Abstract
Outer membrane vesicles (OMV) are critical elements in many host-cell/microbe interactions. Previous studies of the symbiotic association between Euprymna scolopes and Vibrio fischeri had shown that, within 12 h of colonizing crypts deep within the squid’s light organ, the symbionts trigger an irreversible program of tissue development in the host. Here, we report that OMV produced by V. fischeri are powerful contributors to this process. The first detectable host response to the OMV is an increased trafficking of macrophage-like cells called hemocytes into surface epithelial tissues. We showed that exposing the squid to other Vibrio species fails to induce this trafficking; however, addition of a high concentration of their OMV, which can diffuse into the crypts, does. We also provide evidence that tracheal cytotoxin (TCT) release by the symbionts, which can induce hemocyte trafficking, is not part of the OMV cargo, suggesting two distinct mechanisms to induce the same morphogenesis event. By manipulating the timing and localization of OMV signal delivery, we showed that hemocyte trafficking is fully induced only when V. fischeri, the sole species able to reach and grow in the crypts, succeeds in establishing a sustained colonization. Further, our data suggest that the host detection of OMV serves as a symbiotic checkpoint prior to inducing irreversible morphogenesis.
Introduction
The binary association between the Hawaiian bobtail squid, Euprymna scolopes, and its specific bioluminescent symbiont, Vibrio fischeri, provides an opportunity to study how bacteria promote epithelial colonization by establishing a molecular communication with their host. In this symbiosis, the juvenile squid hatch aposymbiotically, and acquire their V. fischeri symbionts from the surrounding seawater. V. fischeri specifically colonize the dedicated light-emitting organ (Nyholm et al. 2000; McFall-Ngai 2014). Within the squid’s seawater-filled mantle cavity, the nascent light organ exposes two pairs of prominent appendages composed entirely of a monolayer of ciliated epithelium wrapped around a fluid-filled sinus. These structures facilitate bacterial recruitment by bringing V. fischeri cells into the vicinity of six pores, each leading to a deep interior crypt, the sites of symbiont colonization. Specifically, after the squid hatches, the ciliated epithelium starts to shed mucus that facilitates the capture of symbionts (Nyholm et al. 2002). By 4 h, V. fischeri cells have formed an aggregate on the epithelium, and begun migrating to and through the surface pores, guided by chemotaxis (Brennan et al. 2013b). After 8 h, a few symbionts have reached the deep crypts and begun to multiply (McFall-Ngai and Ruby 1991). By around 12 h, the symbionts have multiplied to a population of several hundred thousand, filling the crypts, and the colonized organ undergoes an irreversible morphogenesis, leading to the loss of its ciliated epithelial surface (Montgomery and McFall-Ngai 1994; Nyholm et al. 2000; Doino and McFall-Ngai 1995), and the cessation of mucus production (Nyholm et al. 2000). Previous studies showed that this colonization-induced developmental program is due primarily to the synergistic activity of peptidoglycan (PG) and lipopolysaccharide (LPS) derivatives (Foster, et al. 2000; Koropatnick et al. 2004); however, it has remained unclear how and where these signaling molecules are presented to the host. Within 4 days, initial light-organ maturation is achieved, and is characterized by both a cessation of mucus shedding and the regression of the appendages (Montgomery and McFall-Ngai 1994; Koropatnick et al. 2004; Brennan et al. 2014), events that lower the probability of further inoculation from the environment (Foster and McFall-Ngai 1998; Nyholm et al. 2002). An early indication of these developmental changes in the organ is an increased presence of macrophage-like blood cells called hemocytes, which typically occurs concomitantly with symbiont colonization. Hemocytes begin to infiltrate the two superficial epithelial fields of inoculated light organs as early as 2 h and, if the colonization is sustained, reach a maximum presence by 18 h (Koropatnick et al. 2007). Addition of Vibrio fischeri PG, and in particular the monomeric form called ‘tracheal cytotoxin’ (TCT), specifically triggers this developmental event to a level comparable to that induced by the symbionts (Koropatnick eta l. 2007), whereas V. fischeri LPS does not induce this phenotype.
Both pathogens and non-pathogens export PG derivatives that act as strong agonists of host cells (Koropatnick et al. 2004; Johnson et al. 2013; Adin et al. 2009; Boudreau et al. 2012). Its importance in beneficial microbial relationships was first described in the squid/vibrio model (Koropatnick et al. 2004); however, it has remained a mystery how such PG fragments are delivered across the outer membrane of Gram-negative bacteria to trigger responses in eukaryotic cells. One possible mechanism is the release of outer membrane vesicles (OMV), which can deliver a suite of molecular cargo to nearby cells and induce the PG-reactive NOD-like receptors of non-phagocytic animal cells (Bielig et al. 2011; Kaparakis et al. 2010; Mashburn-Warren et Whiteley 2006). OMV are secreted continuously, and contain surface-associated molecules such as outer membrane proteins, lipids and LPS, as well as periplasmic components and quorum-signaling molecules (Kuehn et Kesty 2005; Kulp et Kuehn 2010). While first reported to deliver virulence factors (Ellis et Kuehn 2010), OMV also function in beneficial associations, e.g., transporting to the epithelium a tolerance-inducing polysaccharide of the common human-gut bacterium Bacteriodes fragilis, or selectively delivering enzymes that function in nutrient acquisition (Shen et al. 2012; Elhenawy et al. 2014).
It has become clear that the reaction of a host’s immune system to microbe-associated molecular patterns (MAMPs) like PG or LPS fragments is complex: the nature of the response depends upon the signal’s microbial context (Casadevall and Pirofski 2015). We believe that an understanding of when and where such signals are perceived will help reveal how the host modulates and differentiates its responses toward either a pathogenic infection or a beneficial partnerships.
Vibrio fischeri was been reported to produce OMV that are part of the symbiont’s biofilm formation; electron micrographs indicated them to average 30 nm in diameter (Shibata and Visick 2012). We hypothesized here that OMV are a vehicle by which V. fischeri delivers signals that participate in the triggering of developmental maturation of the squid light organ. In this study, we focus on the induction of hemocyte trafficking. We provide evidence that OMV are capable of triggering hemocyte trafficking into surface epithelia, and experimentally manipulated the delivery of the OMV’s morphogenic signal(s) to determine how it is perceived and processed by the host. The data suggest that (i) hemocyte trafficking is fully induced only after the symbionts reach and proliferate within the deep crypts, (ii) the level of trafficking depends on the signal intensity, (iii) host cells must internalize OMV to induce trafficking, and (iv) OMV synergize with other symbiont MAMPs, such as TCT and light, to drive symbiont-induced developmental processes. Further, we show that V. fischeri OMV does not carry the previously recognized squid morphogen TCT. Taken together, the data suggest that, to participate in light-organ maturation, OMV have both a specific timing and a specific tissue target. In this way, the symbiosis uses OMV delivery as a checkpoint by which to assure that an irreversible maturation of the light organ is not triggered before a successful colonization is firmly established.
Results
OMV are sufficient to induce light-organ maturation
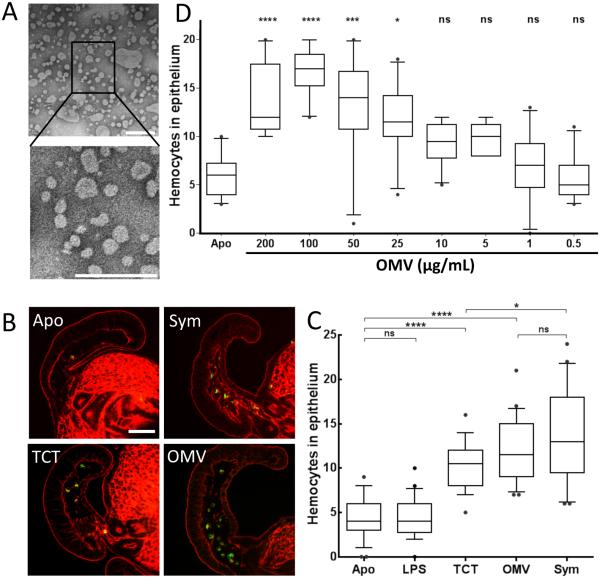
To determine whether OMV produced by V. fischeri (Fig. 1A) might present MAMPs associated with the light organ maturation, we first asked whether these OMV can, by themselves, trigger hemocyte trafficking, a morphogenic change known to be specifically induced by PG fragments (Koropatnick et al. 2004). The other morphogenetic signal LPS (up to 50 mg/mL) induces late apoptosis in synergy with TCT, but is not involved in the induction of hemocyte trafficking (Koropatnick et al. 2004). As a measure of trafficking we quantified the average number of hemocytes present in the appendages after 18 h of exposure to either the symbiont, purified TCT, a previously defined active PG derivative (Koropatnick et al. 2004), purified V. fischeri LPS, or OMV. We found that OMV induced infiltration to a similar level as either TCT or the presence of symbionts, whereas LPS alone did not induce infiltration (Fig. 1B,C). We then exposed juvenile squids to different concentrations of OMV, and showed that the extent of hemocyte trafficking increased in intensity with the level of OMV exposure, reaching a maximum at a dose of 100 μg of OMV protein/ml (Fig. 1D). Such a concentration is consistent with what a population of 104 symbionts could be producing in the light-organ crypts (see details in Experimental procedures). These data show that OMV could be sufficient to induce a PG-associated morphogenic change characteristic of normal light-organ maturation.
Fig. 1.
OMV induce a phenotype associated with PG-linked light-organ morphogenesis. Influence of OMV addition on hemocyte trafficking. (A) Negative-stained TEM of purified OMV produced by wild-type V. fischeri ES114; boxed area enlarged below. Scale bars indicate 200 nm. (B) Confocal micrographs of one appendage of a juvenile light organ: red, rhodamine phalloidin (filamentous actin); green, DNAse I (hemocytes). Scale bar = 50 μm. (C) Quantification of hemocyte trafficking in symbiotic animals (exposed to 104 V. fischeri cfu/mL), or in animals treated with either TCT (1 μM), LPS (10 μg/mL) or OMV (100 μg of protein/mL), after 18 h; these levels of TCT and LPS are in the range that elicits other host responses (Foster et al. 2000; Koropatnick et al. 2004). Hemocytes were counted in the sinuses of the anterior appendage of one epithelial field per light organ. n=20; One-way ANOVA analysis (F=37; p<0.0001). (D) Levels of hemocyte trafficking in the anterior appendages of animals exposed for 18 h to a range of OMV concentrations, from 0.5 to 200 μg of protein/mL. n=20. One-way ANOVA analysis (F=28; p<0).
The induction of hemocyte trafficking is dependent on the intensity and location of signal delivery
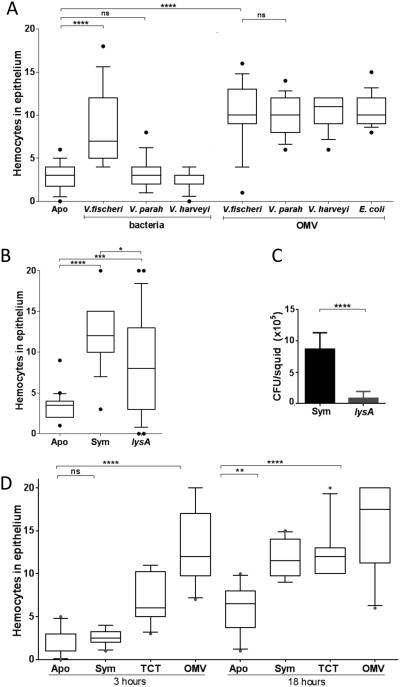
Because OMV are released by many bacteria, we asked why exposure to symbiotic V. fischeri, but not other closely related vibrios (e.g., V. parahaemolyticus and V. harveyi), induces a full level of hemocyte trafficking (Fig. 2A). First, we inoculated seawater containing juvenile squid to the same dose (100 μg of protein/ml) of OMV isolated from each one of several Gram-negative bacteria. Such treatments all resulted in a similar level of hemocyte trafficking (Fig. 2A), suggesting that the ability to signal is not due to a unique chemistry of the V. fischeri OMV, but rather is because (i) only the large population (>105) symbionts, growing within the crypts, can normally deliver OMV at a level sufficient for signaling, and (ii) only V. fischeri can colonize and proliferate within the light organ (Ruby and McFall-Ngai, 1992). Consistent with this notion, a V. fischeri lysine auxotroph (lysA) that colonizes to only 1% of wild-type levels induces a detectable, but significantly lower, trafficking response (Fig. 2B,C). Further, inoculation of seawater with a high dose of OMV, which can then equilibrate quickly into the crypt space, induces maximum hemocyte appearance as early as 3 h (Fig. 2D), rather than the 18-24 h required by the typical colonization by a few V. fischeri cells (Koropatnick et al. 2007). We conclude that the delay reflects how the few cells that inoculate the crypts must divide 10-12 times before they have populated the crypts to a level sufficient to produce an inducing level of OMV (see details in Experimental procedures).
Fig. 2.
Hemocyte trafficking is dependent on the intensity and location of signal delivery. The extent of trafficking was determined by counting hemocytes in the anterior appendage of one epithelial field per light organ. (A) Juvenile squid were exposed for 18 h to either 104 cfu of V. fischeri, V. parahaemolyticus (V. parah), or V. harveyi per ml, or 100 μg of OMV produced by these strains, or by E. coli. One-way ANOVA analysis (F=21; p<0.0001). (B) Juvenile squid were exposed to 104 cfu of wild-type V. fischeri (Sym), or a isogenic lysA derivative, per ml. One-way ANOVA analysis (F=20; p<0.0001). (C) Symbiont population levels after 48 h in squid exposed to either the wild-type (Sym) or the auxotrophic lysA strain, determined in three independent experiments, representing a total of 60 squids per condition. Starting inoculum levels ranged from 8-11 × 103 cfu/mL. Graphical and errors bars indicate average and standard deviation of data. p<0.05. (D) Juvenile squid were exposed for either 3 or 18 h to seawater only (Apo), or seawater containing 104 V. fischeri cfu/mL (Sym), or to 1 μM TCT, or 100 μg of OMV protein/mL. One-way ANOVA analysis (F=27; p<0.0001).
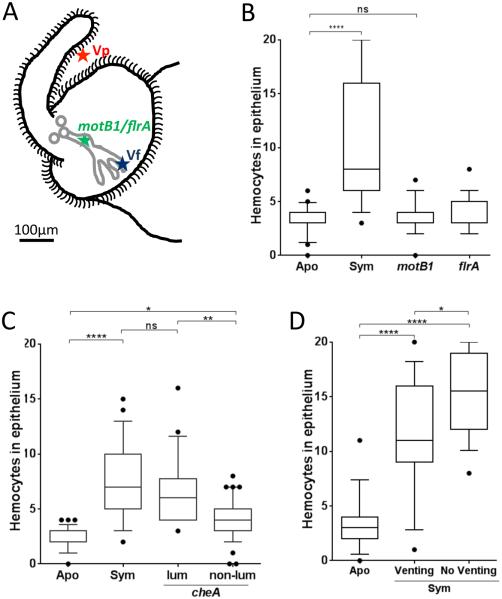
Only V. fischeri cells are capable of successfully migrating through the pores, ducts and antechambers to reach and populate the deep crypts. To differentiate whether the signal is delivered on the way to, or within, the crypts, we asked how the level of hemocyte-trafficking induction is affected in V. fischeri mutants with different abilities to migrate there (Fig. 3A). Mutants that either do not express any flagella (flrA), or are unable to rotate them (motB1), migrate no farther than the antechambers (Brennan et al. 2013A) and are unable to induce hemocyte infiltration (Fig. 3B). Similarly, with a chemotaxis mutant (cheA) that has a colonization success rate of <30% (Brennan et al. 2013B), only those cells that manage to establish a sustained colonization induce a normal level of hemocyte trafficking (Fig. 3C). Finally, in response to the light cue of dawn, the squid expels ~90% of its symbionts out into the seawater through the pores (Lee and Ruby 1994). However, if this light cue is withheld, the expulsion is delayed, and the crypts remain full. We showed that the absence of an expulsion event has only a minor effect on the response (Fig. 3D), indicating that the required signal is not delivered along the venting route as the expelled symbionts exit. Taken together, these data suggest that the hemocyte-trafficking response, while beginning at inoculation (Koropatnick et al. 2007), is only fully induced (i) when a sustained symbiont colonization is established, and (ii) by signal(s) that are delivered within the crypts spaces, probably by OMV, TCT and perhaps other MAMPs shed from the dense symbiont population located there.
Fig. 3.
Bacterial delivery of the hemocyte-trafficking signal. The extent of hemocyte trafficking was determined by counting hemocytes in the sinuses of the anterior appendage of one epithelial field per light organ. (A) Diagram of the left side of a juvenile light organ showing both the surface in contact with the seawater (black outline) and the internal structures (pores, ducts, antechamber and deep crypts) through which symbionts migrate (gray). Indicated are the limits of migration of V. parahaemolyticus (Vp), which like other non-symbionts doesn’t enter the three pores; V. fischeri wild type (Vf), which migrates to and grows in the deep crypts; and, motility mutants (flrA; motB1), which cannot pass beyond the organ’s antechamber. The localization of the cheA mutant, which has been found to stochastically enter the pore (Mandel et al. 2012), is complex and has not been indicated here. When added to the surrounding seawater small particles, like OMVs, can diffuse into the crypts. See text for a full explanation. (B) Juvenile squid were exposed to motB1 and flrA mutants unable to complete the migration into the crypts. (C) Juvenile squid were exposed to 104 cfu of a V. fischeri cheA mutant per mL. After 18 h, animals were sorted into two groups: detectably luminescent (Lum) or not (Non-lum); in the former group, the cheA cells were presumed to have reached a crypt and proliferated, while in the latter, the cheA cells were presumed to have been unable to establish a sustained colonization. n=60. One-way ANOVA analysis (F=31; p<0.0001). (D) After inoculation, animals were either kept 12 h in the dark, followed by 4 h in the light to induce venting through the ducts and pores (Venting), or kept 16 h in the dark (No Venting) before the level of hemocyte trafficking was determined. One-way ANOVA analysis (F=37; p<0.0001).
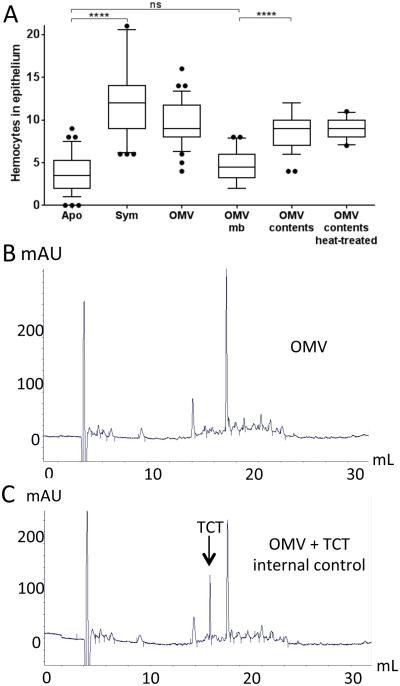
V. fischeri OMV and TCT induce hemocyte trafficking via two separate ways. To examine the chemical nature of the hemocyte trafficking signal(s) delivered by V. fischeri OMV, and determine whether OMV carry TCT, we first asked whether purified membrane and/or protein components of the vesicles can induce hemocyte trafficking. Separation of the OMV’s membrane fraction from their contents indicated that the activity inducing hemocyte trafficking is associated solely with the latter; in addition, treatment with heat (or proteinase-K; data not shown) did not significantly affect the ability of the OMV contents to induce trafficking (Fig. 4A). Thus, we conclude that a non-protein component(s) of the V. fischeri OMV cargo is responsible for inducing hemocyte trafficking. Next, using a previously well-established reverse-phase HPLC method (Cookson et al. 1989), we asked whether TCT itself was present within the OMV by comparing the elution profile obtained for a sample of the contents of OMV (Fig. 4B) with that of the same sample mixed with purified TCT, used here as an internal control (Fig. 4C). This comparison revealed no detectable TCT in the OMV (Fig. 4B,C), and indicated that any TCT produced by V. fischeri is not released within OMV. Not surprisingly, the same amount of TCT (248 and 240 pmol/mL, respectively) could be detected in V. fischeri culture supernatants before and after OMV removal by ultracentrifugation and filtration, confirming that V. fischeri cells most likely release TCT as a soluble molecule.
Fig. 4.
OMV induce hemocyte infiltration, but do not contain TCT. (A) To quantify hemocyte trafficking, 30 animals were exposed either to 104 V. fischeri cfu/mL (Sym), or to purified OMV (100 μg/mL), OMV membranes (mb), OMV contents, or heat-treated OMV contents. Lipid and protein concentrations after fractionation were determined by the FM4-based fluorescence assay and Qubit. Fluorescence associated with the OMV content is 8% and 92% with OMV membranes. One-way ANOVA analysis (F=34; p<0.0001). (B-C) Evidence for the presence of TCT in OMV contents was determined by reversed-phase HPLC. (B) HPLC profile of OMV sample (100 μg of protein). (C) Internal control of 10 nmole of TCT was loaded with the same OMV sample.
We then showed that the release of TCT and of OMV signals were two separate and apparently redundant systems for signaling morphogenesis. Using V. fischeri mutants that are colonization competent, but release either less or more free TCT into the supernatant than wild-type cells (Adin et al. 2009), we showed that modifying TCT production in the range of 15-200% of normal does not significantly affect induction of hemocyte trafficking (Fig. S1A). Interestingly, both over- and under-producing mutants release only 30-40% as much OMV material as the wild type (Fig. S1B). These data suggest that the mechanism responsible for TCT release is independent of the process of OMV production.
OMV internalization is required to induce hemocyte trafficking
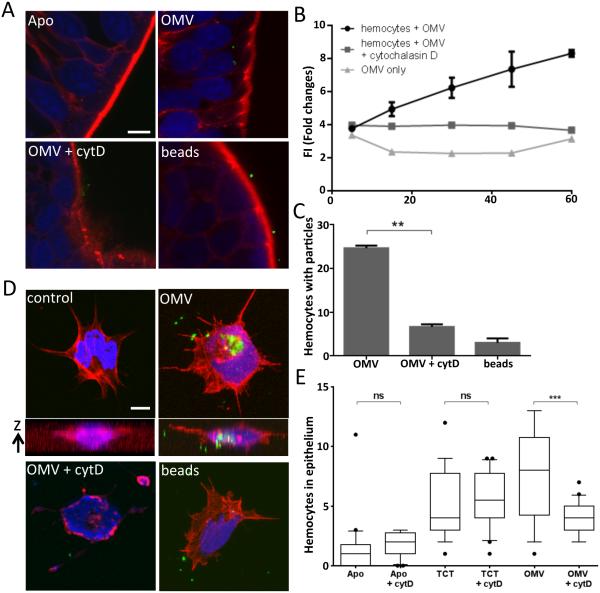
To investigate whether OMV are internalized by hemocytes, and/or epithelial cells on the light organ’s ciliated surface, fluorescently labeled V. fischeri OMV were added either to isolated hemocytes in vitro, or to seawater containing whole animals. Subsequent internalization of OMV was analyzed either directly using confocal microscopy (Fig. 5A,C & D), or indirectly by measuring hemocyte fluorescence (Fig. 5B). Both approaches provided evidence that the OMV were internalized by these two cell types, while small-diameter fluorescent beads were not, indicating that the internalization process that takes up OMVs displays specificity. Significantly, the appearance of OMV inside host cells was an actin-dependent process: treatment with cytochalasin D, an inhibitor of actin polymerization, prevented OMV uptake in all these assays (Fig. 5), suggesting that internalization involves phagocytosis. In addition, when animals were pre-treated with cytochalasin D, induction of hemocyte trafficking by added OMV, but not by soluble TCT, was reduced (Fig. 5E). These latter data further point to a difference in the mechanisms by which morphogenesis is induced by TCT and OMV, as well as lead us to conclude that internalization is a prerequisite for the participation of OMV in the triggering of this developmental event.
Fig. 5.
Internalization of V. fischeri OMV. (A) Confocal-microscopy sections showing the appearance of OMV in the cytoplasm of epithelial cells of the light-organ appendages after 3 h incubation with: FITC-labeled (green) OMV (OMV), OMV and cytochalasin D (OMV + cytD), or 2-μm diameter FITC-labeled beads (beads). Nuclei were stained with TOTO3 (blue), and F-actin with rhodamine phalloidin (red). (B) FITC-labeled OMV were incubated with hemocytes, and their fluorescence was measured over time as an estimation of OMV internalization. Data are presented as mean fluorescence intensity (FI), and error bars indicate one standard deviation; p < 0.05. (C) The numbers of cells with internalized FITC-labeled OMV or beads were determined for 30 treated hemocytes by confocal imaging. Data analyzed with one-way ANOVA analysis of differences: (**), p < 0.001. (D) Confocal-microscopy sections illustrating the internalization of FITC-labeled OMV by isolated hemocytes 90 min after an in vitro incubation; staining as in (A). A z-stack of sections illustrates internalized OMV fluorescence in the OMV-treated, but not control hemocytes. (E) Effect of cytochalasin D pretreatment on induction of hemocyte trafficking by OMV or TCT. n=20; One-way ANOVA analysis (F=18; p<0.0001). Size bars = 5 μm.
Discussion
The immature light organ of a newly hatched E. scolopes has evolved to interact with the environment and promote symbiont harvesting (McFall-Ngai 2014). Within a few hours of exposure to V. fischeri, hemocytes begin to infiltrate the surface epithelium (Koropatnick et al. 2007). While it remains unclear how these host cells are involved in morphogenesis of the light organ, hemocyte trafficking has been reported to accompany microbial induction of tissue remodeling in other mollusks (Lee et al. 2001), as well as in insect morphogenesis (Okazaki et al. 2006). Over the course of the next 4 days, the light organ’s ciliated surface epithelium regresses, an irreversible process requiring at least a 12-h exposure to V. fischeri (Doino and McFall-Ngai 1995), by which time the symbionts have delivered their morphogenic signal. Natural seawater contains millions of Gram-negative bacteria per ml; nevertheless, only an inoculation with V. fischeri cells will induce light-organ morphogenesis. We believe this specificity indicates that, in the natural environment, only V. fischeri can deliver the signals to the correct location, and thus at sufficiently high levels, to trigger the normal morphogenic response. Because of the irreversibility of this developmental event, the communication between symbionts and host needs to be precise enough that maturation is induced only when the symbionts have fully established themselves. Here, we show that OMV are involved in triggering hemocyte trafficking during the establishment of the squid/vibrio symbiosis; thus, communication via OMV is a shared mechanism in both pathogenic and mutualistic associations (Shen et al. 2012; Elhenawy et al. 2014). Exposure to a high concentration of OMV produced by any of the tested species is sufficient to induce a normal developmental event (i.e., hemocyte trafficking) in the light organ (Fig. 1). Because only V. fischeri can colonize the light-organ crypts, where a high concentration of OMV must be delivered, only an interaction with the correct symbiotic species will trigger morphogenesis.
Determining how V. fischeri can establish a highly specific symbiosis that concomitantly induces tissue maturation has long been a focus of research (McFall-Ngai 2014). We used hemocyte trafficking as a host response that indicates when and where the symbiont’s developmental signal is first delivered. This phenotype, which is well described in the squid/vibrio association, is one whose intensity follows the progression of the bacteria through the light organ (Koropatnick et al. 2007). Indeed, hemocyte infiltration into the light-organ appendages doesn’t simply increase linearly in time, but instead reflects three steps during colonization (Koropatnick et al. 2007): (i) trafficking is first observed as early as two hours after inoculation with V. fischeri, when bacteria are first forming an aggregate; (ii) a second increase in the number of hemocytes occurs after 10 h, as bacteria enter the crypts and begin to proliferate; and, finally, (iii) the highest level of hemocyte infiltration is detectable at 18 h, only after the symbionts have filled the crypts.
If the delay in reaching this highest level of hemocyte trafficking simply reflected the time needed to achieve a full symbiont population in the crypts, we would predict that, by adding OMV to a high concentration, similar to what the crypts are likely exposed to, the highest level of trafficking would be triggered sooner. As this hypothesis predicts, when the normal migration and growth process associated with a V. fischeri colonization is bypassed, maximum hemocyte infiltration occurs at least 10 h earlier (Fig. 2D). This finding further suggests that the signal for trafficking is delivered within the crypt, rather than elsewhere along the migration route, a prediction that was supported by the behavior of V. fischeri mutants (Fig. 3). Taken together, these data suggest that full hemocyte trafficking is induced within the deep crypts, in which a sustained colonization must have been established. Finally, because each dawn squid expel 95% of the symbionts through the pores, by delaying that event, we showed that the signal is not delivered as the bacteria reverse their migration path during expulsion. In fact, it seems that when a reduction in the population level is postponed, an even higher degree of trafficking is induced (Fig. 3D), perhaps due to the longer contact time with the full population of symbionts. Alternatively, because hemocytes are part of the host’s immune system, and deferring the daily expulsion delays restoration of the light-organ’s epithelial lining (E. Heath-Heckman, pers. comm.), the host may be responding with an increased immune presence.
It was previously shown that TCT, the monomeric form of PG, is the minimal structure needed to induce hemocyte trafficking (Koropatnick et al. 2004). Only a few studies have attempted to describe the mechanism(s) by which Gram-negative bacteria deliver PG fragments like TCT to host cells. In H. pylori, TCT is delivered in part by a bacterial secretion system, but PG was also apparently associated with OMV (Kaparakis et al. 2010; Viala et al. 2004); unfortunately, the nature of the PG associated with OMV was not investigated. Here, we showed that OMV produced by V. fischeri do not contain the monomer fragment, TCT (Fig. 4B-C). Previous studies showed that a V. fischeri strain that releases only low levels of TCT still induced significant regression of the ciliated appendages (Adin et al. 2009), suggesting that other signals or other forms of PG may be released and induce this morphogenic event. While hemocyte trafficking can be triggered by PG alone, the rest of light-organ development (e.g., late-stage apoptosis and full tissue regression) require the synergic activity of both PG and LPS. Because, the flagella sheath of Vibrio fischeri is involved in LPS release (Brennan et al. 2014), we are currently determining whether OMV also induce other phenotypes associated with the synergic activity of these two MAMPs. In addition, the results presented here raise further questions about the nature of signal delivery, such as: what are the other, non-TCT, PG fragments carried by OMV and, is there a link between flagellar rotation and OMV release? Finally, OMV are internalized by the superficial, ciliated epithelial cells of the light-organ, and such internalization is required to induce hemocyte trafficking (Fig. 5). Interestingly, we observed that while in the hemocytes, the OMV fluorescence marker appeared more diffuse over time. This observation is presents the possibility that OMV may lyse within the hemocytes, releasing their cargo into the cytoplasm. Thus, the nature of both the chemical complexity of OMV MAMP cargo, and the mechanisms of internalization and cargo delivery, remain open questions.
Because the process of light-organ maturation is irreversible, its induction must be tightly controlled (Koch et al. 2014). To ensure that only an effective, well established symbiont population will induce maturation, the host has evolved the ability to identify that the symbionts are properly localized, and that they have the capacity to fill the crypts. Consistent with this idea, symbiotic animals that are cured of their symbionts before maturation is complete will begin to shed mucus again, promoting the capture of new bacteria (Nyholm et al. 2002). These findings suggest a rationale for why signaling from a sustained colonization is required to induce complete light-organ maturation. In short, the level of OMV in the crypts reflects the status of a symbiotic V. fischeri population and, thus, detection of OMV by the host could be used as a checkpoint that indicates a well-established colonization and, in response, triggers organ maturation.
In conclusion, we provide evidence here that (i) V. fischeri produces OMV, which serve to establish molecular communication with its host; (ii) Vibrio OMV are sufficient to trigger a developmental event associated with light-organ maturation; (iii) these OMV are delivered in sufficient quantity to induce maturation only when colonization of the crypts is well established; and (iv) OMV signaling requires internalization, and appears to serve as a checkpoint to regulate light-organ morphogenesis.
Experimental procedures
Bacterial strains and media
The strains used in this study are listed in Table 1. V. fischeri ES114 was the wild-type strain. All marine vibrios were grown in either complex LB-salt (LBS) medium, or seawater tryptone (SWT) medium (Graf et al. 1994), while Escherichia coli DH5α was cultured in LB medium (Bertani 1951). When appropriate, antibiotics were added to media at the following concentrations: chloramphenicol (Cam) 2.5 μg/ml, erythromycin (Erm) 5 μg/ml, and kanamycin (Kan) 50 μg/ml.
Table 1.
Strains used in this study
| Strain | Description | reference |
|---|---|---|
|
Vibrio fischeri
ES114 |
ES114, sequenced wild-type E. scolopes light-organ isolate | (Boettcher and Ruby 1990) |
| ES114 cheA | VF_1831::Tnerm; chemotaxis histidine autokinase | (Brennan et al. 2013a) |
| ES114 motB1 | VF_0715::Tnerm; flagellar motor protein | (Brennan et al. 2013a) |
| ES114 flrA | VF_1856::Tnerm; σ54-dependent flagellum-synthesis regulator | (Brennan et al. 2013a) |
| ES114 lysA | VF_2485::Tnkan; lysine auxotroph | (Koropatnick et al. 2004) |
| DMA388 | ES114 ΔltgA ΔltgD ltgY::pDMA90; transglycosylases | (Adin et al. 2009) |
| DMA352 | ES114 ΔVF_0720 (ΔampG); muropeptide transporter | (Adin et al. 2009) |
|
V. parahaemolyticus
KNH1 |
Environmental isolate from the coast of Oahu, HI | (Nyholm et al. 2000) |
| V. harveyi B392 | Environmental isolate from the Gulf of Mexico, USA | (Reichelt and Baumann 1974) |
|
Escherichia coli
DH5α |
Lab collection |
Preparation, quantification and fluorescent-labeling of outer membrane vesicles (OMV)
OMV were isolated from culture supernatants using a modified version of a previously described procedure (Kulp and Kuehn 2010). OMV were produced to similar levels under all conditions tested, including suspension in sea water or growth in LBS (data not shown). Because the deep crypts provide a nutrient-rich environment to V. fischeri, we chose to grow all strains in complex media (LBS or LB) to an optical density at 600 nm (OD600) of approximately 4.0. The cells were removed by centrifugation at 4,500 x g for 15 min. The resulting supernatant was successively filtered through 0.45 μm and 0.22 μm pore-size PVDF membrane filters (Millipore Corp., Billerica, MA). OMV were separated from other extracellular products by ultracentrifugation at 173,000 x g for 2 h at 4°C in a 90 Ti rotor (Beckman Coulter, Inc., Brea, CA). The resulting pellet was washed and resuspended in Dulbecco’s phosphate buffered saline (dPBS; 0.2 g KCl, 0.2 g KH2PO4, 11.7 g NaCl, 1.1 g Na2HPO4, 0.1 g MgCl2*6H2O, and 0.1 g CaCl2 per liter deionized water), supplemented with an additional 11.7 g NaCl/L, and filter-sterilized. The protein concentration of OMV was estimated using the Qubit 2.0 fluorometer (Life Technologies, Grand Island, NY) following the manufacturer’s protocol. The relative amount of OMV material was also quantified using a lipid stain-binding assay: OMV were incubated with FM4-64 (Life Technologies/Molecular Probes) at a concentration of 3.3 μg/ml of dPBS for 10 min at 37 °C. After excitation at 535 nm, the emission at 670 nm was measured using a Tecan Genios Pro plate-reader fluorometer (Tecan Group, Männedorf, Switzerland) on three replicate samples. Controls included vesicles alone and FM4-64 probe alone. Wild-type V. fischeri strain ES114 was used as the reference to compare relative vesicle production by other strains and mutants. OMV preparations were stored at −20 °C until use. For fluorescence labeling, vesicles were incubated (1 h, 25 °C) with fluorescein isothiocyanate (FITC; Sigma–Aldrich, St. Louis, MO), and pelleted, washed and resuspended in mPBS as above to remove unbound FITC.
To predict the OMV concentration produced by a symbiotic population of V. fischeri within the light-organ crypts, we first estimated the volume of the deep crypts to be approximately 5 × 105 μm3. Because a culture containing 2 × 108 cells produces approximatively 1 μg of OMV protein, we calculated that 5 × 105 cells in 5 × 105 μm3 (corresponding to 1012 cells per mL) will produce a concentration of 5 mg of OMV protein/mL.
Transmission electron microscopy (TEM)
OMV were further purified using a sucrose gradient as previously described in Shibata et al., 2011. The purified OMV were applied to Pioloform-coated copper grids (Ted Pella Co., Tustin, CA) for 1 min, and then negatively stained with NanoW (Nanoprobes, Yaphank, NY) for 1 min. Grids were examined using a Philips CM120 transmission electron microscope (University of Wisconsin – Electron Microscope Facility, Madison, WI).
TCT quantification and HPLC analysis
V. fischeri cells were grown to an OD600 of ~2.0 in LBS. Cells and supernatant were separated by centrifugation, and the cell pellet was washed twice in sea water. OMV were purified from the supernatant as described above. High-performance liquid chromatography (HPLC) analysis and quantification were carried out as previously described (Kohler et al. 2007). Briefly, the contents of purified OMV were released by sonication (two 30-sec pulses at 20% power; Vibra Cell, Sonics material), and separated from the envelope by centrifugation. The components in the supernatant were then fractionated by C18 reverse-phase HPLC, using a 2 to 30% acetonitrile gradient over 60 min. TCT was purified as previously described (Koropatnick et al. 2004; Cookson et al. 1989) at a concentration of 0.6 μM. For an internal control, 10 μL of purified TCT were mixed into 100 μL of purified OMV contents (50 μg/mL) before being subjected to HPLC.
Squid procedures
Newly hatched juvenile squids were transferred to filter-sterilized Instant Ocean (Aquarium Systems, Inc., Mentor, OH) (FSIO). Animals were either maintained aposymbiotic, or made symbiotic by placing them in FSIO containing ~104 V. fischeri cells of the appropriate strain per milliliter. The appearance and level of light-organ colonization was monitored by measuring squid luminescence with a TD20/20 photometer (Turner Designs, Sunnyvale, CA), and the number of colony-forming units (cfu) in the symbiosis was determined by plating dilutions of the light-organ contents of individual squid on LBS agar as previously described (Naughton and Mandel 2012). The addition of TCT, or purified OMV, to FSIO containing newly hatched squid was made either alone, or after a 30-min pretreatment of the animals with cytochalasin D (1 μg/mL seawater).
Hemocyte visualization
Each hemocyte trafficking experiment was done in triplicate and each graph shows one representative replicate. 30 squids were used per condition tested, except if stated otherwise. Statistical analyses were performed using GrahPad software (GraphPad, La Jolla, CA). Asterisks indicate groups of statistically different mean, determined with One-way ANOVA analysis of differences, a posthoc Bonferroni correction and a Tukey’s test when appropriate (****), p < 000.1; (***), p < 0.01; (**),p < 0.001; (*), p < 0.01; (ns), not significant. To visualize the extent of hemocyte trafficking in the epithelial tissues of their ciliated appendages (Koropatnick et al. 2007; Heath-Heckman and McFall-Ngai 2011), juvenile squid were anaesthetized, and then fixed with 4% paraformaldehyde in marine phosphate-buffered saline (mPBS; 50 mM sodium phosphate buffer pH 7.4, containing 0.45 M NaCl) for 18 h at 4 °C. The light organ was then exposed by dissection, and permeabilized for 18 h with a solution of mPBS containing 1% Triton-X at 4 °C. To stain hemocytes and F-actin, respectively, the organ was incubated with 0.64 mM Alexa Fluor 488 conjugated DNase I, and 0.19 mM TRITC-rhodamine phalloidin (Invitrogen, Carlsbad, CA), for 48 h at 4°C. After washing four times with mPBS (15 min each), the organs were mounted on a glass depression slide using Vectashield medium (Vector Laboratories, Inc., Burlingame, CA) to reduce photobleaching. We also confirmed that exposure of the surface appendages to inocula larger than 104 cfu/mL does not influence the level of hemocyte-trafficking response during the first 3 h, at which time no bacteria have reached the crypts (Fig. S2A-B).
OMV Internalization by hemocytes and appendage epithelial cells
Hemocytes were obtained from the cephalic artery of adult squid, and prepared as previously described (Heath-Heckman and McFall-Ngai 2011). After the hemocytes adhered to glass coverslips, an addition of either FITC-labeled OMV (50 μg protein/mL) or FITC-labeled beads (2-μm diameter; Invitrogen) was made, followed by an incubation for 2 h. The coverslips were then washed twice at room temperature for 5 min in Squid Ringer’s solution, which consists of 530 mM NaCl, 10 mM KCl, 25 mM MgCl2, 10 mM CaCl2 and 10 mM HEPES buffer (pH 7.5) (Nyholm et al. 2009). The cells were then fixed on the coverslips by incubation for 30 min at room temperature in mPBS containing 4% paraformaldehyde. The fixed cells were then washed three times for 10 min in mPBS, and incubated for 1 h at room temperature. The buffer was then removed and replaced with permeabilization buffer (1% Triton X-100 in mPBS) and incubated for another 1 h. Finally, the coverslips were placed in fresh permeabilization buffer containing rhodamine phalloidin (0.19 mM) either for 1 h at room temperature or overnight at 4 °C. To visualize nuclei, samples were stained with 1 μM TOTO-3 (Invitrogen) for 20 min. The coverslips were then washed three times for 5 min in mPBS, and mounted onto glass slides coated with Vectashield, and examined by confocal microscopy. To measure fluorescence-associated phagocytosis, FITC-labeled OMV (50 μg protein/well) were incubated with purified hemocytes for between 30 min and 2 h with agitation. All incubations were done in triplicate. Cells were washed twice with mPBS, and then solubilized in 100 μL of 2% Triton X-100 in mPBS. Fluorescence was detected using a Tecan fluorometer (excitation, 485 nm; emission, 535 nm).
Animals were incubated with purified OMV at a final concentration of 50 μg protein/mL for 4 h. Squids were washed twice in fresh FSIO for 5 min and then fixed in mPBS containing 4% paraformaldehyde overnight. Light organs were then dissected and labeled, and visualized similarly to purified hemocytes.
Supplementary Material
Acknowledgements
The authors wish to thank the members of the Ruby and McFall-Ngai laboratories for their insight into this research and, specifically, E. Heath-Heckman, S. Moriano-Gutierrez, and J. Schwartzman for technical training. We also appreciate the help provided by J. Dillard and his laboratory, especially K. Hackett and J. Lenz, for providing assistance separating peptidoglycan fragments by HPLC. Funding for this work was provided by NIH grants AI50661 to M. McFall-Ngai, GM008505 to E.G. Ruby, and OD011024 to E.G. Ruby and MM-N. Portions of this study were supported by a University of Wisconsin Steenbock Professorship to EGR.
References
- Adin DM, Engle JT, Goldman WE, McFall-Ngai MJ, Stabb EV. Mutations in ampG and lytic transglycosylase genes affect the net release of peptidoglycan monomers from Vibrio fischeri. J Bacteriol. 2009;191:2012–2022. doi: 10.1128/JB.01547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielig H, Rompikuntal PK, Dongre M, Zurek B, Lindmark B, Ramstedt M, et al. NOD-like receptor activation by outer membrane vesicles from Vibrio cholerae Non-O1 Non-O139 strains is modulated by the quorum-sensing regulator HapR. Infect Immun. 2011;79:1418–1427. doi: 10.1128/IAI.00754-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher KJ, Ruby EG. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau MA, Fisher JF, Mobashery S. Messenger functions of the bacterial cell wall-derived muropeptides. Biochemistry. 2012;51:2974–2990. doi: 10.1021/bi300174x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CA, Mandel MJ, Gyllborg MC, Thomasgard KA, Ruby EG. Genetic determinants of swimming motility in the squid light-organ symbiont Vibrio fischeri. MicrobiologyOpen. 2013a;2:576–594. doi: 10.1002/mbo3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CA, DeLoney-Marino CR, Mandel MJ. Chemoreceptor VfcA mediates amino acid chemotaxis in Vibrio fischeri. Appl Environ Microbiol. 2013b;79:1889–1896. doi: 10.1128/AEM.03794-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CA, Hunt JR, Kremer N, Krasity BC, Apicella MA, McFall-Ngai MJ, Ruby EG. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. eLife. 2014;3:e01579. doi: 10.7554/eLife.01579. doi:10.7554/eLife.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson BT, Cho HL, Herwaldt LA, Goldman WE. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989;57:2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L-A. What is a host? Incorporating the microbiota into the damage-response framework. Infect Immun. 2015;83:2–7. doi: 10.1128/IAI.02627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doino JA, McFall-Ngai MJ. A transient exposure to symbiosis-competent bacteria induces light organ morphogenesis in the host squid. Biol Bull. 1995;189:347–355. doi: 10.2307/1542152. [DOI] [PubMed] [Google Scholar]
- Elhenawy W, Debelyy MO, Feldman MF. Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. mBio. 2014;5:e00909–14. doi: 10.1128/mBio.00909-14. doi:10.1128/mBio.00909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JS, Apicella MA, McFall-Ngai MJ. Vibrio fischeri lipopolysaccharide induces developmental apoptosis, but not complete morphogenesis, of the Euprymna scolopes symbiotic light organ. Dev Biol. 2000;226:242–254. doi: 10.1006/dbio.2000.9868. [DOI] [PubMed] [Google Scholar]
- Foster JS, McFall-Ngai MJ. Induction of apoptosis by cooperative bacteria in the morphogenesis of host epithelial tissues. Devel Gene Evol. 1998;208:295–303. doi: 10.1007/s004270050185. [DOI] [PubMed] [Google Scholar]
- Graf J, Dunlap PV, Ruby EG. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J Bacteriol. 1994;176:6986–6991. doi: 10.1128/jb.176.22.6986-6991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath-Heckman EAC, McFall-Ngai MJ. The occurrence of chitin in the hemocytes of invertebrates. Zoology (Jena) 2011;114:191–198. doi: 10.1016/j.zool.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Fisher JF, Mobashery S. Bacterial cell-wall recycling.”. Ann New York Acad Sci. 2013;1277:54–75. doi: 10.1111/j.1749-6632.2012.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cellul Microbiol. 2010;12:372–385. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- Koch EJ, Miyashiro T, McFall-Ngai MJ, Ruby EG. Features governing symbiont persistence in the squid-vibrio association. Mol Ecol. 2014;23:1624–1634. doi: 10.1111/mec.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler PL, Hamilton HL, Cloud-Hansen K, Dillard JP. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae Type IV secretion system. J Bacteriol. 2007;189:5421–5428. doi: 10.1128/JB.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- Koropatnick TA, Kimbell JR, McFall-Ngai MJ. Responses of host hemocytes during the initiation of the squid-vibrio symbiosis. Biol Bull. 2007;212:29–39. doi: 10.2307/25066578. [DOI] [PubMed] [Google Scholar]
- Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Ruby EG. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl Environ Microbiol. 1994;60:1565–1571. doi: 10.1128/aem.60.5.1565-1571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-K, Cho B-Y, Lee S-J, Kang J-Y, Jeong HD, Huh SH, et al. Histopathological lesions of Manila clam, Tapes philippinarum, from Hadong and Namhae coastal areas of Korea. Aquaculture. 2001;201:199–209. [Google Scholar]
- Mandel MJ, Schaefer AL, Brennan CA, Heath-Heckman EAC, DeLoney-Marino CR, McFall-Ngai MJ, Ruby EG. Squid-derived chitin oligosaccharides are a chemotactic signal during colonization by Vibrio fischeri. Appl Environ Microbiol. 2012;78:4620–4626. doi: 10.1128/AEM.00377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol. 2006;61:839–846. doi: 10.1111/j.1365-2958.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai MJ. The importance of microbes in animal development: lessons from the squid-vibrio symbiosis. Annu Rev Microbiol. 2014;68:177–194. doi: 10.1146/annurev-micro-091313-103654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai MJ, Ruby EG. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, McFall-Ngai MJ. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development. 1994;120:1719–1729. doi: 10.1242/dev.120.7.1719. [DOI] [PubMed] [Google Scholar]
- Naughton LM, Mandel MJ. Colonization of Euprymna scolopes squid by Vibrio fischeri. JoVE. 2012;(61):e3758. doi: 10.3791/3758. doi:10.3791/3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm SV, Deplancke B, Gaskins HR, Apicella MA, McFall-Ngai MJ. Roles of Vibrio fischeri and nonsymbiotic bacteria in the dynamics of mucus secretion during symbiont colonization of the Euprymna scolopes light organ. Appl Environ Microbiol. 2002;68:5113–5122. doi: 10.1128/AEM.68.10.5113-5122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm SV, Stabb EV, Ruby EG, McFall-Ngai MJ. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc Natl Acad Sci U S A. 2000;97:10231–10235. doi: 10.1073/pnas.97.18.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ Microbiol. 2009;11:483–493. doi: 10.1111/j.1462-2920.2008.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Okudaira N, Iwabuchi K, Fugo H, Nagai T. Apoptosis and adhesion of hemocytes during molting stage of silkworm, Bombyx mori. Zool Sci. 2006;23:299–304. doi: 10.2108/zsj.23.299. [DOI] [PubMed] [Google Scholar]
- Reichelt JL, Baumann P. Effect of sodium chloride on growth of heterotrophic marine bacteria. Arch Microbiol. 1974;97:329–345. doi: 10.1007/BF00403071. [DOI] [PubMed] [Google Scholar]
- Ruby EG, McFall-Ngai MJ. A squid that glows in the night: development of an animal-bacterial mutualism. J Bacteriol. 1992;174:4865–4870. doi: 10.1128/jb.174.15.4865-4870.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Torchia MLG, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Visick KL. Sensor kinase RscS induces the production of antigenically distinct outer membrane vesicles that depend on the symbiosis polysaccharide locus in Vibrio fischeri. J Bacteriol. 2012;194:185–194. doi: 10.1128/JB.05926-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori Cag pathogenicity island. Nature Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.