration, CAPS, baseline BDI, adherence, GIC included as covariates for mixed model. Covariates removed from model if p>0.10. F statistics reported are from mixed model with significant covariates. CAPS was a significant covariate for mindful nonjudging and awareness.
p<0.05,
p<0.001.
Respiration rate
Baseline respiration rates were the same between-group (p=0.69). Changes in resting respiration rate from Baseline to Endpoint were significant (F(3,88)=3.91, p=0.01) and MM and MM+SB participants had lower respirations rates compared to SQ in post-hoc comparisons (MM, p=0.04; MM+SB, p=0.02). During the training sessions, differences in respiration from the first minute of training (Start) to the last minute of training (End) were calculated (MM: Start = 13.5 ± 3.4 versus End = 13.9 ± 4.0; SB: Start = 13.3 ± 4.8 versus End = 8.8 ± 5.0; MM+SB: Start = 14.7 ± 3.8 versus End = 13.2 ± 5.3; SQ: Start = 15.0 ± 4.3 versus End = 15.8 ± 3.8). Respiration rates during trainings were different between-group (F(3,91)=16.83, p<0.00005). SB participants had a greater reduction in respiration rate during the trainings than the MM (p<0.00005), MM+SB (p<0.00005) and SQ (p<0.00005) participants.
Potential Pathway # 1: Autonomic Nervous System
There were no significant between-group differences or post-hoc comparisons on hyperarousal symptoms, heart rate or heart rate variability. Within-group hyperarousal symptoms decreased in the MM, MM+SB and SQ arms (Table 4).
Table 4.
Potential pathway outcomes.
| MM | SB | MM+SB | SQ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Pre | Post | Statistic | Pre | Post | Statistic | Pre | Post | Statistic | Pre | Post | Statistic | Mixed Model Statistics | |
| Pathway #1 Autonomic Nervous System | |||||||||||||
| Hyperarousal (PCL) | 17.7 (2.8) | 15.7 (4.1) |
t(23)= −2.32 p=0.03* |
17.4 (4.4) | 17.0 (3.1) |
t(21)= −0.10 p=0.33 |
17.2 (4.2) | 14.5 (3.8) |
t(20)= −4.24 p=0.0004** |
18.5 (4.1) | 16.8 (4.3) |
t(20)= −2.54 p=0.02* |
F(3,95)=0.92, p=0.43 CAPS |
| Heart Rate | 72.5 (11.5) | 72.7 (10.6) |
t(24)= −0.01 p=0.92 |
74.8 (7.7) | 74.1 (9.8) |
t(23)=0.73 p=0.47 |
76.2 (15.8) | 73.6 (14.7) |
t(22)= −0.84 p=0.41 |
74.4 (12.3) | 74.1 (11.1) |
t(20)= −0.82 p=0.42 |
F(3,94)=0.48, p=0.69 Age |
| Heart Rate Variability | 3.7 (4.0) | 2.8 (2.7) |
t(24)=0.35 p=0.73 |
4.0 (4.5) | 2.2 (1.4) |
t(23)=1.7 p=0.10 |
2.6 (2.2) | 3.3 (3.4) |
t(20)= −0.94 p=0.36 |
1.8 (1.4) | 3.2 (2.6) |
t(20)= −1.98 p=0.06 |
F(3,84)=0.36 p=0.78 Age, time since trauma |
|
| |||||||||||||
| Pathway #2 Mental state and frontal cortex activity | |||||||||||||
| Conflict effect score | 214.3 (89.9) | 204.3 (91.7) |
t(25)=0.53 p=0.61 |
206.3 (90.1) | 230.7 (97.7) |
t(22)= −1.27 p=0.22 |
230.3 (78.7) | 211.9 (103.4) |
t(19)= −2.19 p=0.04* |
234.0 (84.7) | 217.5 (7.9) |
t(20)= −0.84 p=0.41 |
F(3,92)=0.89, p=0.45 Gender, CES, adherence |
| Intrusive Thoughts Score | 26.1 (105.8) | 24.0a* (7.6) |
t(26)= −2.34 p=0.03* |
24.6 (5.8) | 24.8b* (7.4) |
t(22)=0.25 p=0.81 |
21.9 (6.2) | 20.6a*b* (6.9) |
t(22)= −1.53 p=0.14 |
24.2 (7.3) | 23.9 (7.4) |
t(24)= −0.20 p=0.85 |
F(3,97)=1.97, p=0.12 CAPS |
| ERN No Cue | 0.48 (3.0) | −0.21 (3.8) |
t(12)= −0.01 p=0.99 |
0.74 (2.8) | 0.12 (4.2) |
t(14)=0.60 p=0.56 |
−0.80 (2.5) | −0.79 (2.0) |
t(12)= −0.89 p=0.39 |
−0.32 (2.2) | 0.06 (2.0) |
t(15)= −0.94 p=0.36 |
F(3,76)=1.65, p=0.19 CAPS |
| ERN Cue | 0.21 (2.5) | −0.13 (2.8) |
t(12)=1.2 p=0.25 |
0.48 (1.7) | 0.47 (2.6) |
t(12)= −0.34 p=0.74 |
0.76 (2.6) | −0.21a* (3.8) |
t(10)= −0.17 p=0.87 |
−1.30 (2.4) | −0.28a* (2.5) |
t(16)= −1.7 p=0.11 |
F(3,66)=1.49, p=0.23 |
|
| |||||||||||||
| Pathway #3 HPA Axis | |||||||||||||
| Awakening Cortisol | 0.13 (0.21) | 0.07 (0.16) | z(22)= −1.9, =p=0.05* | 0.08 (0.15) | 0.03 (0.10) |
z(23)= −1.30 p=0.21 |
0.05 (0.16) | 0.08 (0.19) | z(19)= −0.26, p=0.79 | 0.09 (0.27) | −0.01 (0.28) | z(23)= −0.40, p=0.69 |
F(3,79)=0.59, p=0.62 Time of Collection |
Note: Age, gender, CES, PTSD duration, CAPS, baseline BDI, adherence, GIC included as covariates for the mixed models. Age, gender, BMI, Smoking status, sleep duration, Season, PSS, CES, PTSD duration, CAPS, baseline BDI, adherence, GIC, time of waking collection were used for the cortisol analysis. Covariates removed from model if p>0.01. F statistics reported are from mixed model with significant covariates. Note-there were no differences between groups on heart rate and heart rate variability at baseline. One participant’s HRV data was removed from this analysis because it was considered an outlier with values being 10 standard deviations from the mean. For the ERN data, participants were excluded for various reasons, as described in methods section. Final participant counts are as follows (MM n=17; SB n=20 v1, n=16 v2; MM+SB n=20 v1, n=16 v2; SQ n=18 v1, n=21 v2). Although awakening cortisol was natural log transformed for the statistical analysis, actual values are reported in this table. MM-Mindfulness Meditation, SB-Slow Breathing, MM + SB-Mindfulness Meditation plus Slow Breathing, SQ-Sitting Quietly, PCL-PTSD Checklist. CES-Combat Exposure Scale, CAPS-Clinician Administered PTSD Scale, ERN-event related negativity, HPA-hypothalamic-pituitary adrenal axis.
p<.05
p<0.001
Potential Pathway # 2: Mental state and frontal cortex activity
For the ANT, reaction times were shorter for cued than uncued targets across all participants as expected. The conflict effect score, intrusive thoughts score and ERN were not different between-group (Table 4). MM had a greater decrease in intrusive thoughts compared to MM+SB (p=0.04), which in turn had a greater decrease than SB (p=0.04). Intrusive thought scores decreased in MM within-group (p=0.03).
Potential Pathway #3: The HPA axis
There was no between-group or post-hoc difference on awakening cortisol. MM participants had lower awakening cortisol values after the intervention (Table 4).
Correlations between the pathway measures
Baseline and Endpoint values demonstrated high test-retest variability for hyperarousal, heart rate, heart rate variability and conflict effect score. Intrusive thought scores and hyperarousal scores were correlated at Baseline and Endpoint. Conflict effect score at endpoint was negatively correlated with hyperarousal at baseline and intrusive thoughts at endpoint.
There were also a number of correlations between change scores. For pathway 1, HRV change was negatively correlated with the hyperarousal change score. Namely, as heart rate variability increased, hyperarousal scores decreased (r=−0.26, p=.03). For pathway 2, the conflict effect change score was positively correlated with the hyperarousal change score. The conflict effect change score was also negative correlated with the HRV change score (greater improvement on the conflict effect score reflected greater improvement in the HRV measure) (r=−0.24, p=0.03). For pathway 3, awakening cortisol change score was positively correlated with the ERN cue change score (r=0.31, p=.03) (greater cortisol values at Endpoint were correlated with lower performance at Endpoint of the ERN cue trials). (Please see Supplement Data Table 1 and 2 for detailed results).
Discussion
In summary, 102 mostly male combat veterans completed the study. Arms were well matched on demographics and important variables for our measures.
PTSD and related symptoms had within-group differences on many outcomes for the mindfulness arms demonstrating a clinical effect from the interventions. Participants in the MM arm had the most improvements in PTSD and related symptom scores, followed by the MM+SB arm, then SQ and finally SB. A 5–10 point change on the PCL represents reliable change not due to chance and a 5-point change is the minimum threshold for determining whether an individual has responded to treatment (Monson et al., 2008). The MM arms demonstrated a modest response to treatment (MM - 5.6; MM + SB - 5.1). The perceived impression of clinical change was also most improved for the MM and MM+SB arms. Other studies have found improvement in PTSD-related symptoms with mindfulness as well (Bhatnagar et al., 2013; Bormann et al., 2014; Kearney et al., 2012, 2013; Omidi, Mohammadi, Zargar, & Akbari, 2013; Polusny et al., 2015).
Adherence
Most participants were adherent. Average adherence levels ranged from 15–30 minutes per day with the SQ arm participants practicing more than 20 minutes per day. SQ activities included making jewelry, tying flies, reading magazines or books, doing crossword puzzles, playing solitaire, and observing nature. Although these activities require attention —an element of mindfulness—they do not qualify as mindfulness practice, per se. While the SQ arm had improvements in PTSD symptoms and perceived stress, the other PTSD-related symptoms did not improve. The SQ was intended to be a non-active time and attention control arm but ended up being a more active control. It appears that for combat veterans, the act of regularly sitting quietly and engaging in a chosen activity has some therapeutic effect.
Mindfulness
The Acting with Awareness and Non-Judging mindfulness parameters increased in the mindfulness arms but not in the no-mindfulness arms although the between-group differences were smaller than expected. Perhaps there was some indirect training in mindfulness elements just through the act of paying attention to one’s breath or sitting quietly. Even though the mindfulness questionnaire was not labeled with the word “mindfulness,” some self-report bias may have occurred from the study title on advertisements.
Respiration rate
We did observe appropriate decreases in respiration rates in the SB and MM+SB participants during trainings. Interestingly, both meditation arms had reduced Endpoint respiration rates at rest compared to Baseline values. As far as we know, this is the first study to report decreased resting respiration rates from a short MM intervention. This finding, along with evidence that respiration slows during meditation regardless of whether explicit instruction to do so exists (Ahani et al., 2014; Ditto, Eclache, & Goldman, 2006), and that experienced meditators have lower resting respiration rates compared to controls (Oken, Zajdel, & Wild, 2007), alludes to the idea that deconstructing mindfulness concept effects from slow breathing effects may in fact not be possible. Continued systematic research is necessary to determine the effects of each component of multi-component interventions like MBSR and MBCT. We’ve gleaned from this study that learning mindfulness concepts and practicing structured meditations may be helpful for PTSD and related symptoms but not more so than other active controls. Future research will examine other specific components incorporating different doses to further elucidate these interventions. We already know that group process and social interactions may have therapeutic benefit, but what of the other specific mindfulness meditation aspects to these interventions (Allen, Chambers, & Knight, 2006). This study looked at only two of the many common components of these structured classes. More research is needed.
Pathway outcomes
The self-report hyperarousal symptoms decreased within (but not between) the MM, MM+SB and SQ arms; there was no change in the objective measures of heart rate or HRV. The reaction times for cued/non-cued and congruent/incongruent trials reflected proper task administration and execution. However, there was no change in conflict effect score or event-related potentials. Interestingly, there was also low test-retest reliability of this measure. A longer and more intense intervention may be needed to create physiological changes. The lack of between-group difference may in part reflect the active nature of our control arm. Future studies should include assessment in the participant’s natural environment or acute responses to a laboratory stressor to more fully examine changes in ANS activity from MM.
CAR was also unchanged from before to after the intervention period. When examined cross-sectionally, CAR is usually noted to be lower in people with PTSD (Chida & Steptoe, 2008; Klaassens, Giltay, Cuijpers, van Veen, & Zitman, 2012; Morris et al., 2012). Very few PTSD treatment studies have examined CAR changes. One treatment study found no CAR changes even though PTSD symptoms significantly improved after prolonged exposure or sertraline therapy (Pacella, Feeny, Zoellner, & Delahanty, 2014). Another highly relevant study examined CAR after a short-term mindfulness program in veterans with PTSD. CAR was reduced post-treatment with changes being moderated by treatment engagement and dosing (Bergen-Cico et al., 2014). The lack of CAR changes in this study may be due to variability in factors affecting cortisol. For example, veterans collected their morning samples at very different times of the day depending on when they awoke. While we covaried for wake time, the time of day the participant woke up may affect the awakening cortisol level in ways we do not understand. Other cortisol measures should be examined such as area under the curve according to the ground and increase (Wahbeh & Oken, 2013c). Due to the complex nature of cortisol’s morning and diurnal rhythms, a multi-level hierarchical analysis may help elucidate any HPA influence of mindfulness (Hruschka, Kohrt, & Worthman, 2005). Also, significant changes in diurnal cortisol related to stress reduction may take longer to occur than this study duration. Future analysis of this data set and work by others may help elucidate these complexities.
Pathway relationships
The correlational analysis of the pathway measures demonstrated some interesting findings. First, HRV improvements were correlated with improvements in hyperarousal scores. Multiple studies have associated lower HRV with PTSD symptoms and diagnosis but not with hyperarousal symptoms specifically (Chalmers, Quintana, Abbott, & Kemp, 2014; Sammito, Thielmann, Zimmermann, & Bockelmann, 2015). Second, improvements in the conflict effect score correlated with improvements in the HRV. Others have postulated that individual differences in HRV are reflected in one’s ability to regulate unwanted and intrusive thoughts and our data are consistent with this (Gillie & Thayer, 2014). These results support the known interrelationships between our proposed pathways.
This study has a number of limitations to consider when interpreting the results. First, the RA who conducted the trainings also conducted the Baseline and Endpoint Visits introducing a risk in bias. This risk was mitigated by educating the RA about equipoise, having evaluations that did not depend on her subjective ratings (e.g. physiological measures, computer tasks and self-report questionnaires) and administering an instructor evaluation to participants at the end of training that demonstrated equality across all study arms.
Another limitation is that what we thought was an inactive control, SQ, was actually an active control for this population. Clinically, this inadvertent finding may be helpful to understand. Maybe a simple prescription of sitting quietly and engaging in a chosen activity for 20 minutes a day may be helpful in improving PTSD symptoms. More research is needed to test this more directly. Additionally, providing daily structure, weekly social engagement and an opportunity to contribute to a project that could potentially help other veterans may be helpful for combat veterans with PTSD.
Unexpectedly, some participants in the SB arm experienced fear and anxiety rather than relaxation when their breathing slowed. After more direction in using the device and encouragement to breathe naturally, most of the anxiety dissipated for all but one participant. As others have also noted, some people develop fear when experiencing relaxation-related sensations (Luberto, 2012). A paradoxical reaction to an intervention meant to induce relaxation should be considered, especially in this population.
Furthermore, MM and MM+SB were not the complete MBSR or MBCT structured programs. We focused on the mindfulness content and actual practice of the intervention rather than other components, such as group process, education about stress reduction or discussion of cognitive restructuring. Perhaps those other pieces alone or in synergy with other components are what mediate clinical change. While our MM interventions included guided meditations and education and discussion about mindfulness, this content was much more limited than what is delivered in a full MBSR or MBCT class. The sessions were approximately 1-hour weekly for six weeks rather than the 2.5-hour weekly classes for eight weeks plus a full-day retreat in the more intensive programs. Many clinicians and researchers have shortened and adapted the full clinical MBSR and MBCT programs with positive results and potential participants express a desire for alternative delivery formats because of the intensity and lack of accessibility of the standard group formats (Wahbeh, Svalina, & Oken, 2014). More specifically, some our subjects did not even like going out in public and did not want to engage in group settings. Further research is needed to optimize delivery of MM in different populations. Many participants in MM programs may be receiving other therapies simultaneously which could influence MM effects. Our study required stable treatments and medications during the course of the study. Future studies should also control in some way for this potential confounder.
Home practice was not equal between arms. SQ participants practiced more than the other participants (@30 minutes/day) who practiced on average closer to the requested amount of time (@15–20 minutes/day). This may be due in part to the nature of the activities. For example, reading for two hours is reasonable, but listening to a 20-minute guided meditation six times (for a total of two hours) is not. Regardless, this was addressed by including adherence as a covariate. Adherence was a significant covariate for sleep, perceived impression of clinical change and the conflict effect score.
Finally, the physiological measures were conducted before and after the six-week intervention. Pre-post evaluations may not be a sensitive enough to detect change in an abbreviated intervention. A number of factors influence physiological measures (e.g. gender, age, BMI, health status) that are not at play for self-report measures. In fact, a meta-analysis found that significant correlations between physiological responses and perceived emotional stress variables were only evident in 25% of the 49 studies examined (Campbell & Ehlert, 2012). The authors suggest various factors, such as assessment features, underlying psychological traits and states, gender and physiological dispositions may account for the discrepancies. Longer-term measurement of physiology or acute measurement of physiological changes when exposed to stressors may better elucidate changes from mindfulness training.
Further research is warranted into self-report versus physiological outcomes. We observed greater change in self-report measures (but still not significant between-group) than the physiological outcomes, so a top-down mechanism is suggested but unclear. These results are mirrored in other studies/meta-analyses that demonstrate greater effect sizes in improvement in self-report psychological measures than physiological measures and may also reflect that self-report measures are more sensitive to expectancy effects (Chiesa, Anselmi, & Serretti, 2014; Chiesa & Serretti, 2010; Goyal et al., 2014; Khoury et al., 2013).
Future research to build upon this study’s results would include the following. 1) Examining predictors that may influence treatment response; 2) Analyzing qualitative data from participant interviews to give a richer commentary on how the interventions may have improved their lives. Many veterans in the mindfulness arms shared that even though they did not see drastic improvements in their PTSD symptoms, their reactions and relationship to their symptoms improved; and 3) Collecting ecological momentary assessment data collected at home before and after the intervention. Outcome measures assessed in a laboratory setting may not fully reflect the perceived changes of PTSD in the participants’ natural environments. Also, using other analysis techniques that better account for the systems aspect of stress responses may be helpful (Oken, Chamine, & Wakeland, 2015). While the data presented in this paper describe a priori research questions and analysis of a National Institutes of Health grant study, future analyses will be conducted on additional biological, qualitative, ecological momentary assessment and predictor data that was collected.
Supplementary Material
Acknowledgments
The authors would like to thank Roger Ellingson, Jennifer Bishop, Joshua Leventhal, and Tabatha Memmott for their help with this project. We would like to thank all the Veterans who participated in the study. Research reported in this publication was supported by National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000128 and the National Center for Complementary and Alternative Medicine of the National Institutes of Health [Grant numbers T32AT002688, K01AT004951, K24AT005121]. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Power calculations used a two-tailed fixed effects analysis of covariance model adjusting for baseline values with the criterion for significance (alpha) set at 0.05. Preliminary data for primary measures were used. With 25 participants in each group, the PCL hyper-arousal scale adjusted effect size (f) is 0.46 which yields power of >0.99, the Attentional Network Task-conflict effect score adjusted effect size (f) is 0.69, which yields power > 0.99, and the Awakening Cortisol adjusted effect size (f) is 0.31, which yields power of 0.85.
None of the authors have financial or other conflicts of interest related to this work.
Contributor Information
Elena Goodrich, Email: goodrice@ohsu.edu.
Elizabeth Goy, Email: elizabeth.goy@va.gov.
Barry S. Oken, Email: oken@ohsu.edu.
References
- Ahani A, Wahbeh H, Nezamfar H, Miller M, Erdogmus D, Oken B. Quantitative change of EEG and respiration signals during mindfulness meditation. J Neuroeng Rehabil. 2014;11:87. doi: 10.1186/1743-0003-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NB, Chambers R, Knight W. Mindfulness-based psychotherapies: a review of conceptual foundations, empirical evidence and practical considerations. Australian and New Zealand Journal of Psychiatry. 2006;40(4):285–294. doi: 10.1080/j.1440-1614.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329–342. doi: 10.1177/1073191107313003. [DOI] [PubMed] [Google Scholar]
- Barnhofer T, Duggan D, Crane C, Hepburn S, Fennell MJ, Williams JM. Effects of meditation on frontal alpha-asymmetry in previously suicidal individuals. Neuroreport. 2007;18(7):709–712. doi: 10.1097/WNR.0b013e3280d943cd. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory -Second Edition Manual. San Antonio: Harcourt Brace & Company; 1996. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Benson H, Beary JF, Carol MP. The relaxation response. Psychiatry. 1974;37(1):37–46. doi: 10.1080/00332747.1974.11023785. [DOI] [PubMed] [Google Scholar]
- Bergen-Cico D, Possemato K, Pigeon W. Reductions in Cortisol Associated With Primary Care Brief Mindfulness Program for Veterans With PTSD. Medical Care. 2014;52(Suppl 5):S25–31. doi: 10.1097/MLR.0000000000000224. [DOI] [PubMed] [Google Scholar]
- Bhatnagar R, Phelps L, Rietz K, Juergens T, Russell D, Miller N, et al. The effects of mindfulness training on post-traumatic stress disorder symptoms and heart rate variability in combat veterans. Journal of Alternative and Complementary Medicine. 2013;19(11):860–861. doi: 10.1089/acm.2012.0602. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bormann JE, Oman D, Walter KH, Johnson BD. Mindful attention increases and mediates psychological outcomes following mantram repetition practice in veterans with posttraumatic stress disorder. Medical Care. 2014;52(Suppl 5):S13–18. doi: 10.1097/MLR.0000000000000200. [DOI] [PubMed] [Google Scholar]
- Bower JE, Irwin MR. Mind-body therapies and control of inflammatory biology: A descriptive review. Brain, Behavior and Immunity. 2015 doi: 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132(2):180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Cai H, Xia J, Xu D, Gao D, Yan Y. A generic minimization random allocation and blinding system on web. J Biomed Inform. 2006;39(6):706–719. doi: 10.1016/j.jbi.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37(8):1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety Disorders are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front Psychiatry. 2014;5:80. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol Psychol. 2008 doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Anselmi R, Serretti A. Psychological mechanisms of mindfulness-based interventions: what do we know? Holistic Nursing Practice. 2014;28(2):124–148. doi: 10.1097/HNP.0000000000000017. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Brambilla P, Serretti A. Neuro-imaging of mindfulness meditations: implications for clinical practice. Epidemiol Psychiatr Sci. 2011;20(2):205–210. doi: 10.1017/s204579601100028x. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol Med. 2010;40(8):1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7(1):29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S. Perceived Stress in Probability Sample of the United States. In: SaO Spacapan S, editor. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Department of Veteran Affairs & Department of Defense. Bacteriologia, Virusologia, Parazitologia, Epidemiologia. Washington, DC: Veterans Health Administration, Department of Veteran Affairs, Department of Defense; 2010. VA/DoD Clinical Practice Guideline for the Management of Post-Traumatic Stress, Revista de Igiena, Bacteriologie, Virusologie, Parazitologie, Epidemiologie, Pneumoftiziologie. [Google Scholar]
- Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Ditto B, Eclache M, Goldman N. Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Ann Behav Med. 2006;32(3):227–234. doi: 10.1207/s15324796abm3203_9. [DOI] [PubMed] [Google Scholar]
- Ducla-Soares JL, Santos-Bento M, Laranjo S, Andrade A, Ducla-Soares E, Boto JP, et al. Wavelet analysis of autonomic outflow of normal subjects on head-up tilt, cold pressor test, Valsalva manoeuvre and deep breathing. Experimental Physiology. 2007;92(4):677–686. doi: 10.1113/expphysiol.2007.038026. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, et al. Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J Neurosci. 2007;27(9):2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders-Patient Edition (SCID-I/P, 11/2002 revision) New York, New York: Biometrics Research Department; 2002. [Google Scholar]
- Fischer D, Stewart A, Lorig K, Holman H. In Patient perceptions of clinical change and its meaning correlate poorly with change measured by conventional instruments. Paper presented at the AHSR FHSR Annu Meet Abstr Book.1995. pp. 56–57. [Google Scholar]
- Fox KC, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience and Biobehavioral Reviews. 2014 doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Research. 2015;229(1–2):37–48. doi: 10.1016/j.psychres.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Gillie BL, Thayer JF. Individual differences in resting heart rate variability and cognitive control in posttraumatic stress disorder. Front Psychol. 2014;5:758. doi: 10.3389/fpsyg.2014.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, et al. Meditation Programs for Psychological Stress and Well-being: A Systematic Review and Meta-analysis. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the life events checklist. Assessment. 2004;11(4):330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- Grunert BK, Weis JM, Smucker MR, Christianson HF. Imagery rescripting and reprocessing therapy after failed prolonged exposure for post-traumatic stress disorder following industrial injury. J Behav Ther Exp Psychiatry. 2007;38(4):317–328. doi: 10.1016/j.jbtep.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164(1):150–153. doi: 10.1176/ajp.2007.164.1.150. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421(1):16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30(7):698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Jerath R, Edry JW, Barnes VA, Jerath V. Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses. 2006;67(3):566–571. doi: 10.1016/j.mehy.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Dell Publishing; 1990. [Google Scholar]
- Kang HK, Natelson BH, Mahan CM, Lee KY, Murphy FM. Post-traumatic stress disorder and chronic fatigue syndrome-like illness among Gulf War veterans: a population-based survey of 30,000 veterans. American Journal of Epidemiology. 2003;157(2):141–148. doi: 10.1093/aje/kwf187. [DOI] [PubMed] [Google Scholar]
- Keane T, Fairbank JA, Caddell J, Zimering R, Taylor K, Mora C. Clinical Evaluation of a Measure to Assess Combat Exposure. A Journal of Consulting and Clinical Psychology. 1989;1(1):53–55. [Google Scholar]
- Kearney DJ, McDermott K, Malte C, Martinez M, Simpson TL. Association of participation in a mindfulness program with measures of PTSD, depression and quality of life in a veteran sample. J Clin Psychol. 2012;68(1):101–116. doi: 10.1002/jclp.20853. [DOI] [PubMed] [Google Scholar]
- Kearney DJ, McDermott K, Malte C, Martinez M, Simpson TL. Effects of participation in a mindfulness program for veterans with posttraumatic stress disorder: a randomized controlled pilot study. Journal of Clinical Psychology. 2013;69(1):14–27. doi: 10.1002/jclp.21911. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clinical Psychology Review. 2013;33(6):763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology. 2012;37(3):317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Kulka R, Schlenger W, Fairbank J, Hough R, Jordan B, Marmar C, et al. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. New York: Routledge; 1990. [Google Scholar]
- Little R, Rubin D. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- Liu J, Chaplin TM, Wang F, Sinha R, Mayes LC, Blumberg HP. Stress reactivity and corticolimbic response to emotional faces in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(3):304–312. doi: 10.1016/j.jaac.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberto CM. Development and Validation of the Relaxation Sensitivity Index. 2012 doi: 10.1007/s41811-020-00086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychological Assessment. 2008;20(2):131–138. doi: 10.1037/1040-3590.20.2.131. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Chamine I, Wakeland W. A systems approach to stress, stressors and resilience in humans. Behavioural Brain Research. 2015;282:144–154. doi: 10.1016/j.bbr.2014.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Zajdel D, Wild J. In Induced and trait differences in EEG phase synchrony and power for experienced meditators versus non-meditators. Paper presented at the Cognitive Neuroscience Society Annual meeting.2007. p. 266. [Google Scholar]
- Omidi A, Mohammadi A, Zargar F, Akbari H. Efficacy of mindfulness-based stress reduction on mood States of veterans with post-traumatic stress disorder. Arch Trauma Res. 2013;1(4):151–154. doi: 10.5812/atr.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella ML, Feeny N, Zoellner L, Delahanty DL. The impact of PTSD treatment on the cortisol awakening response. Depression and Anxiety. 2014;31(10):862–869. doi: 10.1002/da.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- Polusny MA, Erbes CR, Thuras P, Moran A, Lamberty GJ, Collins RC, et al. Mindfulness-Based Stress Reduction for Posttraumatic Stress Disorder Among Veterans: A Randomized Clinical Trial. JAMA. 2015;314(5):456–465. doi: 10.1001/jama.2015.8361. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61(2):197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Sammito S, Thielmann B, Zimmermann P, Bockelmann I. Influence of post-traumatic stress disorder on heart rate variability as marker of the autonomic nervous system - a systematic review. Fortschritte der Neurologie-Psychiatrie. 2015;83(1):30–37. doi: 10.1055/s-0034-1398779. [DOI] [PubMed] [Google Scholar]
- Schein MH, Gavish B, Herz M, Rosner-Kahana D, Naveh P, Knishkowy B, et al. Treating hypertension with a device that slows and regularises breathing: a randomised, double-blind controlled study. J Hum Hypertens. 2001;15(4):271–278. doi: 10.1038/sj.jhh.1001148. [DOI] [PubMed] [Google Scholar]
- Schwarzer R, Jerusalem M, editors. Generalized self-efficacy scale. 1995. [Google Scholar]
- Segal ZV, Williams MG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press; 2002. [Google Scholar]
- Tanielian T, Jaycox LH, Schell TL, Marshall GN, Burnam A, Eibner C, et al. Invisible Wounds: Mental Health and Cognitive Care Needs of America’s Returning Veterans. 2008 2015, from http://www.rand.org/pubs/research_briefs/RB9336.
- Travis F, Arenander A. EEG asymmetry and mindfulness meditation.[comment] Psychosomatic Medicine. 2004;66(1):147–148. doi: 10.1097/00006842-200401000-00020. author reply 147–148. [DOI] [PubMed] [Google Scholar]
- Vujanovic, Niles, Pietrefesa, Potter, Schmertz Potential of Mindfulness in Treating Trauma Reactions. 2014 Retrieved January 3, 2014, from http://www.ptsd.va.gov/professional/treatment/overview/mindful-PTSD.asp.
- Wahbeh H. Mindfulness meditation for posttraumatic stress disorder. In: Ie A, Ngnoumen CT, Langer E, editors. The Wiley Blackwell Handbook of Mindfulness. 1. Chichester, UK: John Wiley & Sons, Ltd; 2014. pp. 776–793. [Google Scholar]
- Wahbeh H, Oken BS. Mindful awareness and non-judging in relation to postraumatic stress disorder symptoms. Mindfulness. 2011;2:219–227. doi: 10.1007/s12671-011-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Oken BS. Peak high-frequency HRV and peak alpha frequency higher in PTSD. Applied Psychophysiology and Biofeedback. 2013a;38(1):57–69. doi: 10.1007/s10484-012-9208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Oken BS. A pilot study of clinical measures to assess mind-body intervention effects for those with and without PTSD. Alternative and Integrative Medicine. 2013b;2(4) doi: 10.4172/2327-5162.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Oken BS. Salivary cortisol lower in posttraumatic stress disorder. Journal of Traumatic Stress. 2013c;26:1–8. doi: 10.1002/jts.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Senders A, Neuendorf R, Cayton J. Complementary and alternative medicine for posttraumatic stress disorder symptoms: a systematic review. Journal of Evidence-Based Complementary & Alternative Medicine. 2014;19(3):163–177. doi: 10.1177/2156587214525403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Svalina MN, Oken BS. Group, One-on-One, or Internet? Preferences for Mindfulness Meditation Delivery Format and their Predictors. Open Medicine Journal. 2014;1:66–74. doi: 10.2174/1874220301401010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Zwickey H, Oken BS. One method for objective adherence measurement in mind-body medicine. J Altern Complement Med. 2011;17(2):175–177. doi: 10.1089/acm.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality & Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD checklist:reliability, validity, and diagnostic utility. Paper presented at the Annual Meeting of the International Society for Traumatic Stress Studies; San Antonia, TX. Oct, 1993. [Google Scholar]
- Weiss D, Marmar C. The Impact of Event Scale-Revised. New York: Guildford; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.