Abstract
Disseminated tumor cells (DTC) are detected early in the disease process in prostate cancer (PCa) patients and can persist after radical prostatectomy. DTC can remain dormant in patients with no evidence of disease for a prolonged period of time only to recur 10 or more years later. Recent advances in single cell genomics and transcriptomics have provided much needed insight into DTC biology and cancer dormancy in patients. With the development of new in vitro and preclinical models, researchers recapitulate the clinical events in patients and therefore allow further elucidation of the molecular mechanisms underlying cancer dormancy and escape. In this review we explore novel ideas on the detection, heterogeneous transcriptomic profiles, molecular and cellular mechanisms of dormancy, and potential mechanisms underlying dormancy escape by DTC. As such there is hope that identifying and targeting novel dormancy-associated pathways in patients with residual disease will have significant clinical implications for the treatment of PCa patients in the future.
Keywords: Disseminated tumor cells, dormancy, microenvironment, bone marrow, prostate cancer, metastasis
Introduction
Prostate cancer (PCa) disseminated tumor cells (DTC) have been detected in patient bone marrow prior to radical prostatectomy (RP) [1,2]. Based on historic data, depending on pathological stage and grade, 16–29% of patients who undergo RP experience biochemical recurrence and approximately 80% of them recur within 5 years [3,4]. However, many patients remain clinically disease-free for years until an increase in serum prostate-specific antigen (PSA) or overt metastases are detected. Though the majority of patients relapse within 5 years, 1 in 5 men recurs after 5 years, and 1 in 20 recurs after 10 years [3,4]. In a study by Amling et al., the hazard ratio of developing biochemical recurrence either stayed constant for organ-confined disease or decreased only slightly after the peak recurrence during the first 2 years post-RP [3]. Furthermore, Ahove et al. determined that among men with an undetectable PSA 5 years after RP, Gleason score, extracapsular extension, and seminal vesicle invasion were still significant predictors of subsequent late recurrence [5].
These data raise many questions concerning the prolonged disease relapse such as: can we determine which patients harbor dormant DTC? What are the DTC doing during that period of dormancy? What triggers dormant DTC to regrow? Can we target dormant DTC with novel therapies when they are held in the quiescent state? In this review, we will address the most updated advances in the detection of DTC and reveal the heterogeneous nature of DTC at a single cell transcriptomic level. We will also discuss emerging hypotheses underlying PCa dormancy and its escape. Finally, the important clinical implications of dormant DTC in PCa will be evaluated.
Detection of DTC
The persistence and implications of DTC are still not completely understood. Our recent study reported detection of DTC in the bone marrow of PCa patients who have no evidence of disease (NED) for up to 18 years after surgery [6]. Controversies persist in whether the detection of PCa DTC pre- or post-surgery correlate with clinical parameters or outcomes, partly due to the lack of standardized methodologies to detect DTC [7,8]. In the past, detection and analysis of DTC has been limited by technology. Bone marrow aspirates were analyzed initially for PSA mRNA expression [9–11] but this approach relies on the cells being PSA-positive. PSMA is another PCa-associated marker but it is also expressed by endothelial cells in cancer [12], therefore it would have to be used in combination with other epithelial markers like EpCAM or pan-CK for DTC detection. Later on, DTC were detected using antibodies against pan-CK/EpCAM on bone marrow aspirates by immunocytochemistry [13]. Until recently, we and others used antibody-conjugated magnetic beads to further enrich for CD45-negative and CK/EpCAM-positive cells for molecular analysis in individual DTC [1,2]. More recently, advances in single cell technology have provided whole genomic and transcriptomic analyses of one cell [6,14–16], and demonstrated the heterogeneity as well as the plasticity of individual DTC.
However, single DTC analysis has its limitations. This approach relied on the successful identification of DTC using epithelial markers including cytokeratin (CK) or epithelial cell adhesion molecule (EpCAM). A recent study by Todenhöfer et al. summarized DTC studies that assessed the percentage of positive DTC detected in PCa patients pre- or post-surgery [8]. The rates of detection ranged from 13–45% using pan-CK staining [1,9,11,13,17–22] and 72–90% using EpCAM staining [2,10].
Several recent observations may help explain the observed high proportion of EpCAM-positive cells present in the bone marrow even in early disease. Our subsequent study on a single cell transcriptomic analysis demonstrated that a significant portion of the EpCAM-positive cells expressed a signature associated with erythroid progenitor-like cells [6,23,24]. An independent single cell gene expression study by Gužvić et al. reported an interesting population of cells that expressed prostate epithelial markers, genomically aberrant, EpCAM-positive but CK8- and CK18-negative, suggesting a potential loss of CK epithelial markers in these DTC [15]. Therefore, while anti-EpCAM antibody may capture some non-epithelial cells (e.g. erythroid progenitor-like cells) it may also capture a subgroup of PCa DTC that are CK8/18-negative. Alternatively, a subgroup of DTC that displays a neuroendocrine (NE) or a cancer stem-like cell (CSC) phenotype do not usually express EpCAM but are positive for CK [25,26]. To further complicate the situation, non-epithelial cells (e.g. plasma cells) in the bone marrow may be CK positive [27].
Based on what we have learned to date, we can no longer rely on the use of anti-EpCAM antibodies alone for the identification and isolation of PCa DTC from the bone marrow. We have known for years that the plasticity of DTC would result in the loss of detection of some epithelial tumor cells which no longer express routine epithelial markers. Our most recent data from individual cell gene expression complicate this phenomenon further by suggesting that a rare population of non-epithelial cells in the bone marrow also expresses EpCAM. These two biological sources of error prompt us to urge the field to use a more robust cocktail of antibodies to identify and capture DTC which have lost EpCAM expression and to identify those rare EpCAM-positive bone marrow progenitor cells as non DTC. For example, to include NE cells or CSCs which may well be within the DTC population, one could add to the cocktail antibodies that target NE markers like chromogranin A [25,28], synaptophysin [25], and neuron-specific enolase [29], and CSC markers like CD44 [26]. With future advances in single cell technology, coupled genomic and transcriptomic analysis, and multispectral imaging will allow a more complete definition of molecular profiles of DTC with the range of phenotypes observed in PCa.
The transcriptomic profile of DTC is heterogeneous in patients
To understand the biology behind the DTC and its relation to tumor dormancy, we [6,30] and others [15] recently profiled single DTC in the bone marrow of PCa patients at different stages of disease progression. DTC displayed high intra- and inter-patient heterogeneity [6,15]. Gužvić et al., using a single cell genomic and gene expression analysis, reported that the genomically aberrant DTC isolated from PCa patients regardless of the metastatic status were highly plastic [15]. Furthermore, a global gene expression analysis on DTC isolated from PCa NED patients 7–18 years post-RP was compared with those from patients with advanced disease (i.e. biochemical recurrence or known bone metastasis) in an attempt to uncover clinical dormancy-associated gene signatures [6]. Interestingly, we identified two groups of DTC in individual patients with advanced disease and the DTC in one of these groups display a gene expression pattern similar to those isolated from patients with NED [6]. This single DTC analysis suggests that at least two populations of ADV DTC co-exist in the same microenvironment. While the microenvironment is permissive for the development of bone metastasis, clearly not all DTC entering the bone form metastasis suggesting these cells enter a dormant/quiescent state.
Molecular and cellular mechanisms of PCa dormancy
We define a dormant cell as one that remains in a quiescent state, but has the potential to proliferate. PCa cell dormancy and its reactivation may be governed by cell intrinsic factors or cues from the microenvironment. Thus, signals from the microenvironment may alter the molecular and cellular pathways of the DTC which results in a dormant or proliferating phenotype.
1. Tumor microenvironment
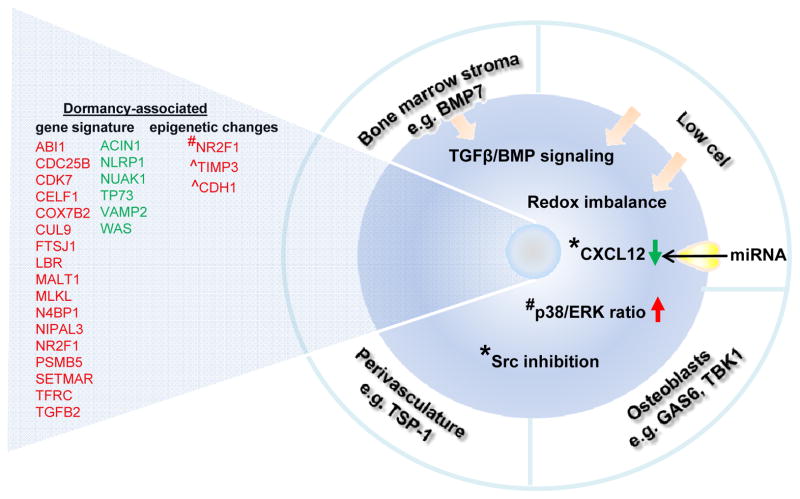
Tumor microenvironments contain a multiplicity of cells including tumor cells, target organ cells, CSC, endothelial cells, pericytes, immune inflammatory cells, and cancer-associated fibroblasts among others [31]. Previous studies from Taichman’s group have demonstrated that in preclinical models, the hematopoietic stem cell niche in the bone marrow promoted tumor dormancy. Briefly, osteoblast-derived growth-arrest specific 6 (GAS6) ligands induced a subset of PCa cells (expressing high GAS6 receptor Axl/Tyro3 ratio) to express a dormant phenotype (Fig. 1) [32]. Additionally, ‘dormancy-enriched’ populations of human PCa cells binding to mouse niche osteoblasts expressed a higher TANK binding kinase 1 level, suggesting at least a subset of PCa cells are under osteoblast-derived growth suppression (Fig. 1) [33]. Examining the perivascular niche, breast cancer (BCa) DTC have been demonstrated by Ghajar et al. to reside in a dormant state adjacent to mature blood vessels in the lung, bone marrow, and brain, associated with the local endothelial-derived factors such as thrombospondin-1 produced by the mature blood vessels (Fig. 1) [34]. In the same study, apparently dormant DTC were stimulated to grow when attaching to sprouting microvasculature which secreted periostin and transforming growth factor-beta 1 (TGF-β1) [34].
Fig. 1. Molecular and cellular mechanism of PCa dormancy.
A schematic diagram summarizing the current evidence of molecular and cellular events in maintaining dormancy in PCa cells. Gene expression signature was adopted from references [6, 27]. Red: upregulated; green: downregulated. * in breast cancer; # in head and neck squamous carcinoma; ^ in ovarian cancer. TSP-1: thrombospondin-1; GAS6: Growth arrest-specific 6; TBK1: TANK-binding kinase 1; BMP7: Bone morphogenetic protein 7.; TGFβ: transforming growth factor beta; CXCL12: C-X-C motif chemokine 12; Src: Proto-oncogene tyrosine-protein kinase Src.
CSCs are defined as cells that retain the ability to self-renew and efficiently seed new tumors [35]. Emerging evidence links the CSC hypothesis with dormancy and metastasis because both CSCs and dormant cells share features like quiescence and immune evasion [36]. On the other hand in a recent publication by Wang et al. comparing mitotic quiescent subpopulations of PCa tumor cells to a rapidly dividing population showed that the quiescent cells were not stem-like, with no expression of CD133 and similar levels of CD44 and integrins α2/β1, when compared to the rapidly dividing population [37]. Their model argues that mitotic quiescence, but not unique “stemness,” marked the phenotype of bone metastasis-initiating cells in PCa [38].
2. Cellular pathways
Once the DTC arrive at a foreign microenvironment or niche, they have to adapt before they can proliferate. One would anticipate these DTC may remain dormant in response to inhibitory signals (e.g. bone morphogenetic protein, BMP) or when deprived of activating niche signals (e.g. Wnt and Notch) [39,40]. In PCa, BMP7 secreted from bone stromal cells induced senescence through activation of p38 MAPK in human PCa cells as well as CSCs (Fig. 1) [41]. Recently, we have shown that in cells derived from PCa patient-derived xenografts, they failed to divide when seeded in low density and these apparently dormant cells showed an upregulated level of TGFB2 [42]. Low cell density also triggered TGFβ/BMP signaling and redox imbalance to prime cells for dormancy [43]. In a head and neck squamous carcinoma model TGF-β2 signaling in the bone marrow has been shown to activate p38 MAPK, inducing a high p38/ERK signaling ratio and consequently dormancy (Fig. 1) [44]. Similarly, in a BCa dormancy model Touny et al. have shown p38 MAPK is inhibitory [45]. Furthermore, they determined that proto-oncogene tyrosine-protein kinase Src (Src) inhibition can block proliferation (Fig. 1), but blocking Src in combination with MEK1/2 which is upstream of ERK 1/2 results in not just growth inhibition, but apoptosis [45]. Additionally, in BCa, bone marrow stroma has been purported to promote tumor dormancy by reducing CXCL12 and cell cycling in cancer cells (Fig. 1) [46,47]. In contrast, TGFβ2-triggered CXCL12-CXCR4 signaling is crucial for the PCa DTC to maintain a slow-cycling state in the bone marrow [48]. A recent review has comprehensively summarized the enhanced survival signaling and stress signaling in maintaining dormancy of a cancer cell before it is ready to proliferate [40].
3. Transcriptomics
Recent advances in single cell genomics and transcriptomics, and the successful isolation of DTC from patients provide novel insights into transcriptomes of PCa DTC and dormancy. PCa DTC isolated from the bone marrow of NED patients for 7–18 years post-RP displayed higher expression of the dormancy-associated markers including NR2F1 and TGFB2 than those from patients with biochemical recurrence or bone metastasis (Fig. 1) [30]. Global transcriptomic comparison between DTC from these two groups of patients revealed a deregulation in the p38 stress response pathway, suggesting the p38 pathway may play a role in regulating PCa DTC dormancy in the patient bone marrow [6]. Furthermore, cell culture experiments showed a consistent upregulation of TGFB2 gene expression among apparently dormant cells isolated from three PCa patient-derived xenograft models [42].
4. Epigenetics
The tumor microenvironment and cellular pathways potentially alter the epigenetics of a cell. A recent review by Crea et al. proposes that cancer dormancy is a nongenetic disease driven by flexible epigenetic and non-coding interactome [49]. In head and neck squamous carcinoma, Sosa et al. demonstrated that the established dormancy-inducing gene NR2F1 was regulated by histone modifications and was upregulated by 5-azacytidine (a DNA demethylating agent) followed by retinoic acid (Fig. 1) [30]. DNA demethylation and histone acetylation has also recently been shown to upregulate the anti-angiogenic genes tissue inhibitor of metalloproteinases-3 (TIMP3) and E-cadherin (CDH1) during ovarian cancer dormancy (Fig. 1) [50].
5. microRNAs
Emerging evidence support the role of microRNAs (miRNAs) in the switch of the tumor from a dormant to a fast-growing phenotype. Recently, an unbiased approach has identified miRNAs (e.g miR- 138 and miR-246) as drivers of metastatic reactivation of BCa in the lung in vivo [51]. In multiple cancers, Almog et al. showed that the loss of a set of dormancy-associated miRNAs that downregulated anti-angiogenic factors such as TIMP3, basic fibroblast growth factor (bFGF) and TGFα resumed the growth of multiple dormant cancer cell types (e.g. BCa, glioblastoma, osteosarcoma, and liposarcoma) [52]. The same group later determined that cell cycle arrest was not involved in the miR-190-expressing dormant glioblastoma [53]. In BCa, CXCL12-specific miRNAs were proposed to be transported from healthy bone marrow stroma to BCa cells via gap junctions, leading to reduced CXCL12 expression and decreased cell proliferation (Fig. 1) [46]. In addition, exosomal transfer of miRNAs (e.g. miR-23b) from the bone marrow may promote BCa cell dormancy via suppression of a target cell cycle gene MARCKS [47]. However, these studies were done on healthy bone marrow stroma. Whether dormant cancer cells can be reactivated when cultured in the cancer-associated bone marrow stroma remains unexplored. Studies on the impact of miRNAs on PCa dormancy are not currently available in the literature.
Escaping dormancy
Emerging preclinical and clinical evidence supports the possible role of an unstable bone microenvironment that favors cancer dormancy reactivation and the outgrowth of metastasis. Zoledronic acid, the osteoclast inhibitor, is routinely prescribed to PCa patients with active bone metastasis or skeletal-related complications to dampen bone resorption and limit bone loss in men receiving androgen deprivation therapy [54]. A model of PCa bone metastasis recently revealed that after androgen ablation, the high bone turnover environment increased the outgrowth of DTC and this outgrowth was reversed by zoledronic acid [55]. Similarly, the slow- or non-growing BCa cells attached to the matrix produced by osteoblasts resumed growth after the addition of bone remodeling cytokines, TNFα and interleukin 1-beta [56]. A BCa clinical trial supported that adjuvant zoledronic acid treatment for those patients with DTC detected during surgery increased progression-free survival [57], further supporting the potential benefit of inhibiting bone turnover to prevent metastatic outgrowth. Another clinical study in BCa even showed that zoledronic acid contributed to the eradication of DTC [58], although in PCa a preclinical study failed to show that zoledronic acid eliminated the pre-existing DTC [37]. Nonetheless, the potential use of zoledronic acid as an adjuvant therapy to androgen deprivation therapy in minimally metastatic PCa patients will be particularly interesting – one would anticipate it may help patients preserve a more intact bone environment that is unfavorable to the outgrowth of DTC and therefore prevent the development of bone metastasis.
In PCa, reactivating dormant cells at the cellular level has been proposed by two independent groups reporting that high level of CXCR4 was associated with mitotic quiescence [37] or slow-cycling [48]. Inhibition of CXCR4 activity reactivated cell proliferation in vitro [48]. However, when these quiescent cells with high CXCR4 were injected into the circulation, the tumorigenicity of the cells in the bone increased in vivo [37]. While it is likely that the high level of CXCR4 simply increases the bone propensity of this PCa cell line PC3-NW1 and hence its outgrowth in the bone, whether or not the increase in CXCR4 level in the PCa cell impacts the dormancy switch in DTC that are already in the bone marrow remains unexplored.
Recent research supports that dormant cells maintain reversible cell cycle arrest, a low reactive oxygen species state, and a low metabolic state featured with decreased glycolysis, reduced translation rates, and activation of autophagy [59–61]. Whether a disruption of these dormancy-maintaining cellular states will promote metastatic reactivation warrants investigation. Alternatively, tumor senescence could be achieved by switching on a senescence-like program via activation of Ras signaling [62], suggesting that dormant cells can potentially be converted to senescent–like cells to irreversibly prevent cancer outgrowth.
Clinical implications of dormant DTC
To assess the burden and biology of minimal residual disease after RP, DTC residing at distant sites offer a valid surrogate to be sampled. Although the detection of DTC does not necessarily imply the development of clinically overt metastasis, the presence of DTC is unarguably a marker for metastatic burden.
With the relative ease of acquisition of DTC from the bone marrow and the recent advance in technology to molecularly characterize DTC at a single cell level, investigation of DTC by liquid biopsy before the detection of overt metastasis will be attractive to assess individual prognosis and stratify patients at risk to systemic adjuvant anti-cancer therapies [63]. However, we still face two major technical challenges: (1) molecular characterization of DTC sampling at easily accessible sites like the iliac crest may not represent DTC burden in other osseous tissues or in visceral tissues, and (2) current positive selection of DTC using EpCAM or CK could lead to misidentification of epithelial cells [23,24,27]. We urge the field to improve methods of identifying DTC using multiple markers and this improved method should also ideally allow downstream biological characterization of DTC using genomic and transcriptomic approaches.
The novel molecular and cellular information generated from DTC in patient specimens will facilitate biomarker discovery and/or clinical diagnosis and prognosis. We propose that the long latency for PCa reactivation is possibly tightly regulated by multiple mechanisms. We may identify actionable targets to prevent metastasis by maintaining cells in a dormant state, driving proliferating cells into dormancy, or eradicating DTC. To keep or push PCa DTC towards a dormant state, based on the current literature, one may consider upregulating the p38 stress response pathway, reactivating BMP7 signaling in the bone marrow niche, inhibiting Src, MEK 1/2, or bone turnover, or target the DTC at the epigenetic or miRNA level. On the other hand, if the enhanced survival pathways identified in other cancers are common to PCa dormancy, it may be possible to eradicate PCa DTC by inhibiting these pathways. Importantly, we believe we have only explored the tip of the iceberg of what promotes PCa dormancy in patients post-RP. Unraveling the biology behind DTC and tumor dormancy will allow us to target early disseminated disease, which should be more effective than targeting the late-stage clinically-apparent metastasis.
Acknowledgments
The work was supported by NIH PO1 CA85859, the Pacific Northwest Prostate Cancer SPORE NIH P50 CA097186, and the Richard M. LUCAS Foundation. H.M.L. is a recipient of the Young Investigator Award from the Prostate Cancer Foundation, and a Career Development Award from the Pacific Northwest Prostate Cancer SPORE (P50 CA097186). This material is also the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington (R.L.V. is a VA Biomedical Laboratory R&D senior research career scientist and P.H.L. is a staff physician).
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Weckermann D, Muller P, Wawroschek F, Krawczak G, Riethmuller G, Schlimok G. Micrometastases of bone marrow in localized prostate cancer: correlation with established risk factors. J Clin Oncol. 1999;17:3438–3443. doi: 10.1200/JCO.1999.17.11.3438. [DOI] [PubMed] [Google Scholar]
- 2.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15:677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101–105. [PubMed] [Google Scholar]
- 4.Loeb S, Feng Z, Ross A, Trock BJ, Humphreys EB, Walsh PC. Can we stop prostate specific antigen testing 10 years after radical prostatectomy? J Urol. 2011;186:500–505. doi: 10.1016/j.juro.2011.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahove DA, Hoffman KE, Hu JC, Choueiri TK, D’Amico AV, Nguyen PL. Which patients with undetectable PSA levels 5 years after radical prostatectomy are still at risk of recurrence?--implications for a risk-adapted follow-up strategy. Urology. 2010;76:1201–1205. doi: 10.1016/j.urology.2010.03.092. [DOI] [PubMed] [Google Scholar]
- 6.Chery L, Lam HM, Coleman I, Lakely B, Coleman R, Larson S, Aguirre-Ghiso JA, Xia J, Gulati R, Nelson PS, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014;5:9939–9951. doi: 10.18632/oncotarget.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilleby W, Stensvold A, Mills IG, Nesland JM. Disseminated tumor cells and their prognostic significance in nonmetastatic prostate cancer patients. Int J Cancer. 2013;133:149–155. doi: 10.1002/ijc.28002. [DOI] [PubMed] [Google Scholar]
- 8.Todenhofer T, Hennenlotter J, Faber F, Wallwiener D, Schilling D, Kuhs U, Aufderklamm S, Bier S, Mischinger J, Gakis G, et al. Significance of apoptotic and non-apoptotic disseminated tumor cells in the bone marrow of patients with clinically localized prostate cancer. Prostate. 2015;75:637–645. doi: 10.1002/pros.22947. [DOI] [PubMed] [Google Scholar]
- 9.Wood DP, Jr, Banerjee M. Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J Clin Oncol. 1997;15:3451–3457. doi: 10.1200/JCO.1997.15.12.3451. [DOI] [PubMed] [Google Scholar]
- 10.Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003;61:277–281. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- 11.Pfitzenmaier J, Ellis WJ, Hawley S, Arfman EW, Klein JR, Lange PH, Vessella RL. The detection and isolation of viable prostate-specific antigen positive epithelial cells by enrichment: a comparison to standard prostate-specific antigen reverse transcriptase polymerase chain reaction and its clinical relevance in prostate cancer. Urol Oncol. 2007;25:214–220. doi: 10.1016/j.urolonc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 13.Weckermann D, Muller P, Wawroschek F, Harzmann R, Riethmuller G, Schlimok G. Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: detection and prognostic value. J Urol. 2001;166:699–703. [PubMed] [Google Scholar]
- 14.Klein CA, Schmidt-Kittler O, Schardt JA, Pantel K, Speicher MR, Riethmuller G. Comparative genomic hybridization, loss of heterozygosity, and DNA sequence analysis of single cells. Proc Natl Acad Sci U S A. 1999;96:4494–4499. doi: 10.1073/pnas.96.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzvic M, Braun B, Ganzer R, Burger M, Nerlich M, Winkler S, Werner-Klein M, Czyz ZT, Polzer B, Klein CA. Combined genome and transcriptome analysis of single disseminated cancer cells from bone marrow of prostate cancer patients reveals unexpected transcriptomes. Cancer Res. 2014;74:7383–7394. doi: 10.1158/0008-5472.CAN-14-0934. [DOI] [PubMed] [Google Scholar]
- 16.Welty CJ, Coleman I, Coleman R, Lakely B, Xia J, Chen S, Gulati R, Larson SR, Lange PH, Montgomery B, et al. Single cell transcriptomic analysis of prostate cancer cells. BMC Mol Biol. 2013;14:6. doi: 10.1186/1471-2199-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg A, Berner A, Lilleby W, Bruland OS, Fossa SD, Nesland JM, Kvalheim G. Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int J Cancer. 2007;120:1603–1609. doi: 10.1002/ijc.22488. [DOI] [PubMed] [Google Scholar]
- 18.Kollermann J, Weikert S, Schostak M, Kempkensteffen C, Kleinschmidt K, Rau T, Pantel K. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol. 2008;26:4928–4933. doi: 10.1200/JCO.2007.15.0441. [DOI] [PubMed] [Google Scholar]
- 19.Weckermann D, Polzer B, Ragg T, Blana A, Schlimok G, Arnholdt H, Bertz S, Harzmann R, Klein CA. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009;27:1549–1556. doi: 10.1200/JCO.2008.17.0563. [DOI] [PubMed] [Google Scholar]
- 20.Cher ML, de Oliveira JG, Beaman AA, Nemeth JA, Hussain M, Wood DP., Jr Cellular proliferation and prevalence of micrometastatic cells in the bone marrow of patients with clinically localized prostate cancer. Clin Cancer Res. 1999;5:2421–2425. [PubMed] [Google Scholar]
- 21.Bianco FJ, Jr, Wood DP, Jr, Gomes de OJ, Nemeth JA, Beaman AA, Cher ML. Proliferation of prostate cancer cells in the bone marrow predicts recurrence in patients with localized prostate cancer. Prostate. 2001;49:235–242. doi: 10.1002/pros.10018. [DOI] [PubMed] [Google Scholar]
- 22.Mitsiades CS, Lembessis P, Sourla A, Milathianakis C, Tsintavis A, Koutsilieris M. Molecular staging by RT-pCR analysis for PSA and PSMA in peripheral blood and bone marrow samples is an independent predictor of time to biochemical failure following radical prostatectomy for clinically localized prostate cancer. Clin Exp Metastasis. 2004;21:495–505. doi: 10.1007/s10585-004-3217-0. [DOI] [PubMed] [Google Scholar]
- 23.Lammers R, Giesert C, Grunebach F, Marxer A, Vogel W, Buhring HJ. Monoclonal antibody 9C4 recognizes epithelial cellular adhesion molecule, a cell surface antigen expressed in early steps of erythropoiesis. Exp Hematol. 2002;30:537–545. doi: 10.1016/s0301-472x(02)00798-1. [DOI] [PubMed] [Google Scholar]
- 24.Eisenwort G, Jurkin J, Yasmin N, Bauer T, Gesslbauer B, Strobl H. Identification of TROP2 (TACSTD2), an EpCAM-like molecule, as a specific marker for TGF-beta1-dependent human epidermal Langerhans cells. J Invest Dermatol. 2011;131:2049–2057. doi: 10.1038/jid.2011.164. [DOI] [PubMed] [Google Scholar]
- 25.Brownback KR, Renzulli J, Delellis R, Myers JR. Small-cell prostate carcinoma: A retrospective analysis of five newly reported cases. Indian J Urol. 2009;25:259–263. doi: 10.4103/0970-1591.52940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 27.Shetye JD, Liljefors ML, Emdin SO, Frodin JE, Strigard K, Mellstedt HT, Porwit A. Spectrum of cytokeratin-positive cells in the bone marrows of colorectal carcinoma patients. Anticancer Res. 2004;24:2375–2383. [PubMed] [Google Scholar]
- 28.Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12:181–187. doi: 10.1210/edrv-12-2-181. [DOI] [PubMed] [Google Scholar]
- 29.Danza G, Di SC, Rosati F, Lonetto G, Sturli N, Kacer D, Pennella A, Ventimiglia G, Barucci R, Piscazzi A, et al. Notch signaling modulates hypoxia-induced neuroendocrine differentiation of human prostate cancer cells. Mol Cancer Res. 2012;10:230–238. doi: 10.1158/1541-7786.MCR-11-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sosa MS, Parikh F, Maia AG, Estrada Y, Bosch A, Bragado P, Ekpin E, George A, Zheng Y, Lam HM, et al. NR2F1 controls tumour cell dormancy via. Nat Commun. 2015;6:6170. doi: 10.1038/ncomms7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, Yumoto K, Berry JE, Shiozawa Y, Pienta KJ. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One. 2013;8:e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JK, Jung Y, Wang J, Joseph J, Mishra A, Hill EE, Krebsbach PH, Pienta KJ, Shiozawa Y, Taichman RS. TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia. 2013;15:1064–1074. doi: 10.1593/neo.13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 36.Kleffel S, Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin? Adv Exp Med Biol. 2013;734:145–179. doi: 10.1007/978-1-4614-1445-2_8. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Docherty F, Brown HK, Reeves K, Fowles A, Lawson M, Ottewell PD, Holen I, Croucher PI, Eaton CL. Mitotic quiescence, but not unique “stemness,” marks the phenotype of bone metastasis-initiating cells in prostate cancer. FASEB J. 2015 doi: 10.1096/fj.14-266379. [DOI] [PubMed] [Google Scholar]
- 38.Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology. 2007;50:648–658. doi: 10.1111/j.1365-2559.2007.02665.x. [DOI] [PubMed] [Google Scholar]
- 39.Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, Giancotti FG. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruppender N, Larson S, Lakely B, Kollath L, Brown L, Coleman I, Coleman R, Nguyen H, Nelson PS, Corey E, et al. Cellular Adhesion Promotes Prostate Cancer Cells Escape from Dormancy. PLoS One. 2015;10:e0130565. doi: 10.1371/journal.pone.0130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bui AT, Laurent F, Havard M, Dautry F, Tchenio T. SMAD signaling and redox imbalance cooperate to induce prostate cancer cell dormancy. Cell Cycle. 2015;14:1218–1231. doi: 10.1080/15384101.2015.1014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, Schewe DM, Aguirre-Ghiso JA. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Touny LH, Vieira A, Mendoza A, Khanna C, Hoenerhoff MJ, Green JE. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J Clin Invest. 2014;124:156–168. doi: 10.1172/JCI70259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 47.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura T, Shinriki S, Jono H, Guo J, Ueda M, Hayashi M, Yamashita S, Zijlstra A, Nakayama H, Hiraki A, et al. Intrinsic TGF-beta2-triggered SDF-1-CXCR4 signaling axis is crucial for drug resistance and a slow-cycling state in bone marrow-disseminated tumor cells. Oncotarget. 2015;6:1008–1019. doi: 10.18632/oncotarget.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crea F, Nur Saidy NR, Collins CC, Wang Y. The epigenetic/noncoding origin of tumor dormancy. Trends Mol Med. 2015;21:206–211. doi: 10.1016/j.molmed.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Lyu T, Jia N, Wang J, Yan X, Yu Y, Lu Z, Bast RC, Jr, Hua K, Feng W. Expression and epigenetic regulation of angiogenesis-related factors during dormancy and recurrent growth of ovarian carcinoma. Epigenetics. 2013;8:1330–1346. doi: 10.4161/epi.26675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao H, Chakraborty G, Lee-Lim AP, Mavrakis KJ, Wendel HG, Giancotti FG. Forward genetic screens in mice uncover mediators and suppressors of metastatic reactivation. Proc Natl Acad Sci U S A. 2014;111:16532–16537. doi: 10.1073/pnas.1403234111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almog N, Ma L, Schwager C, Brinkmann BG, Beheshti A, Vajkoczy P, Folkman J, Hlatky L, Abdollahi A. Consensus micro RNAs governing the switch of dormant tumors to the fast-growing angiogenic phenotype. PLoS One. 2012;7:e44001. doi: 10.1371/journal.pone.0044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almog N, Briggs C, Beheshti A, Ma L, Wilkie KP, Rietman E, Hlatky L. Transcriptional changes induced by the tumor dormancy-associated microRNA-190. Transcription. 2013;4:177–191. doi: 10.4161/trns.25558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saad F, McKiernan J, Eastham J. Rationale for zoledronic acid therapy in men with hormone-sensitive prostate cancer with or without bone metastasis. Urol Oncol. 2006;24:4–12. doi: 10.1016/j.urolonc.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Ottewell PD, Wang N, Meek J, Fowles CA, Croucher PI, Eaton CL, Holen I. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer. 2014;21:769–781. doi: 10.1530/ERC-14-0199. [DOI] [PubMed] [Google Scholar]
- 56.Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis. 2015;32:335–344. doi: 10.1007/s10585-015-9710-9. [DOI] [PubMed] [Google Scholar]
- 57.Hartkopf AD, Taran FA, Wallwiener M, Hahn M, Becker S, Solomayer EF, Brucker SY, Fehm TN, Wallwiener D. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients - results from a large single-centre analysis. Eur J Cancer. 2014;50:2550–2559. doi: 10.1016/j.ejca.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 58.Banys M, Solomayer EF, Gebauer G, Janni W, Krawczyk N, Lueck HJ, Becker S, Huober J, Kraemer B, Wackwitz B, et al. Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: results of a prospective clinical trial. BMC Cancer. 2013;13:480. doi: 10.1186/1471-2407-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kangwan N, Park JM, Kim EH, Hahm KB. Chemoquiescence for ideal cancer treatment and prevention: where are we now? J Cancer Prev. 2014;19:89–6. doi: 10.15430/JCP.2014.19.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valcourt JR, Lemons JM, Haley EM, Kojima M, Demuren OO, Coller HA. Staying alive: metabolic adaptations to quiescence. Cell Cycle. 2012;11:1680–1696. doi: 10.4161/cc.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dey-Guha I, Wolfer A, Yeh AC, Albeck G, Darp R, Leon E, Wulfkuhle J, Petricoin EF, III, Wittner BS, Ramaswamy S. Asymmetric cancer cell division regulated by AKT. Proc Natl Acad Sci U S A. 2011;108:12845–12850. doi: 10.1073/pnas.1109632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bihani T, Chicas A, Lo CP, Lin AW. Dissecting the senescence-like program in tumor cells activated by Ras signaling. J Biol Chem. 2007;282:2666–2675. doi: 10.1074/jbc.M608127200. [DOI] [PubMed] [Google Scholar]
- 63.Pantel K, Alix-Panabieres C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep. 2014;3:584. doi: 10.1038/bonekey.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]