Abstract
Background
Slow gait is a component of frailty assessment in older adults, but its prognostic value in predicting outcomes one year following acute myocardial infarction (AMI) is unknown.
Design
Observational cohort with longitudinal follow up.
Setting
24 US Hospitals participating in the TRIUMPH Registry.
Participants
338 older adults (age ≥ 65 years) with in-home gait assessment one month following acute myocardial infarction.
Measurements
Baseline characteristics and one-year mortality or hospital readmission adjusted using Cox proportional hazards regression among older adults with slow (<0.8m/s) vs. preserved (≥0.8m/s) gait speed.
Results
Slow gait was present in 181/338 older adults (53.6%). Those with slow gait were older, more often female and nonwhite, and had a higher prevalence of heart failure and diabetes. They were also more likely to experience one-year mortality or readmission compared with those with preserved gait (35.4% vs. 18.5%, log-rank P=.006). This association remained significant after adjusting for age, sex, and race (slow vs. preserved gait HR = 1.76, 95% CI 1.08–2.87, P=.02), but was no longer significant after adding clinical factors (HR = 1.23, 95% CI 0.74 to 2.04, P=.43).
Conclusion
Slow gait, a marker of frailty, is common one month after AMI in older adults and is associated with a nearly twofold increased risk for mortality or readmission at one year. Understanding its prognostic importance independent of comorbidities and whether routine testing of gait speed can improve care requires further investigation.
Keywords: Coronary artery disease, older adults, frailty, outcomes
INTRODUCTION
Frailty, defined by an increased physiologic vulnerability to stressors, 1 is playing an increasingly important role in clinical risk stratification of older adults1–4. Gait speed, a single performance measure for identifying physical frailty across clinical settings, is associated with mortality4,5, loss of independence6, hospitalization7, and nursing home placement8,9. Gait speed is an accepted health metric among community-dwelling older adults, yet its utility among older adults with acute cardiac conditions has only recently been explored10.
To better characterize the prognostic importance of frailty in older adults recovering from an acute myocardial infarction (MI), we conducted a multi-center study and hypothesized that slow gait speed at one month would be associated with an increased likelihood of mortality or hospital readmission at one year. An association of slow gait with outcomes would substantiate its importance as a marker of risk in a cardiac population. It would also have implications for identifying vulnerable individuals who stand to benefit from closer monitoring and rehabilitation in the post-MI period.
METHODS
Study Design and Participants
Details of the Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction: Patients’ Health status (TRIUMPH) registry have been previously described11. Briefly, TRIUMPH is a prospective, multi-center registry of AMI patients from 24 study sites across the United States. Patients were ≥18 years of age and met objective criteria for AMI (biomarker evidence of myocardial injury and clinical features of ischemia), and presented to the enrolling institution within 24 hours of symptom onset. Enrollment occurred between April 2005 and December 2008.
Patients underwent detailed interviews by trained research personnel within 24 to 72 hours of initial presentation, with additional information obtained by chart abstraction. Data were collected on an array of variables, including sociodemographic characteristics, medical comorbidities, AMI severity, interventions and events during hospitalization, and discharge medications. At 1, 6, and 12 months following AMI discharge, patients were interviewed to obtain follow-up clinical and health status data.
At 1 month, all patients who were ≥65 years of age were invited to participate in an additional in-home assessment of gait speed. Participants who consented to the in-home assessment, but were unable to complete the walk test due to self-reported physical limitations (N=6), were included in the sample and their gait speed was scored as zero.
All participants provided written informed consent, and the institutional review board at each participating site approved the protocols.
Gait Speed
To measure gait speed, a flat space 6 meters in length, free of obstructions for half a meter on either side, was identified in the home. The course was marked with masking tape at four points such that the internal space between markers 2 and 3 measured 5 meters. Participants were instructed to start at the first point and walk at their usual pace from one end of the course to the other. They were timed in seconds between markers 2 and 3. Gait speed was calculated as the average velocity in meters per second (m/s) of three of these timed walks with a brief recovery period between each walk. Slow gait was defined by gait speed <0.8 m/s. 5
Outcomes
The primary outcome was the combination of mortality or hospital readmission at one year following hospitalization for AMI. Mortality was assessed through follow-up interviews and a query of the Social Security Death Masterfile12. Data on hospital readmissions were collected during follow-up interviews. If a hospital readmission was reported, charts were requested and adjudicated according to previously described methods11. For descriptive purposes, readmissions were categorized as cardiovascular (a primary diagnosis of AMI, unstable angina, sudden cardiac death, heart failure, or arrhythmia) or not cardiovascular (all other primary diagnoses).
Statistical Analysis
We compared characteristics of patients with slow gait versus preserved gait using the t-test for continuous variables and the Pearson’s Chi-squared or Fisher’s exact test for categorical variables, as appropriate. For the primary outcome of mortality or hospital readmission within 1 year of the AMI, we generated survival curves comparing patients with and without slow gait identified at the 1 month assessment using the Kaplan-Meier method and tested significance using the log-rank test. Patients were censored at the time of first readmission or mortality event. We then adjusted the association of gait speed with the primary outcome by using a multivariable Cox proportional hazards model adjusting for age, sex, and race (Model 1), and additionally adjusting for clinically relevant covariates (atrial fibrillation, heart failure, hypertension, peripheral vascular disease, diabetes, chronic lung disease, advanced renal dysfunction [creatinine clearance <30]) (Model 2). All tests for statistical significance were two-tailed with an alpha level of 0.05.
Analyses were conducted using SAS software, release 9.3 (SAS Institute, Cary, North Carolina) and R version 2.14.113.
To account for potential bias attributable to those with missing follow-up data, we calculated a non-parsimonious propensity score with successful follow-up as the dependent variable. An inversely weighted propensity score was assigned to each participant 14 to provide greater weight to the gait speed of patients who were most like those without follow-up. Results were comparable with and without weighting, so only the unweighted analyses are presented.
RESULTS
Study sample characteristics
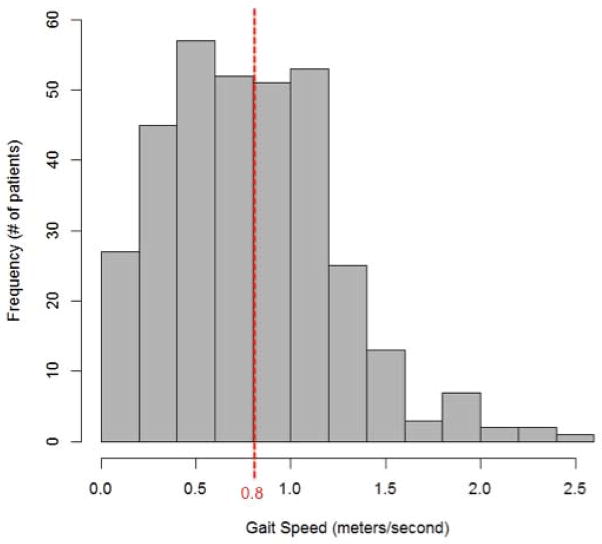
Of the 1,314 older adults (age ≥65) in TRIUMPH, 994 could not be assessed for gait speed, leaving a cohort of 338 participants with 1 month gait speed tested. Of the 994 without gait speed at one month, 37 had died prior to 1 month, 596 had a phone interview only (so were missing the in-home assessment), and 90 had the in-home assessment but declined the gait speed test. Those without an in-home assessment were less likely to have at least a high school education (43.7% vs. 52.5%, P=.005), less likely to have been revascularized at the time of initial MI hospitalization (65.5% vs. 75.4%, P<.001), and more likely to have reported problems with usual activities, self-care and mobility at baseline (P<.001) compared with those who did. (Appendix) Among the older adults with 1-month gait assessment, 181 (53.6%) had slow gait speed (Figure 1). Compared with participants with preserved gait, those with slow gait speed were older, more likely to be nonwhite, female and had higher comorbidity burden (Table 1).
Figure 1.
Distribution of gait speed among study participants (N=338). Patients with gait speed <0.8 m/sec were classified as having slow gait.
Table 1.
Study Sample Characteristics
| Slow gait (N=181) | Preserved gait (N=157) | P Value | |
|---|---|---|---|
| Age (mean ± SD) | 74.9 ± 6.5 | 72.2 ± 5.4 | <.001 |
| Female | 52.5% | 27.4% | <.001 |
| Nonwhite race | 29.3% | 14.0% | <.001 |
| Comorbid diseases | |||
| Atrial fibrillation | 7.7% | 4.5% | .213 |
| Heart failure | 12.7% | 5.7% | .029 |
| Hypertension | 80.7% | 70.1% | .023 |
| Diabetes | 35.9% | 24.2% | .020 |
| Chronic lung disease | 12.2% | 5.7% | .041 |
| Advanced renal dysfunctiona | 9.7% | 4.5% | .074 |
| Peripheral vascular disease | 8.3% | 5.7% | .362 |
| AMI presentation | |||
| ST-elevation AMI | 33.7% | 46.5% | .016 |
| Shock | 2.8% | 3.8% | .584 |
| In-hosp. revascularization | 70.7% | 80.9% | .030 |
| Participated in rehab (6 mo.) | 40.0% | 52.4% | .040 |
Defined as calculated creatinine clearance <30 mL/min
SD=standard deviation
Death or Readmission at 1-year
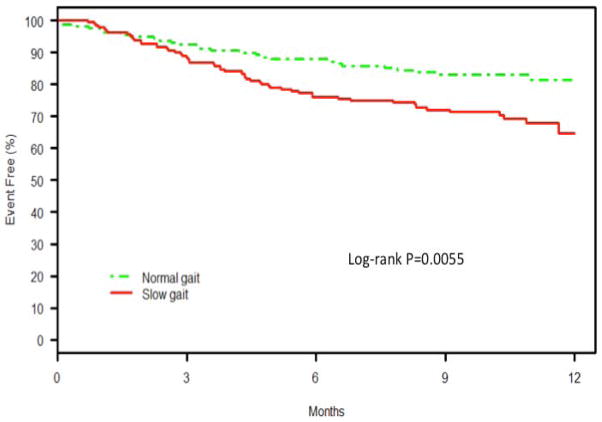
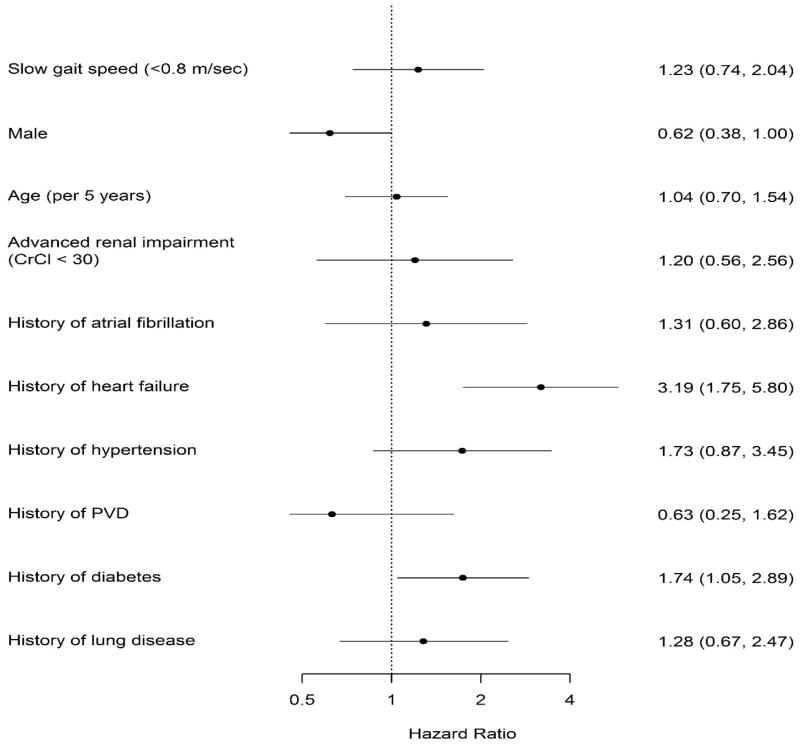
At one year, older adults with slow gait were significantly more likely to experience mortality or hospital readmission compared with older adults with preserved gait (KM-estimated rates 35.4% vs. 18.5%, log-rank P=.006). (Figure 2) The majority of these adverse events in both groups were readmissions, with mortality being a relatively uncommon contributor to the composite (slow gait: 12/55 events [21.8%]; preserved gait: 6/27 events [22.2%]). Older adults with slow gait had a two-fold higher unadjusted hazard for mortality or hospital readmission than those with preserved gait (HR 1.90 (95% CI 1.20 to 3.01), P=.01). This association remained significant after adjustment for age, sex, and race (HR 1.76, 95% CI 1.08–2.87, P=.02) but not after adding clinical characteristics (atrial fibrillation, heart failure, hypertension, peripheral vascular disease, diabetes, chronic lung disease, advanced renal dysfunction) (HR = 1.23, 95% CI 0.74 to 2.04 P=.43) (Figure 3). Sensitivity analyses using both a lower cut point for gait speed at <0.65m/s (data not shown) and using continuous gait per 0.2m/s decline demonstrated similar results. For each 0.2m/s decline in gait speed, the age/sex and race adjusted HR for death or rehospitalization was 1.18 (1.05–1.33); p=0.005. After full adjustment this association was no longer significant (HR 1.04 (0.93–1.16); p=0.507).
Figure 2.
Kaplan-Meier survival curves for combined endpoint of mortality or hospital readmission among patients with slow gait (<0.8 m/sec) versus normal gait (≥0.8m/sec) within 1 year of AMI.
Figure 3.
Multivariable association of slow gait and other baseline characteristics with one year mortality or hospital readmission.
Hospital readmissions at 1 year were significantly higher among those with slow gait speed (34% vs. 16%, p= .01). Readmissions were classified as cardiovascular (MI, unstable angina, sudden cardiac arrest, heart failure, arrhythmia) and non-cardiovascular (all other diagnoses). Overall, fewer than half of the readmissions were cardiovascular in either group (18/43 [41.9%] slow gait, 10/21 [47.6%] preserved gait, p=.66). The hazard for readmission with slow gait persisted after adjustment for age, sex and race (HR 1.87 (95% CI 1.07 to 3.24), P=.026), but after full adjustment (HR = 1.45, 95% CI 0.82 to 2.59 P= .202) was no longer significant.
DISCUSSION
Slow gait speed, an indicator of physical frailty, assessed one month after discharge is a predictor of adverse outcomes in older MI survivors. Older adults with slow gait speed post-MI experienced greater mortality or hospital readmission at one year after adjusting for age, sex and race. This association was no longer significant after adjusting for comorbidities, perhaps due to limited sample size, or the fact that comorbidities are along the causal pathway of frailty and adjusting for them diminishes the independent association of frailty with outcomes. However, biologic plausibility and the well-established relationship between frailty and outcomes in community-dwelling older adults4,5,15 and recent studies in STEMI patients10,16 lend context to interpreting these results. More than half of the older adult population who underwent gait speed testing had slow gait, despite having less mobility impairment and higher education than those who did not have a gait speed, further underscoring the benefits of directly measuring gait speed.
The risk associated with slow gait speed in older adults is partly attributable to comorbidities, including heart failure and diabetes. Heart failure commonly co-exists with frailty17, and is an independent driver of hospital readmissions and mortality18. Diabetes has been previously described as a risk factor for hospital readmissions in older adults.19,20 Diabetics are at risk of developing frailty 21 and both conditions share common inflammatory pathways.22 Adjusting for diabetes, or heart failure, may be in the same causal pathway therefore diminishing the association between gait and outcomes with the fully adjusted model in this relatively small sample. Disentangling slow gait speed attributable to frailty from that attributable to other comorbid conditions is challenging. Gait speed as a predictor independent of comorbidities may be less important than gait speed as a stratification tool, identifying those who would benefit from follow up targeted to improve outcomes.
Our findings extend prior work on the association between performance measures in hospitalized older adults and short-term outcomes. Two studies found gait speed and short physical performance battery were predictive of length of stay or likelihood of home discharge.23,24 Another study found the short physical performance battery performed at discharge was predictive of rehospitalization, death and difficulty in ADLs during follow up. 25 While it is premature to recommend routine gait speed screening post-MI, the ongoing NIH-funded SILVER-AMI trial (NCT01755052), may provide further insight into the association of this measure with long-term outcomes. Risk stratification may enable the use of a number of interventions for vulnerable older adults with acute cardiac conditions, including improving transitions of care at hospital discharge26,27, implementing early follow-up after hospitalization27, and increasing referrals to cardiac rehabilitation.28 There is also growing evidence that physical activity may prevent or reverse the frailty phenotype29,30 and that structured physical activity should be recommended. Therefore, further development of gait speed as an identifier of vulnerable cardiac patients is warranted.
Our study has several limitations. First, the small sample size limited our ability to adjust for multiple potentially important covariates. Second, gait speed was performed one month after AMI hospitalization to allow for recovery from the acute event. This limited participation with a significant number of participants who either could not be contacted or declined an in-home assessment. While there were differences between those who did and did not have the gait speed assessment, our use of inverse propensity weighting to obtain a more generalizable assessment of the association of gait speed with outcomes suggests no meaningful impact related to this association.
CONCLUSION
Slow gait speed, an indicator of physical frailty, was present in half of older adults recovering from an MI hospitalization and was associated with a nearly twofold increase in mortality or hospital readmission over the subsequent year. Larger, prospective studies with complete ascertainment of gait speed are needed to clarify its optimum use for risk stratifying and guiding therapy in older adults after an MI.
Acknowledgments
Funding Source: TRIUMPH was sponsored by a grant from National Heart, Lung, and Blood Institute (NHBLI) (P50 HL077113). Dr. Dodson is supported by a National Institute of Aging (NIA) grant (R03AG045067) and a T. Franklin Williams Scholarship Award (funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American College of Cardiology). Dr. Arnold is supported by a Career Development Grant Award (K23 HL116799) from the NHLBI. Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the NIA. Dr. Krumholz is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the NHLBI.
Appendix: Missing gait speed and in-home gait speed populations
| No Gait Speed (N=994) | Gait Speed (N=338) | P Value | |
|---|---|---|---|
| Age (mean ± SD) | 73.8 ± 6.8 | 72.2 ± 5.4 | .726 |
| Female | 42.2% | 40.8% | .670 |
| Nonwhite race | 27.7% | 22.2% | .087 |
| >HS education | 43.7% | 52.5% | .005 |
| Revascularization during index hospitalization | 65.5% | 75.4% | <.001 |
| Comorbid diseases | |||
| Atrial fibrillation | 11.3% | 6.2% | .007 |
| Heart failure | 12.4% | 9.5% | .150 |
| Hypertension | 77.9% | 75.7% | .420 |
| Diabetes | 37.1% | 30.5% | .027 |
| Chronic lung disease | 10.2% | 9.2% | .599 |
| Advanced renal dysfunctiona | 11.8% | 8.3% | .075 |
| Peripheral vascular disease | 7.5% | 7.1% | .788 |
| Baseline EQ-5D | |||
| Mobility (some problems or confined) | 47.6% | 36.1% | <.001 |
| Self-care (some problems or unable to do) | 26.0% | 14.3% | <.001 |
| Usual Activities (some problems or unable to do) | 51.1% | 40.9% | <.001 |
Defined as calculated creatinine clearance <30 mL/min
SD=standard deviation
Footnotes
Author Contributions:
John A. Dodson: Conception, interpretation of data, drafting and revising, final approval
Suzanne V. Arnold: Reviewing and revising, final approval
Kensey L. Gosch: Analysis and interpretation of data, final approval
Thomas M. Gill: Interpretation of data, revising critically, final approval
John Spertus: Acquisition of data, interpretation, revising critically, final approval
Harlan M. Krumholz: Acquisition of data, interpretation, revising critically, final approval
Michael W. Rich: Interpretation of data, revising critically, final approval
Sarwat I. Chaudhry: Interpretation of data, revising critically, final approval
Daniel E. Forman: Interpretation of data, revising critically, final approval
Frederick A. Masoudi: Interpretation of data, revising critically, final approval
Karen P. Alexander: Conception, interpretation of data, drafting and revising, final approval
Sponsor’s Role: None.
Conflict of Interest Disclosures:
Dr. Spertus owns the copyright to the Seattle Angina Questionnaire, serves on the Scientific Advisory Board for United Healthcare, and has had grant support from Lilly, Genentech, Abbott Vascular, EvaHeart, Gilead and Amorcyte. Dr. Krumholz is a recipient of research grants from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing, and is chair of a cardiac scientific advisory board for UnitedHealth. Dr. Masoudi has a contract with the American College of Cardiology. The other authors report no conflicts of interest.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Taekema DG, Gussekloo J, Westendorp RG, et al. Predicting survival in oldest old people. Am J Med. 2012;125:1188–1194. doi: 10.1016/j.amjmed.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 4.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: The role of physical performance. J Am Geriatr Soc. 1995;43:603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 7.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 9.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzawa Y, Konishi M, Akiyama E, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61:1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): Design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2:2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Development Core Team. A language and environment for statistical computing [Google Scholar]
- 14.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 15.Dumurgier J, Elbaz A, Ducimetiere P, et al. Slow walking speed and cardiovascular death in well functioning older adults: Prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–2404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 17.Flint KM, Matlock DD, Lindenfeld J, et al. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail. 2012;5:286–293. doi: 10.1161/CIRCHEARTFAILURE.111.963215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang HJ, Stryer D, Friedman B, et al. Multiple hospitalizations for patients with diabetes. Diabetes Care. 2003;26:1421–1426. doi: 10.2337/diacare.26.5.1421. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Ross JS, Melkus GD, et al. Scheduled and unscheduled hospital readmissions among patients with diabetes. Am J Manag Care. 2010;16:760–767. [PMC free article] [PubMed] [Google Scholar]
- 21.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 23.Ostir GV, Berges I, Kuo YF, et al. Assessing gait speed in acutely ill older patients admitted to an acute care for elders hospital unit. Arch Intern Med. 2012;172:353–358. doi: 10.1001/archinternmed.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpato S, Cavalieri M, Guerra G, et al. Performance-based functional assessment in older hospitalized patients: Feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci. 2008;63:1393–1398. doi: 10.1093/gerona/63.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prvu Bettger J, Alexander KP, Dolor RJ, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: A systematic review. Ann Intern Med. 2012;157:407–416. doi: 10.7326/0003-4819-157-6-201209180-00004. [DOI] [PubMed] [Google Scholar]
- 27.Bradley EH, Curry L, Horwitz LI, et al. Contemporary evidence about hospital strategies for reducing 30-day readmissions: A national study. J Am Coll Cardiol. 2012;60:607–614. doi: 10.1016/j.jacc.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammill BG, Curtis LH, Schulman KA, et al. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson MJ, Giuliani C, Morey MC, et al. Physical activity as a preventative factor for frailty: The health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64:61–68. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]