Abstract
Rationale
Anxiety during pregnancy has been linked to adverse maternal health outcomes, including postpartum depression (PPD). However, there has been limited study of biological mechanisms underlying behavioral predictors of PPD during pregnancy.
Objectives
Considering the shared etiology of chronic stress amongst antenatal behavioral predictors, the primary goal of this pilot study was to examine associations among stress-related physiological factors (including GABA-ergic neurosteroids) and stress-related behavioral indices of anxiety during pregnancy.
Methods
Fourteen nulliparous women in their second trimester of a singleton pregnancy underwent speech and mental arithmetic stress, following a two-week subjective and objective recording of sleep-wake behavior.
Results
Lower cortisol, progesterone, and a combined measure of ALLO + pregnanolone throughout the entire stressor protocol (area under the curve, AUC) were associated with greater negative emotional responses to stress, and lower cortisol AUC was associated with worse sleep quality. Lower adrenocorticotropic hormone was associated with greater anxious and depressive symptoms. Stress produced paradoxical reductions in cortisol, progesterone and a combined measure of allopregnanolone + pregnanolone, while tetrahydrodeoxycorticosterone levels were elevated.
Conclusions
These data suggest that cortisol, progesterone and ALLO + pregnanolone levels in the second trimester of pregnancy are inversely related to negative emotional symptoms and the negative impact of acute stress challenge appears to exert its effects by reducing these steroids to further promote negative emotional responses.
Keywords: Pregnancy, Anxiety, Postpartum Depression, Cortisol, Stress, Sleep, neurosteroids, Allopregnanolone (ALLO)
Introduction
Maternal mood and anxiety disorders during pregnancy have been linked to postpartum depression (PPD) (O'Hara 1996; O'Hara and Wisner 2014), which is estimated to affect 10% to 15% of mothers of childbearing age (Gavin et al. 2005; Gaynes et al. 2005; O'Hara 1996). Results of meta-analytic reviews have suggested several factors that may be strongly associated with increased risk for the development of depression during pregnancy and PPD, many of which may be identified early in pregnancy, including a history of chronic stress (O'Hara 1996; O'Hara and Wisner 2014; Verreault et al. 2014), and anxious and depressive symptoms during pregnancy (O'Hara 1996; O'Hara and Wisner 2014; Verreault et al. 2014). In addition, disturbed sleep has been associated with both depressed mood (Jomeen and Martin 2007; Okun et al. 2013; Skouteris et al. 2008) and stress during pregnancy (Okun et al. 2013), and has also been shown to be a potential predictor of depressive mood postpartum (Bei et al. 2010; Goyal et al. 2009; Okun et al. 2009). However, there has been limited study of the biological mechanisms underlying the relationships among chronic stress, sleep disturbances and perinatal mood and anxiety symptoms.
Perhaps most relevant to the interaction among chronic stress, sleep disturbance, and perinatal depression symptomatology, may be the joint stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis, and progesterone (PROG)-derived gamma-amino-butyric acid (GABA)-ergic neurosteroids, each of which has been implicated in reproductive mood disorders (Girdler et al. 2001; Segebladh et al. 2013), including PPD (Nappi et al. 2001; Nierop et al. 2006).
Neuroactive steroids such as the progesterone (PROG) metabolites 3α,5α-3-hydroxypregnan-20-one (3α,5α–THP; allopregnanolone) and 3α,5β-3-hydroxypregnan-20-one (3α,5β–THP; pregnanolone), and the deoxycorticosterone (DOC) metabolite 3α,5α-3,21-dihydroxypregnan-20-one (tetrahydrodeoxycorticosterone; THDOC) are powerful endogenous positive modulators of GABAA receptors with antidepressant, anxiolytic, and sedative properties (Bitran et al. 1995; Crawley et al. 1986; Reddy 2006). These GABAergic neurosteroids, with cortisol, may therefore represent potential candidate mechanisms of PPD risk, since they each are stress responsive, are mutually regulatory during stress (Crowley and Girdler 2014; Mody and Maguire 2011), and are implicated in the development of both PPD and insomnia (Basta et al. 2007; Schiller et al. 2014; Teran-Perez et al. 2012).
To date, no studies have examined stress-induced ALLO, pregnanolone, or THDOC in pregnancy or concurrent indicators of HPA axis activation [e.g., adrenocorticotrophic hormone (ACTH), cortisol] and their relationship to mood and anxiety in pregnancy. One potential pathway underlying the relationship among GABAergic neurosteroids, HPA axis activity, and the development of PPD may reside in their relationship to stress-related behavioral regulation during pregnancy. Considering the biopsychosocial challenges incurred during the transition to motherhood, pregnancy itself might be conceptualized as a chronic stressor and therefore an apt model for the investigation of physiological and behavioral markers of stress that may be predictive of postpartum mood dysregulation.
Using the perinatal first and second trimester as a natural longitudinal model of chronic stress, we examined the relationships among stress reactive neurosteroids, HPA axis hormones, and stress-related behavioral factors (sleep disturbances, emotional responses to stress). The primary goal of this pilot study was to examine the associations among daytime physiological stress reactivity measures and stress-related behavioral indices of anxiety during pregnancy in order to identify potential stress-related biomarkers of risk as a preliminary step to developing a biobehavioral predictive model of PPD that might be tested in future research.
Methods
Participants
Fourteen nulliparous women in the second trimester of a singleton pregnancy were recruited from the community using flyers, email listserv, and local advertisements. Recruitment was targeted so that laboratory testing could be conducted during the second trimester of pregnancy (22–24 weeks gestation). Exclusion criteria included: age < 18 years or > 45 years; multiparity; non-singleton pregnancy; current use of antidepressants, anti-anxiety medications, mood-stabilizers, psychotropic medications, progesterone treatment, or sleep medications (prescription or over the counter); current use of tobacco, alcohol, recreational drugs, or steroids; current or recent history (within the last 6 months) of a depressive disorder; current or recent history (within the last three years) of anxiety disorders, or eating disorders; any history of schizoaffective disorder, bipolar disorder, or neurodevelopmental disorder, confirmed by the Structured Clinical Interview for DSM-IV disorders (SCID); chronic health problems (obtained via self-report) that may affect sleep, endocrine, or cardiovascular function including diagnosed obstructive sleep apnea, diagnosed restless legs syndrome, cancer, pre-gestational diabetes, chronic hypertension (documented or taking medication for hypertension), gestational hypertension, preeclampsia, or anemia; medical high risk pregnancy, as determined from obstetrics clinic assessment; a body mass index of > 40kg/m2 just prior to the pregnancy; and other medical issues that would preclude safe participation in the study.
Screening and Enrollment
From July 2014 through November 2014, 57 women were screened via telephone for inclusion into the study. Of these, 32 (56.14%) met initial screening criteria, 25 (43.86%) did not meet initial screening criteria. Of the 32 women who met the initial screening criteria, four did not meet eligibility criteria following an in-person enrollment session involving a medical screen, and a psychiatric interview (three for medical histories not reported during the phone screen and one for a recent history of anxiety disorder); seven were no longer interested; and four could not be scheduled due to conflicts. In total, 17 women were enrolled into the laboratory-based study protocol. Fourteen women with complete assay data for the laboratory-based study protocol are included in the present report.
Psychiatric History Assessment and Enrollment Session
All procedures were approved by the university institutional review board and all participants underwent informed consent prior to the initiation of the enrollment session. During the enrollment session, participants were evaluated for past depressive disorders (e.g., major depressive disorder, minor depressive disorder, adjustment disorder with depressive features, etc.), anxiety disorders (e.g., panic disorder, generalized anxiety disorder, etc.) and post-traumatic stress disorder (PTSD) using the SCID (Spitzer et al. 1995). Women with DSM-IV (American Psychiatric Association, 1994) Axis I disorders, current (within six months) depression, recent (within three years) Axis I disorders, and/or any history of bipolar, schizoaffective, and neurodevelopmental disorders at study enrollment were excluded. To create clinically and conceptually meaningful categories, all histories of depressive disorders were categorized as ‘any depressive disorder’ and all histories of anxiety disorders were categorized as ‘any anxiety disorder.’ A urine pregnancy test (SA Scientific, San Antonio) was conducted during the enrollment session to confirm pregnancy. Also administered during the enrollment session were the Edinburgh Postnatal Depression Scale (EPDS); the Speilberger State Trait Anxiety Inventory (STAI), trait version; and a questionnaire to assess demographic and socioeconomic information.
Sleep Recording
Following the enrollment session, participants were provided an actiwatch spectrum plus (Philips Respironics, Bend Oregon) and sleep diary for in-home recording of sleep-wake behavior for a period of two weeks. In addition to filling out the prospective sleep diaries daily, morning and night, participants were instructed to wear the actiwatch continuously on their non-dominant wrist, only removing the device should it become immersed in water. Using an accelerometer to record movements of the arm over time, the actiwatch provides a reliable measure of sleep-wake behavior (Cole et al. 1992) that has been validated against polysomnography (Marino et al., 2013). In order to increase accuracy of scoring, participants were asked to press an event marker button on the watch to indicate (1) when the participant intended to initiate sleep at night (e.g., when they turn the lights off with the intention of going to sleep); (2) when the participant woke in the morning with the intention to get out of bed; and (3) before and after any daytime naps. All data was checked against the sleep diaries for scoring of sleep-wake intervals. Data were collected in one-minute epochs, scored for periods of wake and sleep using a standard Philips Respironics Actiware software algorithm. Average sleep onset latency (SOL), or the time (in minutes) from bedtime to sleep onset, was subjectively measured via sleep diaries and objectively measured via actigraphy. In addition, average wake after sleep onset (WASO), or the sum total (in minutes) of wake times from sleep onset to final morning wake time; and average sleep fragmentation index, or the amount of movement/restlessness in a sleep period (where a higher index indicates more restless sleep) was objectively measured via actigraphy. At the end of the sleep recording period, participants completed the Pittsburgh Sleep Quality Inventory (PSQI) as a measure of overall sleep quality.
Stress Testing Laboratory Session
Following the sleep recording period, each subject participated in a single laboratory session that was scheduled between 3:00 p.m. and 5:00 p.m. To ensure that participants were hydrated prior to the laboratory session, each participant was asked to consume eight, eight-ounce glasses of water on the day prior to sampling, and at least five eight-ounce glasses and a low fat breakfast and lunch the day of testing (confirmed via self-report diary). Participants were asked to refrain from over-the-counter medications 24 hours prior to testing; and from caffeine, exercise and alcohol the day of testing (confirmed via interview at study visit). Participants who had been ill within seven days of testing or who had fewer than six hours of sleep the previous night were rescheduled.
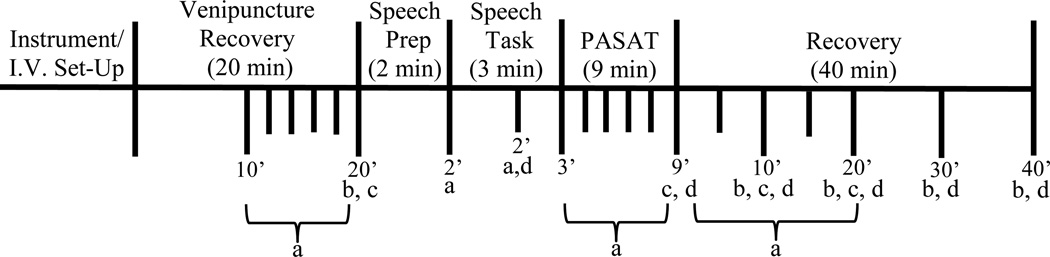
As illustrated in figure 1, our laboratory protocol consisted of the following events in fixed order: (1) instrumentation and venipuncture (that included the insertion of an indwelling catheter for serial blood sampling); (2) venipuncture recovery (20 min); (3) speech preparation (2 min) consisting of silent preparation of speech; (4) speech task (3 min) where the participant was asked to speak about a recent interpersonal stressor [randomly selected (to prevent rehearsal) by research staff from a list of three potential topics provided by the participant] in front of a female evaluator holding a microphone attached to an audio recorder. Participants were told that the evaluator was trained in behavioral analysis and that the audio recording of their speech would be later replayed to research staff for analysis of speech characteristics; (5) paced auditory serial addition task (PASAT, 9 min.) where participants were asked to listen to a tape recording with numbers from 1 to 9 and to add each number presented to the immediately preceding number, stating the answer aloud. The PASAT is comprised of four series of progressively shorter inter-digit intervals (Tombaugh 2006); (6) post-stress recovery (40 min). During the venipuncture recovery period, the participant rested alone quietly in a comfortable chair. A curtain shielded the catheter and the blood sampling from the participant’s view. Cardiovascular measures [heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP)] were collected during the laboratory stress testing paradigm using automated equipment [Suntech Exercise BP monitor, Model 4240 (SunTech Medical Instruments, Inc., Raleigh, NC)] at the following intervals: (1) every two minutes during the last 10-min of the venipuncture recovery period (that constituted the pre-stress baseline comparison period); (2) at the beginning of the speech preparation period; (3) at the beginning and at minute two of the speech task; (4) every two minutes of the PASAT task; (5) every five minutes of the first 20 minutes of the post-stress recovery period. These measures were then averaged to obtain a mean speech preparation, speech, and PASAT task value for each cardiovascular measure. Blood samples were collected from an indwelling catheter at the following intervals: (1) for cortisol: end of venipuncture recovery (min 20) and again 10, 20, 30, and 40 minutes post-stressor completion; (2) for ACTH: end of venipuncture recovery (min 20), immediately following stressor completion, and 10 and 20 minutes post-stressor completion; (3) Progesterone (PROG) and neurosteroids [ALLO, Pregnenolone, Pregnanolone, 3α,5β-THDOC (THDOC)]: end of venipuncture recovery period (min 20), minute two of the speech task, immediately following stressor completion, and 10, 20, 30, and 40 minutes post-stressor completion.
Figure 1.
Schematic representation of stress testing protocol: aCardiovascular measures [heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP)] were collected (1) every two minutes during the last 10-min of the venipuncture recovery period; (2) at the beginning of the speech preparation period; (3) at the beginning and at minute two of the speech task; (4) every two minutes of the PASAT task; and (5) every five minutes of the first 20 minutes of the post-stress recovery period. These measures were then averaged to obtain a mean speech preparation, speech, and PASAT task value for each cardiovascular measure. bBlood for cortisol was collected at the end of venipuncture recovery and again 10, 20, 30, and 40 minutes post-stressor completion. cBlood for adrenocorticotrophic hormone (ACTH) was collected (1) at the end of venipuncture recovery; (2) immediately following stressor completion (after the PASAT task); and (3) 10 and 20 minutes post-stressor completion. Blood for dprogesterone (PROG) and neurosteroids [ALLO, Pregnanolone, 3α,5β-THDOC (THDOC)] was collected (1) at the end of venipuncture recovery period; (2) minute two of the speech task; (3) immediately following stressor completion (after the PASAT task); and (4) 10, 20, 30, and 40 minutes post-stressor completion.
At the end of the stressor period, but before beginning post-stress recovery, participants rated their emotional responses to the stressor battery. Using a Likert scale ranging from 0 (“not at all”) to 10 (“extremely”), participants indicated the extent to which the stressors caused them to feel anxious, overwhelmed, or worried.
Neuroendocrine Assays
Blood for cortisol, PROG, and neurosteroids was collected into serum-separating (SST) tubes, and left at room temperature for 30 minutes. Samples were spun at 3,000 rpm for 10 minutes at room temperature (18°C) and supernatant (serum) was aliquoted into 2mL tubes and frozen at −80°C until assayed. Blood for ACTH was collected into EDTA Vacutainer tubes that were kept on ice. Immediately after blood draw, tubes for ACTH were centrifuged at 3000 rpm for 10 minutes at 4°C. Supernatant (plasma) was aliquoted into tubes, rapidly frozen, and maintained at −80°C until assayed.
Serum levels of cortisol were determined by radioimmunoassay (RIA) using commercial kits from ICN pharmaceuticals (Hampshire, United Kingdom). The sensitivity of the assay is excellent at 0.07µg/dL. The specificity of the RIA for cortisol is high, showing only 0.05%–2.2% cross-reactivity with most similarly structured compounds. Plasma ACTH was determined using RIA techniques from commercially available kits (Nichols Institute Diagnostics). The sensitivity of this assay is high at 1 pg/ml, and the selectivity is excellent showing only 0.0%–0.02% cross-reactivity with steroid compounds. The specificity of the antiserum for PROG is very high, showing only 0.01%–2.5% cross-reactivity with other steroid compounds.
To check for cross-reactivity of cortisol and PROG with neuroactive steroids in our sample of pregnant women, a competition curve using seven concentrations (4000ng/ml to 1ng/ml) of four steroids (ALLO, THDOC, pregnanolone, PROG) was conducted in both the cortisol and PROG RIAs. Little to no cross-reactivity of the steroids was detected in either assay. In the cortisol assay, none of the steroid concentrations measured more than 0.2% of the cortisol concentration. In the PROG assay, none of the steroid concentrations measured more than 0.2% of the PROG concentration, except for ALLO at 4000ng/ml that measured 1.45%.
Neurosteroids were measured in serum by Gas Chromatography – Mass Spectrometry (GCMS) following purification by solid phase extraction, as previously described (Girdler et al. 2012; Porcu et al. 2009).
Data Reduction and Analysis
Sensitivity analyses were used to identify extreme outliers prior to data analysis, defined as values three or more standard deviations above or below the mean. For this sample, one outlier was detected for ACTH, and as such, the outlier was removed from the ACTH dataset prior to analysis. The stereoisomers 3α,5α-THP (ALLO) and 3α,5β-THP (pregnanolone) have similar potency and efficacy as positive modulators of GABA action at GABAA receptors (Majewska et al. 1986; Morrow et al. 1990) and the extent of the stress-induced decrease, from baseline, elicited by our stressor protocol was similar (table 3). Therefore, in line with previous work (Uzunova et al. 1998), in addition to ALLO and pregnanolone, we have presented the combined concentrations of these neuroactive steroids under the acronym 3α,5α + 3α,5β–THP for statistical analyses of significance. Indeed, previous work has shown that cerebral spinal fluid (CSF) measures of this combined value (3α,5α + 3α,5β–THP) are significantly inversely associated with depression severity and also significantly increased following effective treatment with fluoxetine or fluvoxamine in unipolar depressed patients (Uzunova et al. 1998). Descriptive statistics (means, SD’s, proportions) were used to describe demographic and behavioral characteristics of the study population. For each cardiovascular measure, reactivity was defined as the difference between mean stress level and mean baseline level. One participant was missing values for cardiovascular data due to equipment failure. For each neuroendocrine measure, as described elsewhere (Klatzkin et al. 2006a; Pruessner et al. 2003), our primary analyses involved calculation of area under the curve with respect to ground (AUCg), which includes neuroendocrine values integrated across baseline rest, stress, and stress recovery periods, and is therefore an indicator of overall neuroendocrine status. Secondarily, we also examined neuroendocrine stress reactivity, which was defined as the difference between baseline level and the post-stress time point associated with the greatest change from baseline (whether increase or decrease). Consequently, stress-induced levels were measured for PROG and all neurosteroids, at 10 minutes post-stressor completion, for ACTH at 20 minutes post-stressor completion, and for cortisol at 40 minutes post-stressor completion. For the neuroendocrine data, two participants were missing recovery values due to inability to obtain the blood samples. Paired t-tests were used to confirm a significant effect of the modified TSST on cardiovascular and neuroendocrine measures. Where significant (from baseline) stress values were obtained, we conducted correlational analyses with behavioral measures. All p values reported are 2-sided with an alpha level of 0.05. All statistical analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina).
Table 3.
Stress-reactive physiological measurements in second trimester pregnant women.
| Mean change (+/− SEM) from baseline to stressor |
p Value | |
|---|---|---|
| Neuroendocrine measures | ||
| Cortisol (ng/ml) | −42.1 (19.3) | 0.05 |
| ACTH (pg/ml) | −8.7 (4.6) | 0.08 |
| PROG (ng/ml) | −12.3 (4.7) | 0.02 |
| Pregnenolone (pg/ml) | −36.6 (35.0) | 0.31 |
| ALLO (3α,5α–THP) (pg/ml) | −28.9 (28.4) | 0.33 |
| Pregnanolone (3α,5β–THP) (pg/ml) | −38.5 (22.0) | 0.10 |
| 3α,5α + 3α,5β–THP (ALLO + Pregnanolone) (pg/ml) | −67.3 (28.8) | 0.04 |
| 3α,5β–THDOC (pg/ml) | 73.1 (20.3) | 0.003 |
| Cardiovascular Measures | ||
| Heart Rate (bpm) | 10.0 (2.3) | <0.001 |
| Systolic Blood Pressure (mmHg) | 16.4 (2.9) | <0.001 |
| Diastolic Blood Pressure (mm/Hg) | 11.4 (1.9) | <0.001 |
SEM = standard error of the mean; ACTH = Adrenocorticotrophic Hormone; PROG = Progesterone; ALLO = Allopregnanolone; THDOC = tetrahydrodeoxycorticosterone
Results
Participant Characteristics
As reflected in Table 1, the mean age for the participants was 29.9 +/− 4.7, and the mean gestational stage at study enrollment was 21.0 ± 1.0 weeks. Participants were generally well-educated with ~79% of the sample having acquired a master’s degree or higher. The mean EPDS and STAI scores for our sample of women (mean EPDS: 4.1 ± 3.4; mean STAI: 30.0 ± 4.0), were at or below the normative range for women in the second trimester of pregnancy (Bergink et al. 2011; Gunning et al. 2010). Baseline cortisol and PROG concentrations during the second trimester of pregnancy were consistent with expected values (Soldin et al. 2005).
Table 1.
Characteristics of study participants
| Participants, n | 14 |
| Age, years (mean ± SD) | 29.9 ± 4.7 |
| Gestational stage at study enrollment (mean ± SD) | 21.0 ± 1.0 |
| Race | |
| White, n (%) | 9 (64.3) |
| Other, n (%) | 5 (35.7) |
| Education | |
| College degree or lower, n (%) | 3 (21.4) |
| Master’s n (%) degree, n (%) | 9 (64.3) |
| Professional Degree or Doctorate, n (%) | 2 (14.3) |
| Family income (annually) | |
| < $75,000/year, n (%) | 6 (42.9) |
| $75,000/year, n (%) | 8 (57.1) |
| Depression History | |
| yes, n (%) | 2 (14.3) |
| no, n (%) | 12 (85.7) |
| Anxiety History | |
| yes, n (%) | 1 (7.1) |
| no, n (%) | 13 (92.9) |
| EPDS | 4.1 (3.4) |
| STAI (Trait Version; mean ± SD) | 30.0 ± 4.0 |
| PSQI (mean ± SD) | 5.5 ± 3.0 |
| SOL AverageDiary (mean ± SD) | 26.5 ± 16.6 |
| SOL AverageActigraphy (mean ± SD) | 10.0 ± 5.85 |
| WASO AverageActigraphy (mean ± SD) | 48.4 ± 19.5 |
| Sleep Fragmentation Index AverageActigraphy (mean ± SD) | 28.0 ± 9.9 |
| Baseline Cortisol ng/mL (mean ± SD) | 191.7 ± 96.5 |
| Baseline ACTH pg/mL (mean ± SD) | 99.1 ± 46.3 |
| Baseline PROG ng/mL (mean ± SD) | 87.4 ± 30.0 |
| Baseline pregnenolone pg/mL (mean ± SD) | 637.4 ± 179.8 |
| Baseline ALLO (3α,5α–THP) pg/mL (mean ± SD) | 551.8 ± 176.7 |
| Baseline pregnanolone (3α,5β–THP) pg/mL (mean ± SD) | 339.4 ± 146.7 |
| Baseline 3,5β–THDOC pg/mL (mean ± SD) | 299.0 ± 124.8 |
| Baseline 3α,5α + 3α,5β–THP pg/mL (mean ± SD) | 891.2 ± 285.9 |
EPDS = Edinburgh Postnatal Depression Scale; STAI = Speilberger State-Trait Anxiety Inventory, trait version; PSQI = Pittsburgh Sleep Quality Index; SOL = sleep onset latency (in minutes); WASO = wake after sleep onset (in minutes); ACTH = adrenocorticotrophic Hormone; PROG = progesterone; ALLO = allopregnanolone; THDOC = allotetrahydrodeoxycorticosterone
Associations among Neuroendocrine Status and Behavioral Measures
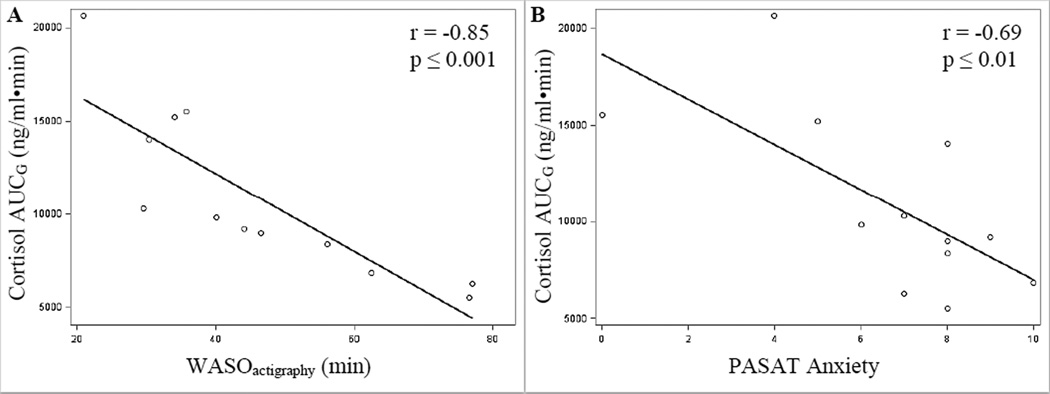
As shown in table 2, lower cortisol AUCg was associated with increased sleep disturbances (SOL: r = −0.64, p = 0.03; WASO: r = −0.85, p ≤ 0.001; fragmentation index: r = −0.66, p = 0.02) and worse self-reported sleep quality (PSQI: r = −0.74, p ≤ 0.001), in addition to increased negative emotional responses to stress (anxiety in response to the PASAT task: r = −0.69, p = 0.01; see Figure 2 for relationship of Cortisol AUCg to WASO and PASAT anxiety). Lower ACTH AUCg was associated with depressive (EPDS: r = −0.58, p = 0.04) and anxious (STAI: r = −0.67, p = 0.01) symptoms in pregnancy. Lower PROG AUCg was associated with increased anxious (STAI: r = −0.66, p = 0.02) symptoms in pregnancy in addition to increased negative emotional responses to stressor tasks (feeling worried and overwhelmed in response to the speech task (r’s = −0.60, p’s ≤ 0.05) and feeling worried in response to the PASAT task (r = −0.66, p = 0.02). Lower ALLO AUCg was associated with more negative emotional responses to the PASAT task (feeling overwhelmed: r = −0.71; p = 0.01), and a trend was observed for lower ALLO AUCg to predict worse sleep quality (diary-measured SOL: r = −0.49, p = 0.10; PSQI: r = −0.52, p = 0.08). Lower ALLO + pregnanolone (3α,5α + 3α,5β–THP) AUCg was associated with a more negative emotional response to the PASAT task (feeling overwhelmed: r = −0.63; p = 0.03). No significant relationships were observed among pregnanolone AUCg and behavioral measures (data not shown). No other significant relationships were observed among neuroendocrine measures and behavioral indices of anxiety.
Table 2.
Pearson’s Correlation Coefficients (r) of neuroendocrine status with mood symptoms, sleep-wake behavior, and emotional responses to stress in second trimester pregnant women
| Cortisol AUCgb |
ACTH AUCga |
PROG AUCgb |
3α,5α – THP AUCgb |
3α,5β– THP AUCgb |
3α,5α + 3α,5β–THP AUCgb |
3α,5β– THDOC AUCgb |
|
|---|---|---|---|---|---|---|---|
| STAI | −0.16 | −0.67* | −0.66** | −0.11 | −0.41 | −0.24 | −0.25 |
| EPDS | −0.20 | −0.58** | −0.23 | −0.13 | −0.35 | −0.23 | 0.28 |
| SOLDiary | −0.64** | −0.01 | 0.33 | −0.49† | −0.10 | −0.37 | 0.45 |
| SOLActigraphy | −0.38 | −0.02 | 0.42 | −0.10 | 0.06 | −0.04 | 0.32 |
| WASOActigraphy | −0.85* | 0.26 | 0.01 | −0.17 | −0.03 | −0.13 | −0.01 |
| Fragmentation IndexActigraphy | −0.66** | 0.19 | −0.01 | −0.06 | 0.09 | 0.00 | −0.17 |
| PSQI | −0.74* | −0.28 | 0.06 | −0.52† | −0.15 | −0.41 | 0.37 |
| Speech Anxiety | −0.09 | 0.05 | −0.47 | −0.14 | −0.31 | −0.22 | −0.30 |
| Speech Worry | −0.25 | −0.20 | −0.60** | −0.28 | −0.30 | −0.31 | −0.17 |
| Speech Overwhelmed | −0.20 | −0.04 | −0.60** | −0.22 | −0.30 | −0.27 | −0.20 |
| PASAT Anxiety | −0.69* | 0.03 | −0.33 | −0.55† | −0.24 | −0.47 | −0.02 |
| PASAT Worry | −0.41 | −0.41 | −0.66** | −0.41 | −0.34 | −0.42 | −0.13 |
| PASAT Overwhelmed | −0.23 | −0.13 | −0.14 | −0.71* | −0.36 | −0.63** | 0.27 |
ACTH = adrenocorticotrophic hormone; PROG = progesterone; ALLO = allopregnanolone; 3α,5β–THP = pregnanolone; THDOC = tetrahydrodeoxycorticosterone; STAI = Speilberger State-Trait Anxiety Inventory, trait version; PSQI = Pittsburgh Sleep Quality Index; SOL = sleep onset latency (in minutes); WASO = wake after sleep onset (in minutes); AUCG = area under the curve with respect to ground.
p ≤ 0.01;
p ≤ 0.05;
p ≤ 0.10;
n=13;
n=12
Figure 2.
Scatterplot and regression line reflecting Pearson correlation (r) of Cortisol AUGG and (A) mean actigraphy-measured wake after sleep onset (WASO) duration (in minutes); and (B) anxiety in response to the Paced Auditory Serial Addition Task (PASAT)
Overall Efficacy of the Stress Protocol
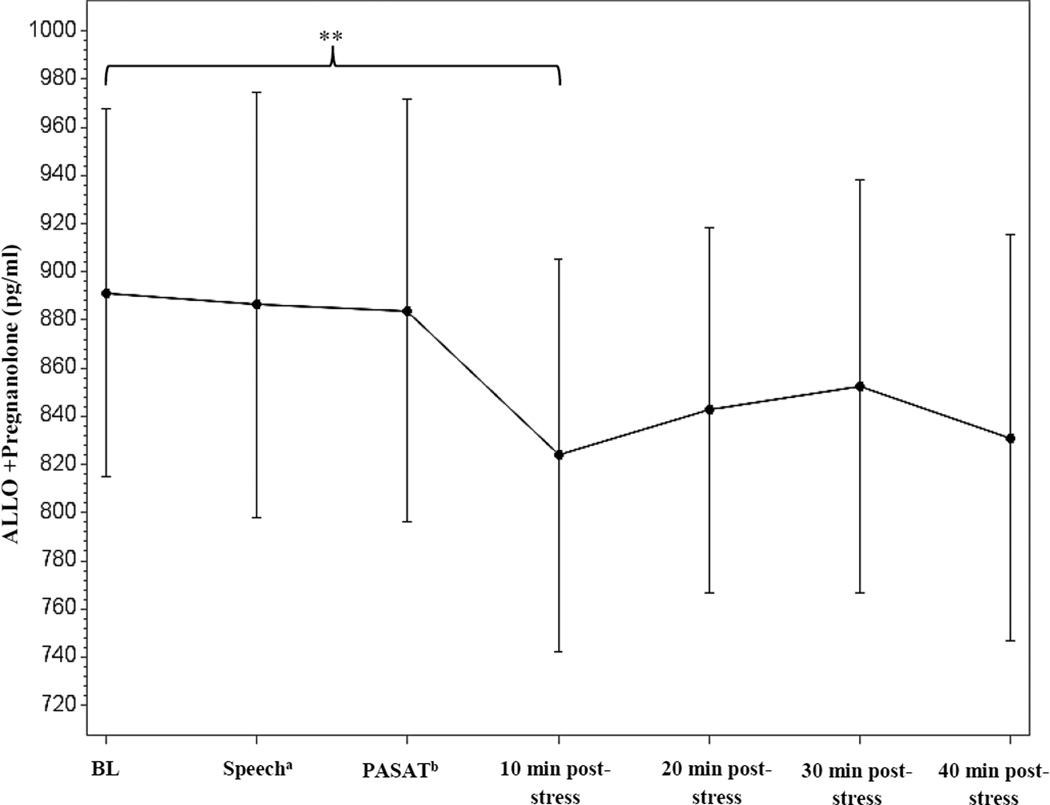
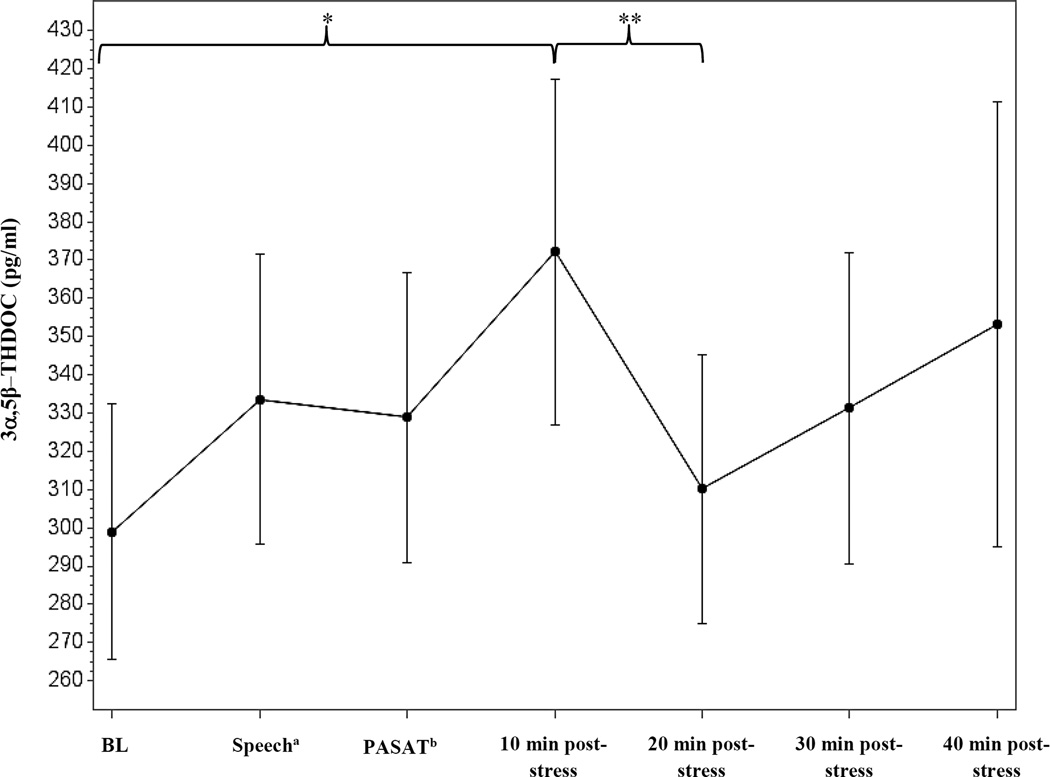
As shown in table 3, SBP, DBP, and HR all significantly increased in response to the stressor task [t (12) = 16.4, 11.4, and 10.0, p’s < 0.001, respectively]. For the neuroendocrine measures, we observed a significant decrease from baseline to stressor for cortisol [t (12) = −2.2, p = 0.05], PROG [t (14) = −2.62, p = 0.02], and the combined ALLO + pregnanolone measure [t (14) = −2.3, p = 0.04] (see Figure 3). While 3α,5α-THDOC could not be detected in the assay, a significant increase in 3α,5β-THDOC was observed from baseline to stressor [t (14) = 3.6, p = 0.003] (see Figure 4). There was no effect of stress on pregnanolone levels (data not shown). No other significant changes from baseline to stressor were observed for the neuroendocrine measures.
Figure 3.
Time course of the ALLO + pregnanolone combined measure stress response in second trimester pregnant women, measured via GC-MS. Data represent mean ± SEM as a function of time across the task. aSample taken during minute 2 of the speech task. bSample taken immediately following the PASAT task. **p ≤ 0.05
Figure 4.
Time course of 3,5β–THDOC stress response in second trimester pregnant women, measured via GC-MS. Data represent mean ± SEM as a function of time across the task. aSample taken during minute 2 of the speech task. bSample taken immediately following the PASAT task. *p ≤ 0.01; ** p ≤ 0.05
Discussion
Our findings indicate that integrated levels of GABAergic neurosteroids and HPA axis hormones (integrated across rest, stress and stress recovery periods) may regulate fundamental processes underlying the core symptoms of perinatal mood and anxiety disorders, and that these relationships are apparent even during the second trimester of pregnancy. Specifically, we observed that lower integrated cortisol, ACTH, PROG, and the combined ALLO + pregnanolone value were associated with increased anxiety-related emotional responses to stress, worse sleep quality, and increased symptom severity in second trimester pregnant women. This is consistent with previous research indicating that lower stress-induced PROG and ALLO levels may predict symptom severity in women with menstrually-related mood disorders (Klatzkin et al. 2006b).
One plausible mechanism by which these biological variables may increase risk for PPD is by altering sensitivity to environmental stressors, such as sleep disturbances during pregnancy, and in this way, increasing risk for the development of psychopathology. Given the bi-directional relationship between sleep disruption and affective dysregulation (Krystal 2012; Slavich and Irwin 2014), the identification of sub-threshold insomnia symptoms (e.g., stress-related sleep disturbances) during pregnancy may help to inform preventative treatment efforts that could significantly impact the clinical course of postpartum depression symptomatology.
Remarkably, we also observed a reversal of the “classic” stress response to a laboratory-based psychosocial stressor (Dickerson and Kemeny 2004) in several neuroendocrine measures (namely cortisol, ACTH, PROG, and the combined ALLO + pregnanolone measure). However, the psychosocial laboratory challenge was an effective stressor for pregnant women in their second trimester of pregnancy as evidenced by significant increases, from baseline, in the cardiovascular measures that reflect sympathetic/autonomic function. Our cardiovascular results are in line with results from other studies conducted in pregnant women during the third trimester of pregnancy (Christian 2012; De Weerth et al. 2007; Monk et al. 2000), which indicates that the cardiovascular response to stress appears to be maintained during pregnancy (albeit attenuated; Matthews and Rodin 1992).
Both animal and human studies suggest that maternal HPA axis responses to stress are markedly suppressed in late pregnancy (e.g., 3rd trimester; Brunton and Russell 2008; Christian 2012), but this is the first study to suggest this may be true in second trimester as well. It has been suggested that this mechanism not only serves to promote appropriate metabolic adaptations necessary for a successful pregnancy outcome, but to also minimize fetal exposure to excessive levels of glucocorticoids in utero that may contribute to detrimental fetal programming (Herrera 2000). While several mechanisms have been proposed to explain the pregnancy-related adaptations in the brain that underpin HPA axis hypo-responsiveness during pregnancy, evidence from animal models suggests that ALLO may play a critical role in suppressed HPA axis responses to stress in pregnancy (Brunton et al. 2009; Brunton and Russell, 2011).
However, given our observations of stress-induced increases in 3α,5β-THDOC, an alternative, though not mutually exclusive possibility, is one of a differential neurosteroid metabolism in response to acute stress in women during pregnancy. During acute stress, PROG and deoxycorticosterone (DOC) undergo sequential metabolic reduction by 5α-and 5β-reductase and 3α- hydroxysteroid dehydrogenase (3α-HSD) to form ALLO, Pregnanolone, and 3α,5α- and 3α,5β-THDOC (Rupprecht and Holsboer 1999). To date, the physiological importance of 3α,5β-THDOC is not well studied, however, it is clearly GABAergic (Morrow et al. 1990) and there is substantial evidence that 3α,5α-THDOC participates in the HPA axis response to acute stress in rodents (Morrow et al. 1995; Purdy et al. 1991; Reddy and Rogawski 2002). It may be possible that the clinical relevance of 3α,5β-THDOC to psychopathology is related to its elevation following stress that associates with the dampening of the HPA axis stress response indicated not only by the reduction in cortisol that we observed but also by the reduction in the progesterone-derived neuroactive steroids, since the adrenals represent the primary source of circulating neuroactive steroids in response to stress (O'Dell et al. 2004; Purdy et al. 1991).
To the extent that lower ALLO and pregnanolone concentrations, especially following stress, are associated with diminished capacity to negatively modulate the HPA axis and facilitate its recovery following stress exposure (Guo et al. 1995; Patchev et al. 1996), then the blunted ALLO + pregnanolone stress response that was observed in our study sample may be one mechanism that may increase vulnerability to stress and stress-related illness (Crowley and Girdler 2014), including PPD. This may be particularly true for those women with a history of depressive illness, which is highly associated with chronic stress (Kendler et al. 2000; McGuffin and Rivera 2015), and in whom we previously observed a blunted ALLO response to mental stress relative to never depressed women (Klatzkin et al. 2006b). This is also consistent with data from animal models of chronic stress and diminished neurosteroid availability/synthesis (Bortolato et al. 2011; Dong et al. 2001; Guidotti et al. 2001; Matsumoto et al. 2005; Serra et al. 2000; Serra et al. 2005). Although only two women in our sample had a history of depression, post-hoc descriptive review of the data indicated that they had lower ALLO + pregnanolone AUCg levels coupled with higher β-THDOC levels than those without a history of depression.
Why we observed a significant stress-induced increase, from baseline, in 3α,5β-THDOC, yet blunting of the neuroendocrine output across the stressor protocol in other neuroendocrine measures, including cortisol, ACTH, PROG, and the combined ALLO + pregnanolone value, (which were inversely associated with negative emotional stress responses), is intriguing. One possible explanation is that the extent of susceptibility to affective dysregulation following stress may depend on the maintenance of an appropriate balance of neuroactive steroids, HPA axis hormones, and/or perhaps other neuromodulatory factors (e.g. pro-inflammatory cytokines), that have also been linked to affective regulation (Raison et al. 2006).
The hypothesis that stress- induced affective dysregulation may be influenced by the balance of neuroactive steroids (Porcu et al. 2006), may be of particular relevance to our finding that, concurrent with a stress-induced decrease in several neuroendocrine measures, we also observed a stress-induced increase in 3α,5β-THDOC. Using the concept of pregnancy as a model of chronic stress, and capitalizing on the opportunity to study challenges to the neuroendocrine system in a natural model of HPA axis suppression (e.g., pregnancy), our results suggest that, during pregnancy, 3α,5β-THDOC is not downregulated during stress as are other GABAergic neurosteroids. Considering the evidence that the HPA axis is blunted in pregnancy, and our finding of general neuroendocrine suppression, it is possible that stress-induced synthesis of 3α,5β-THDOC and/or its precursor, DOC may involve factors outside of adrenal sources. This hypothesis is in line with previous research in non-human primates which has shown that following dexamethasone suppression of the HPA axis and subsequent ACTH administration, DOC levels fail to change in response to exogenous ACTH (Porcu et al. 2006). Though we did not observe any significant relationships between 3α,5β-THDOC levels and behavioral measures in our sample, the predictive power of deoxycorticosterone-derived neuroactive steroids such as THDOC in PPD may rest in their relationship to other GABAergic neurosteroids, or other neuroendocrine factors. This question will require further longitudinal investigation.
Although the strengths of our study consist of a highly novel study design including prospective assessment of subjectively and objectively measured sleep-wake behavior, a laboratory-based psychosocial stress paradigm for the assessment of daytime stress reactivity, and measurement of stress-reactive GABA-ergic neurosteroids, this study is not without its limitations. First, the analyses reported here must be considered preliminary based on our small sample size, and the nature of this study as pilot work that is meant to inform future research. Moreover, compared to a clinical sample, our women, on average, exhibited mild depressive and anxious symptoms (mean EPDS score = 4.1 ± 3.4; mean STAI score = 30.0 ± 4.0), that were at or below the normative range for women in the second trimester of pregnancy (Bergink et al. 2011; Gunning et al. 2010). However, it is interesting that these women displayed sleep characteristics that were clinically significant (Natale et al. 2015), particularly with regards to stress-related sleep disturbances such as prospective diary assessed sleep onset latency. Therefore, while our results may not be generalizable to a clinical population, our study targets an important question regarding the fundamental processes linking behavioral predictors in pregnancy to perinatal affective dysregulation. Using a conceptual model of pregnancy as a chronic stressor, particularly for first-time mothers, further investigation in a clinical population may provide more insight with regards to a “tipping point” where stress-related physiological changes may be associated with increased risk for disease. Although to our knowledge, we are the first to investigate relationships among neuroendocrine stress reactivity (including neurosteroids) and behavioral indices of anxiety in women during the second trimester of pregnancy, a second limitation of the present study is presented in our sample enrollment, which displayed some heterogeneity (e.g., psychiatric history characteristics) that may have influenced our measurements. Nonetheless, the results of the present investigation demonstrate the feasibility of this protocol in pregnant women and provide general proof of concept for potential biomarkers of risk that may bridge the link between behavioral symptoms in pregnancy and risk for PPD. Specifically, our findings that blunted cortisol, PROG, and ALLO + pregnanolone were associated with worse sleep and more anxiety related emotional responses to stress suggests that these neuroendocrine factors may serve as mediators in the link between affective disturbance in pregnancy and the development of PPD. Clearly a larger sample and a longitudinal design would be needed to test whether this profile represents a “biosignature” of risk in pregnancy for the development of PPD, but the results of this study would support such research.
Acknowledgments
This research was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant award number 1UL1TR001111, NIH grant T32-MH09331, and a grant from the Foundation of Hope for the Research and Treatment of Mental Illness. In addition Dr. Schiller was supported by the UNC Building Interdisciplinary Careers in Women’s Health (BIRCWH) Career Development Program (K12 HD001441), and Dr. Stuebe was supported by NIH grant R01HD073220. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would like to thank Chihiro Christmas, Samantha Nau, Carissa Cartelli, Andrea Ramirez, Pallavi Surana, Audra Goldstein, Ariana Rivens, Danyelle Dawson, Julia Paulson and Khanh Nguyen for their help with data collection for the study.
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Shannon K. Crowley, Email: shannon_crowley@med.unc.edu.
Todd K. O’Buckley, Email: okham@med.unc.edu.
Crystal E. Schiller, Email: crystal_schiller@med.unc.edu.
Alison Stuebe, Email: astuebe@med.unc.edu.
A. Leslie Morrow, Email: leslie_morrow@med.unc.edu.
Susan S. Girdler, Email: susan_girdler@med.unc.edu.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM- IV. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic Insomnia and the Stress System. Sleep Med Clin. 2007;2:279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–538. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A, Pop V. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011;70:385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, et al. Isolation rearing-induced reduction of brain 5alpha-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–1308. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nature Nat RevNeurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, McKay AJ, Ochedalski T, Piastowska A, Rebas E, Lachowicz A, Russell JA. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009;29:6449–6460. doi: 10.1523/JNEUROSCI.0708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat. Stress. 2011;14:6–12. doi: 10.3109/10253890.2010.482628. [DOI] [PubMed] [Google Scholar]
- Christian LM. Physiological reactivity to psychological stress in human pregnancy: current knowledge and future directions. Prog Neurobiol. 2012;99:106–116. doi: 10.1016/j.pneurobio.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Research. 1986;398:382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986;398:382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology. 2014;23:23. doi: 10.1007/s00213-014-3572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weerth C, Wied GD, Jansen LM, Buitelaar JK. Cardiovascular and cortisol responses to a psychological stressor during pregnancy. Acta Obstet Gynecol Scand. 2007;86:1181–1192. doi: 10.1080/00016340701547442. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, et al. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A. 2001;98(5):2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics & Gynecology. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence Reports/Technology Assessments. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37:543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Goyal D, Gay C, Lee K. Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Womens Ment Health. 2009;12:229–237. doi: 10.1007/s00737-009-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Gunning M, Denison F, Stockley C, Ho S, Sandhu H, Reynolds R. Assessing maternal anxiety in pregnancy with the State-Trait Anxiety Inventory (STAI): issues of validity, location and participation. J Reprod Infant Psychol. 2010;28:266–273. [Google Scholar]
- Guo AL, Petraglia F, Criscuolo M, Ficarra G, Nappi RE, Palumbo MA, Genazzani AR. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecol Endocrinol. 1995;9:1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000;54:S47–S51. doi: 10.1038/sj.ejcn.1600984. [DOI] [PubMed] [Google Scholar]
- Jomeen J, Martin CR. Assessment and relationship of sleep quality to depression in early pregnancy. J Reprod Infant Psychol. 2007;25:87–99. [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the "kindling" hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006a;31:1208–1219. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol. 2006b;71:2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Krystal AD. Psychiatric disorders and sleep. Neurologic Clinics. 2012;30:1389–1413. doi: 10.1016/j.ncl.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Marino M, Li Y, Rueschman MN, Winkelman JW, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Pinna G, Puia G, Guidotti A, Costa E. Social isolation stress-induced aggression in mice: a model to study the pharmacology of neurosteroidogenesis. Stress. 2005;8:85–93. doi: 10.1080/10253890500159022. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Rodin J. Pregnancy alters blood pressure responses to psychological and physical challenge. Psychophysiology. 1992;29:232–240. doi: 10.1111/j.1469-8986.1992.tb01691.x. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rivera M. The interaction between stress and genetic factors in the etiopathogenesis of depression. World Psychiatry. 2015;14:161–163. doi: 10.1002/wps.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci. 2011;6:30. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Sloan RP, Trien L, Hurtado A. Maternal stress responses and anxiety during pregnancy: effects on fetal heart rate. Dev Psychobiol. 2000;36:67–77. [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Purdy RH, Paul SM. Neuroactive steroid modulators of the stress response. Ann N Y Acad Sci. 1995;771:257–272. doi: 10.1111/j.1749-6632.1995.tb44687.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions with gamma-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum "blues". Obstetrics & Gynecology. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Natale V, Leger D, Bayon V, Erbacci A, Tonetti L, Fabbri M, Martoni M. The consensus sleep diary: quantitative criteria for primary insomnia diagnosis. Psychosom Med. 2015;77:413–418. doi: 10.1097/PSY.0000000000000177. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med. 2006;68:931–937. doi: 10.1097/01.psy.0000244385.93141.3b. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004;484:241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- O'Hara MW. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry (Abingdon, England) 1996;8:37–54. [Google Scholar]
- O'Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Practice & Research Clinical Obstetrics & Gynaecology. 2014;28:3–12. doi: 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Hanusa BH, Hall M, Wisner KL. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behav Sleep Med. 2009;7:106–117. doi: 10.1080/15402000902762394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Kline CE, Roberts JM, Wettlaufer B, Glover K, Hall M. Prevalence of Sleep Deficiency in Early Gestation and its Associations with Stress and Depressive Symptoms. Journal of Womens Health. 2013;12:12. doi: 10.1089/jwh.2013.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Porcu P, Grant KA, Green HL, Rogers LS, Morrow AL. Hypothalamic-pituitary-adrenal axis and ethanol modulation of deoxycorticosterone levels in cynomolgus monkeys. Psychopharmacology. 2006;186:293–301. doi: 10.1007/s00213-005-0132-2. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;138:911–920. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology. 2014;231:3557–3567. doi: 10.1007/s00213-014-3599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segebladh B, Bannbers E, Moby L, Nyberg S, Bixo M, Bäckström T, Poromaa IS. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16:131–137. doi: 10.1007/s00737-013-0327-1. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu M, Floris I, Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress: The International Journal on the Biology of Stress. 2005;8:259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Skouteris H, Germano C, Wertheim EH, Paxton SJ, Milgrom J. Sleep quality and depression during pregnancy: a prospective study. J Sleep Res. 2008;17:217–220. doi: 10.1111/j.1365-2869.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84:701–710. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Gibbon M, Williams J. Structured clinical interview for axis I DSM-IV disorders (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Teran-Perez G, Arana-Lechuga Y, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano JA, Velazquez Moctezuma J. Steroid hormones and sleep regulation. Mini Rev Med Chem. 2012;12:1040–1048. doi: 10.2174/138955712802762167. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Arch Clin Neuropsychol. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault N, Da Costa D, Marchand A, Ireland K, Dritsa M, Khalife S. Rates and risk factors associated with depressive symptoms during pregnancy and with postpartum onset. J Psychosom Obstet Gynaecol. 2014;35:84–91. doi: 10.3109/0167482X.2014.947953. [DOI] [PubMed] [Google Scholar]