Abstract
De novo induction of organized lymphoid aggregates at non-lymphoid sites has been observed in many chronic inflammatory conditions where foreign antigens such as infectious agents, auto- or alloantigens, persist. The prevailing opinion in the field of transplantation is that lymphoid neogenesis within allografts is detrimental to the establishment of immune tolerance. These structures, commonly referred to as tertiary lymphoid organs (TLOs), are thought to contribute to graft rejection by generating and propagating local alloimmune responses. However, recent studies have shown that TLOs rich in regulatory Foxp3+ cells are present in long term accepting allografts. The notion that TLOs can contribute to the local downregulation of immune responses has been corroborated in other chronic inflammation models. These findings suggest that contrary to previous suggestions that the induction of TLOs in allografts is necessarily harmful, the induction of “tolerogenic” TLOs may prove advantageous. In this review, we discuss our current understanding of how TLOs are induced and how they regulate immune responses with a particular focus on alloimmunity.
Tertiary lymphoid organs (TLOs) are accumulations of immune cells at nonlymphoid sites such as lungs, livers and salivary glands that resemble secondary lymphoid organs (SLOs) in their cellular content and organization. These structures are generally associated with states of chronic inflammation such as autoimmunity, atherosclerosis, infections, cancer and graft rejection and are oftentimes linked to a poor clinical prognosis (1). Lymphoid neogenesis might play a role in governing local immune response against self-antigens and infectious agents. To this end, it has been postulated that persistence of the target antigen is a common denominator for the conditions where lymphoid neogenesis is observed. Seminal studies by Randall and colleagues have conclusively demonstrated that productive T and B cell responses can be generated in these structures independent of SLOs, such as lymph nodes and spleen (2). In subsequent studies, these authors showed that TLOs are able to propagate and maintain immunological memory responses (3). Studies in a variety of autoimmunity models have indicated that cellular and humoral responses generated within TLOs in afflicted tissues and organs maintain the reactivity directed against self-antigens and contribute to the progression of disease (4, 5).
Similar to SLOs, TLOs are composed of zones of T and B cells, active germinal centers and a highly organized vascular system of high endothelial venules and lymphatic vessels, which provides an essential role for lymphocyte extravasation and trafficking (1). High endothelial venules that stain positively for peripheral nodal addressin are considered to be a unique feature of SLOs or TLOs. Lymphatic vessels express LYVE-1 and PROX1 and may play a role in cellular trafficking, antigen transport and regulation of fluid balance (6). Thaunat put forward a theory that lymphoid neogenesis in transplanted organs is in part facilitated by disruption of lymphatic drainage from the graft (7). Antigen presenting cells such as dendritic cells are present in TLOs, suggesting that T lymphocytes can be primed in these structures independent of SLOs (6, 8). Furthermore, a network of follicular dendritic cells help B cells to proliferate, mature and undergo selection thereby generating local humoral responses (1).
Signals that induce and maintain TLOs
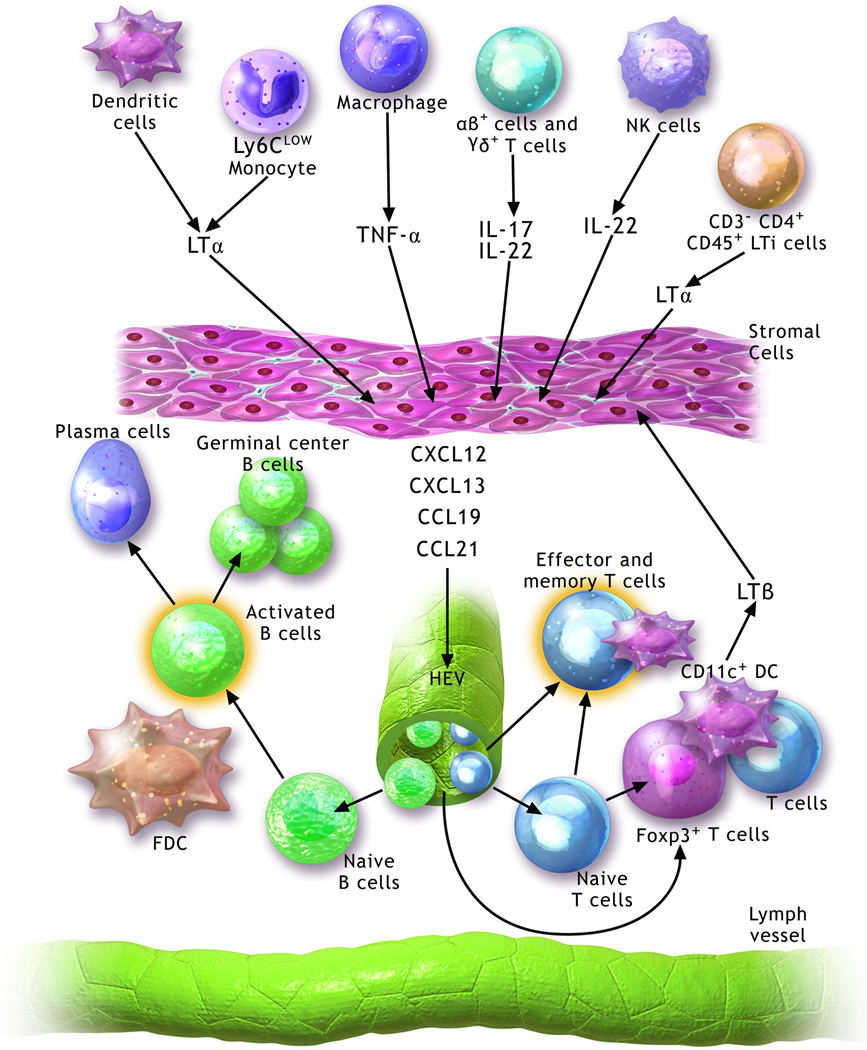
Lymphoid neogenesis is a dynamic process involving a wide range of growth and survival factors, cytokines, chemokines and specific cells that generate these substances. Members of the tumor necrosis factor (TNF)-family-mainly lymphotoxin (LT)-α1β2 and TNF-α have been implicated in TLO development (Figure 1). Binding of TNF-α and LTα1β2 to their respective receptors triggers the expression of several critical homeostatic chemokines (1). In SLO development, expression of LTα1β2 on the surface membrane of CD3−CD4+CD45+ hematopoietic lymphoid tissue inducer cells can activate lymphoid tissue organizer cells to release homeostatic chemokines such as CCL19, CCL21, CXCL12 and CXCL13, which regulate lymphocyte homing and compartmentalization (9).
Figure 1. Molecular and cellular pathways contributing to the development and maintenance of tertiary lymphoid organs.
Several cell populations including lymphoid organ inducer cells (LTi) (CD3−CD4+CD45+), natural killer (NK) cells, dendritic cells (DC), macrophages and Ly6Clow monocytes may contribute to the development of TLOs through the production of various mediators, including lymphotoxin (LT)-α, tumor necrosis factor (TNF)-α, IL-17 and IL-22. These activate stromal cells to secrete homeostatic chemokines (CCL19, CCL21, CXCL12 and CXCL13), which promote the recruitment of B cells and T cells. A well-organized tertiary lymphoid organ is composed of separated T and B cell areas, follicular dendritic cells (FDC), CD11c+ dendritic cells, high endothelial venules (HEVs) and lymphatic channels. CD11c+ dendritic cells contribute to the maintenance of these structures possibly through secretion of LTβ.
Overexpression of lymphotoxin-α or homeostatic chemokines has been shown to result in the induction of lymphoid neogenesis at various sites. For example, transgenic expression of LT-α induces ectopic lymphoid structures in skin allografts and results in their rejection after transplantation onto recipient hosts that lack spleens and peripheral lymph nodes (10). Islet-specific expression of the CCR7 ligand CCL21 leads to de novo formation of organized lymphoid aggregates (11). The critical role for chemokines that attract CCR7-expressing cells in lymphoid neogenesis was supported by studies showing that TLOs did not form in the lungs of plt/plt mice, that lack the expression of CCL19 and the serine isoform of CCL21 (12). Interestingly, unlike wildtype mice where TLOs can be induced in lungs only after exposure to inflammatory stimuli CCR7-deficient mice develop TLOs in their lungs spontaneously (13). Kocks demonstrated that injection of wildtype regulatory T cells into CCR7-deficient mice resulted in a substantial reduction of TLO development in their lungs suggesting that CCR7-dependent homing of regulatory T cells downregulates the formation of these structures.
While some reports have suggested that lymphoid tissue inducer cells play a role in the induction of TLOs this issue remains controversial and many studies have identified other cells as initiators of this process (14). A study by Rangel-Moreno raised the interesting possibility that IL-17-producing T cells rather than LT signaling could initiate the induction of lymphoid neogenesis in the lung by triggering the production of homeostatic chemokines by stromal cells (15). LT-independent induction of TLOs was also recently reported in a mouse model of salivary gland inflammation (16). Here, IL-22 could induce accumulation of B cells within lymphoid follicles and production of autoantibodies by promoting the expression of B cell chemoattractants. Similar to IL-17 in Rangel-Moreno’s study, T cells were mostly responsible for the production of IL-22 with lesser contributions by innate lymphoid cells and NK cells (15). Other cell populations such as M1 macrophages or CD11c+CD68+Ly6Clow monocytes may also have the capacity to induce the formation of TLOs (17, 18). Furthermore, CD11c+ dendritic cells may contribute to lymhangiogenesis in TLOs through expression of LT (6). Lymphoid tissue organizer cells are generally thought to be stromal cells. For example, in the context of tertiary lymphoid aggregates that are present in the adventitia of vessels adjacent to atherosclerotic lesions, vascular smooth muscle cells have the capacity to produce homeostatic chemokines such as CCL21 and CXCL13 through LTβR or TNFRI/II signaling (17, 18). In Barone’s study IL-22 induces production of CXCL13 and CXCL12 by fibroblasts and epithelial cells in salivary glands, respectively (16).
Two studies have demonstrated that CD11c+ dendritic cells are critical for the maintenance of bronchus associated lymphoid tissue, a term that defines TLOs in the lung (6, 8). Two-photon microscopy revealed that CD11c+ dendritic cells interact with T cells within these structures pointing to a role in antigen presentation (19). In addition, depletion of CD11c+ dendritic cells from the bronchus associated lymphoid tissue resulted in substantial diminution of local and systemic humoral immune responses (8). Finally, CD11c+ dendritic cells expressed LT-β suggesting that they contribute to continuous production of homeostatic chemokines through LTβR signaling (8). This notion that LTβR signaling plays an important role in maintaining the structural integrity of TLOs was corroborated by disruption of established bronchus associated lymphoid tissue, organized lymphoid aggregates associated with atherosclerotic plaques or cardiac allograft vasculopathy after treatment with LTβR-fusion protein (8, 18, 20).
Lymphoid neogenesis in allografts promoting rejection
Hallmarks of lymphoid neogenesis have been described in many rodent and human grafts that were undergoing acute or chronic rejection (Table 1). While not demonstrating the presence of TLOs, Renkonen’s group reported that endothelial cells in acutely rejecting kidney and heart grafts stained with MECA-79 indicating expression of peripheral nodal addressin, a characteristic feature of high endothelial venules (21, 22). Of note, for hearts the intensity of this staining correlated with the severity of rejection. Thaunat found germinal center-like structures in surgically removed human heart and kidney grafts that had undergone chronic rejection (23). Immunostaining revealed B lymphocytes at the center of these structures that were juxtaposed to follicular dendritic cells. In a subsequent study, this group provided evidence that the local humoral response in the graft differed qualitatively from the systemic humoral response in the transplant recipients (24). Similarly, Huibers found ectopic lymphoid tissue in close proximity to cardiac allograft vasculopathy lesions in hearts from deceased heart transplant recipients (25). Notably, memory B cells and IgM and IgG producing plasma cells were present in these structures raising the possibility that locally produced alloantibody contributed to damage of the coronary endothelium. These human observations have correlates in experimental studies as Baddoura found evidence of lymphoid neogenesis, either in the form of organized lymphoid aggregates or peripheral nodal addressin-expressing high endothelial venules, in 25% of more than 300 examined mouse heart allografts (26). Remarkably, the presence of these structures was virtually always associated with either acute or chronic graft rejection. The demonstration that skin allografts containing TLOs secondary to transgenic expression of LT-α are rejected by hosts that lack SLOs provided further evidence that intra-graft TLOs can propagate alloimmune responses (10). Furthermore, disruption of TLOs through inhibition of the LTβR pathway attenuated humoral immune responses, but did not prevent the development of vasculopathy in bm12 cardiac grafts after transplantation into B6 recipients (20).
Table 1.
Tertiary lymphoid organs associated with allograft rejection or acceptance
| Promoting Rejection | ||||
|---|---|---|---|---|
| Organ | Species | Supporting evidence | TLO characteristics | Reference |
| Heart | Human | Peripheral nodal addressin is expressed in acutely rejected allografts | PNAd | 22 |
| Human | TLOs containing memory B cells and plasma cells are in proximity to allograft vasculopathy lesions | PNAd, memory B cells, T cells, LT-β, CCL19, CCL21 | 25 | |
| Human | TLOs are present in hearts undergoing chronic rejection | T and B cells, Ki67+ cells, FDC | 23 | |
| Mouse | Presence of TLOs composed of T- and B-cell zones correlates with acute or chronic allograft rejection | T and B cells, PNAd+ HEV | 26 | |
| Kidney | Human | TLOs with evidence of local humoral immune response are present in chronically rejected human allografts | PNAd, podoplanin, B cells (GC),T cells, FDC, CCL21, CXCL13, LTβR | 24 |
| Human | TLOs are present in kidneys undergoing chronic rejection | T cells, B cells, Ki67+ cells, FDC | 23 | |
| Human | Peripheral nodal addressin is expressed in allografts undergoing acute rejection | PNAd | 21 | |
| Lung | Human | TLOs are associated with obliterative bronchiolitis lesions | PNAd+ HEV, memory effector T cells | 27 |
| Rat | TLOs serve as sites of local immune response that accelerate onset of lung rejection | Lymphocyte infiltration into preexisting TLOs | 29 | |
| Trachea | Mouse | TLOs form in lungs after intrapulmonary transplantation of tracheal allografts Rejection of intrapulmonary tracheal allografts is independent of recipient secondary lymphoid organs |
T and B cells, Ki67+ cells PNAd+ HEV |
28 |
| Aorta | Rat | TLOs containing germinal center B cells which produce donor-specific alloantibodies and promote rejection | T cells, B cells (GC), HEV | 23 |
| Skin | Mouse | Donor skin graft bearing TLOs cause robust humoral rejection in an allogeneic host | PNAd+ HEV | 10 |
| Skin, Vascularized composite grafts | Human, Nonhuman Primate, Rat | Expression level of peripheral nodal addressin correlates with rejection | T and B cells, PNAd+ HEV | 30 |
| Promoting Acceptance | ||||
| Lung | Human | TLOs are associated with clinically insignificant acute rejection and are not associated with the development of chronic rejection | T (mainly CD4+) and B cells | 31 |
| Human | Rejection is associated with decrease in TLOs | Plasma cells | 32 | |
| Mouse | TLOs rich in Foxp3+ regulatory T cells are present in long term accepted grafts | Foxp3+ T cells, B cells, DCs, PNAd+ HEV, CCL21 | 33 | |
| Kidney | Mouse | TLOs form in allografts that are spontaneously accepted | Foxp3+ T cells, T and B cells, DCs, macrophages, podoplanin, PNAd+ HEV | 34 |
| Mouse | Nodular infiltrates rich in Foxp3+ regulatory T cells are present in spontaneously accepting allografts | Foxp3+ T cells, T (mainly CD4+) and B cells, DC | 35 | |
(TLO: tertiary lymphoid organ; PNAd: peripheral nodal addressin; HEV: high endothelial venules; FDC: follicular dendritic cells; DC: dendritic cells; GC: germinal center)
Sato and colleagues detected peripheral nodal addressin-expressing high endothelial venules in the small airways of human lungs with an established diagnosis of obliterative bronchiolitis, a manifestation of chronic pulmonary graft rejection (27). Furthermore, the authors demonstrated the development of bronchus associated lymphoid tissue in lungs with accumulation of effector T cells in a rat model of intrapulmonary transplantation of tracheal allografts. Based on these human and rodent observations the authors concluded that the induction of TLOs in lung allografts contributes to chronic deleterious local immune responses. In a subsequent study, the same group showed that T and B cells were undergoing proliferation within these TLOs that formed after intrapulmonary tracheal transplantation in mice (28). Interestingly, intrapulmonary grafts were rejected by recipient mice that lacked SLOs suggesting that lymphocyte priming within TLOs was sufficient to propagate alloimmune responses (28). It is important to note that unlike the case for mice and humans where bronchus associated lymphoid tissue is not expressed at baseline but can be induced in the setting of inflammation, other species including rats express these structures in their lungs constitutively. To this end, seminal studies performed by Prop and colleagues in an orthotopic rat lung transplant model in the 1980’s suggested that a local immune response was induced through infiltration of recipient T cells into the bronchus associated lymphoid tissue of the allograft (29). This mechanism was thought to contribute to the comparatively rapid onset of lung rejection.
In an aortic transplantation model in rats, Thaunat found that endothelial cells in the adventitia of allografts acquired a phenotype consistent with high endothelial venules (23). Furthermore, proliferating B cells formed follicles in the adventitia of these grafts, which produced antibodies against donor MHC class I molecules. In addition, peripheral nodal addressin expression on vascular endothelial cells and lymphoid neogenesis have been recently correlated with graft rejection after vascularized composite transplants in both humans and experimental small and large animals (30). Collectively, these studies have given rise to the notion that de novo formation of TLOs within transplanted organs propagate detrimental local immune responses that contribute to rejection. Therefore, it has been suggested that targeting these structures could represent a novel avenue to prevent chronic graft failure.
Lymphoid neogenesis in allografts promoting acceptance
There exist several reports in the literature that induction of TLOs in allografts is not necessarily associated with harmful immune responses and may even be beneficial. For example, Hasegawa reported that the presence of bronchus associated lymphoid tissue in transbronchial biopsies of lung transplant recipients was associated with clinically insignificant acute cellular rejection and was not associated with the development of chronic rejection (31). Based on these observations the authors suggested that lymphoid neogenesis may be a manifestation of immune tolerance and should not trigger augmentation of immunosuppression. Similarly, Hruban demonstrated in a small number of human heart-lung transplant recipients that pulmonary graft rejection was associated with a decrease in bronchus associated lymphoid tissue (32). As this resulted in a depletion of IgA- and IgG-bearing plasma cells in the graft the authors speculated that a deficiency in TLOs within lung allografts associated with rejection may predispose these patients to pulmonary infections (32). Our laboratory has recently demonstrated that immunosuppression-mediated long term acceptance of mouse lung allografts was associated with de novo induction of bronchus associated lymphoid tissue (Figure 2)(33). Intravital two-photon microscopy demonstrated that these structures contained abundant CD11c+ cells that were highly motile and displayed probing behavior. Recipient T lymphocytes interacted with these CD11c+ cells providing evidence that they continuously encounter donor antigen within these lymphoid aggregates in the lung allograft. Perhaps more importantly, the bronchus associated lymphoid tissue within these accepted lung allografts was very rich in Foxp3+ regulatory T cells that were in close proximity to CD11c+ antigen presenting cells (33). Similar observations were made in kidney transplants, where Brown found that TLOs formed in renal grafts that were spontaneously accepted by allogeneic hosts (34). The authors provided evidence suggesting that these TLOs participated in both direct and indirect allorecognition. Importantly, the size of the TLOs was associated with improved graft function. Colvin’s group demonstrated nodular infiltrates surrounding cortical arteries in spontaneously accepting mouse kidney allografts that were rich in Foxp3+ regulatory T cells (35). The authors termed these structures “Treg-rich organized lymphoid structures.” Remarkably, depletion of Foxp3+ cells results in dissolution of these structures and acute rejection of the kidney grafts that was associated with diffuse T cell infiltration into the cortex of the kidney. The concept that tertiary lymphoid aggregates could contribute to local immunosuppression was recently corroborated by observations in human cancer patients and studies in murine tumor models. Similar to the case for transplantation, most previous reports suggested that TLOs in tumor-bearing organs promoted anti-tumor responses and that their presence correlated with improved survival (36, 37). However, Joshi demonstrated that activated regulatory Foxp3+ T cells accumulate in TLOs that are associated with adenocarcinomas in murine lungs (38). Importantly, local depletion of these cells resulted in an increase in the expression levels of costimulatory molecules on dendritic cells in the TLOs and triggered potent anti-tumor T cell responses (38). Similarly, while intratumoral intraepithelial CD8+ T cells are associated with an improved prognosis in patients suffering from ovarian cancer, infiltration of Foxp3+ regulatory T cells into these tumors resulting in low ratios of CD8+/regulatory CD4+ T cell adversely impacts survival (39, 40). Expression of CCL21 by melanoma tumors leads to the development of lymph node-like stromal areas, expression of peripheral nodal addressin and the recruitment of regulatory cell populations such as Foxp3+ T lymphocytes and myeloid-derived suppressor cells (41). These changes result in a “tolerogenic” environment that promotes accelerated tumor growth. A recent study also showed that naïve T cells undergo conversion into Foxp3+ regulatory T cells in TLOs associated with atherosclerotic lesions in murine aortas (42).
Figure 2. Tertiary lymphoid organ in accepted mouse lung allograft.
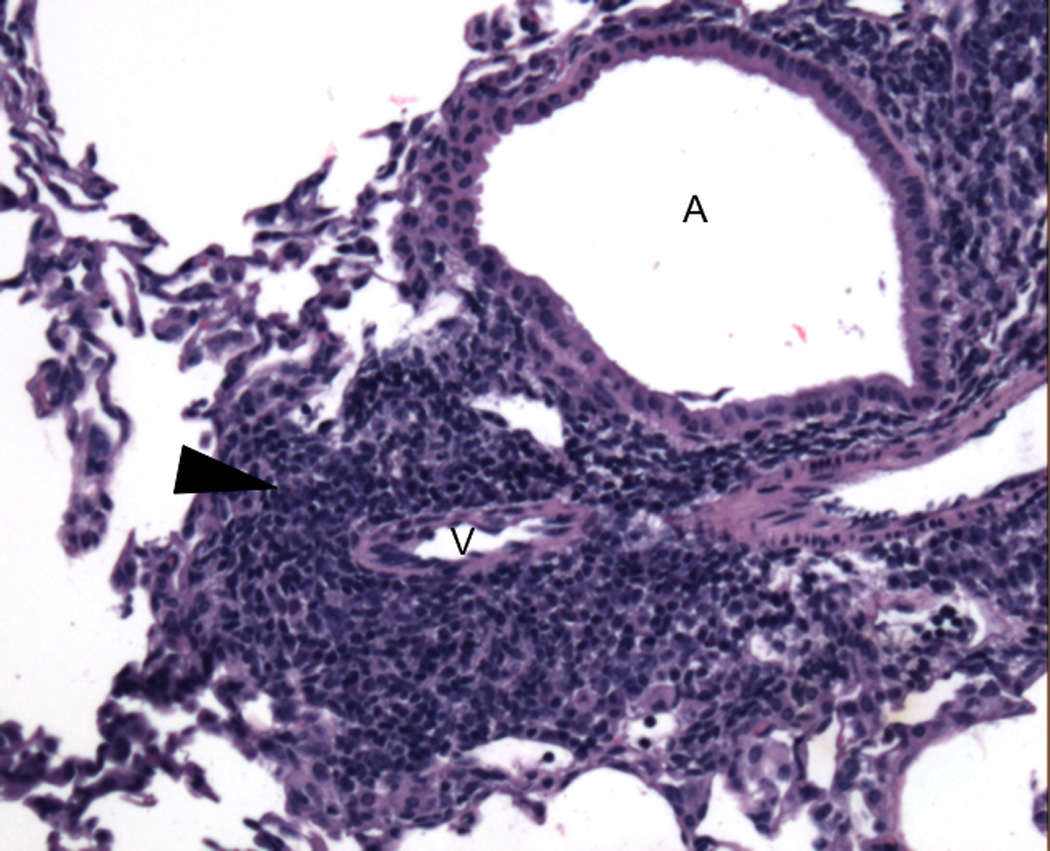
Representative H&E photomicrograph of bronchus associated lymphoid tissue (arrowhead) in an accepted BALB/c lung allograft 30 days after transplantation into an immunosuppressed B6 recipient (MR1 250 µg intraperitoneally day 0, CTLA4-Ig 200 µg intraperitoneally day 2). A: airway; V: vessel. Picture was taken on an Olympus BX51 imaging system under the 10× objective lens (100× total magnification) with an AmScope MT1400-CCD camera.
Genetic elimination of LTβR signaling in vascular smooth muscle cells, which these authors have previously shown to be important for TLO generation and maintenance in this model of inflammation, resulted in disruption of the organized lymphoid structures and, interestingly, in exacerbation of atherosclerosis in aged mice (42). These findings further support the notion that TLOs can protect from disease progression in certain settings of chronic inflammation, possibly through propagating local immune regulatory circuits.
Concluding Remarks
As chronic inflammatory responses at nonlymphoid sites that are associated with persistence of the foreign antigen appear to be a prerequisite for the de novo development of organized lymphoid aggregates it is not surprising that these structures are commonly observed in tissue and organ allografts. In addition, unique features related to the disruption of lymphatic channels, which is a natural consequence of the surgical process of transplantation, may favor their induction at these sites (7). TLOs within allografts have not been regarded simply as an epiphenomenon of chronic low-grade inflammation. It has rather been the prevailing paradigm that TLOs situated within allografts are the site of immune responses that are detrimental to the transplanted tissue or organ (20–30). This view was supported by numerous studies in other inflammatory settings where immune responses generated in such structures could clear infections, reject tumors or contribute to autoimmune destruction of target organs (1). However, few recent studies in transplantation and other areas have raised the intriguing possibility that ectopic lymphoid tissue could be a critical site of immune regulation. At least for lungs, which we have shown provide a suitable environment for interaction between innate and adaptive immune cells and priming of T cells, this challenges the notion that downregulation of alloimmune responses depends on cellular trafficking to draining SLOs (43). Undoubtedly, more mechanistic studies are required to elucidate how TLOs in various grafts differ qualitatively and how they regulate alloimmune responses. However, it appears certain that it may not be appropriate to universally target them therapeutically in an attempt to improve graft survival. It remains to be determined whether specific populations of lymphoid tissue inducer or organizer cells favor the induction of TLOs that promote the expansion and survival of regulatory populations. Once we gain a better understanding of the genesis and maintenance of “tolerogenic” TLOs therapeutic strategies could be developed to induce and stabilize these structures in allografts.
Acknowledgments
We thank Eric T. Olson for assistance with graphic design.
DK is supported by National Institutes of Health grants 1P01AI116501, R01 HL113931, and R01 HL094601.
Abbreviations
- LT
lymphotoxin
- SLO
secondary lymphoid organ
- TLO
tertiary lymphoid organ
- TNF
tumor necrosis factor
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 2.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 3.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25(4):643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Pei G, Zeng R, Han M, Liao P, Zhou X, Li Y, et al. Renal interstitial infiltration and tertiary lymphoid organ neogenesis in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9(2):255–264. doi: 10.2215/CJN.01150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Th1-biased tertiary lymphoid tissue supported by CXC chemokine ligand 13-producing stromal network in chronic lesions of autoimmune gastritis. J Immunol. 2003;171(8):4359–4368. doi: 10.4049/jimmunol.171.8.4359. [DOI] [PubMed] [Google Scholar]
- 6.Muniz LR, Pacer ME, Lira SA, Furtado GC. A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J Immunol. 2011;187(2):828–834. doi: 10.4049/jimmunol.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaunat O, Kerjaschki D, Nicoletti A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006;27(10):441–445. doi: 10.1016/j.it.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206(11):2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MY, Kim KS, McConnell F, Lane P. Lymphoid tissue inducer cells: architects of CD4 immune responses in mice and men. Clin Exp Immunol. 2009;157(1):20–26. doi: 10.1111/j.1365-2249.2009.03932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr IW, Reel M, Oberbarnscheidt MH, Mounzer RH, Baddoura FK, Ruddle NH, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. 2007;7(5):1071–1079. doi: 10.1111/j.1600-6143.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen SC, Vassileva G, Kinsley D, Holzmann S, Manfra D, Wiekowski MT, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168(3):1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 12.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A. 2007;104(25):10577–10582. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocks JR, Davalos-Misslitz AC, Hintzen G, Ohl L, Forster R. Regulatory T cells interfere with the development of bronchus-associated lymphoid tissue. J Exp Med. 2007;204(4):723–734. doi: 10.1084/jem.20061424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004;21(5):655–667. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner KM, et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1503315112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guedj K, Khallou-Laschet J, Clement M, Morvan M, Gaston AT, Fornasa G, et al. M1 macrophages act as LTbetaR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis. Cardiovasc Res. 2014;101(3):434–443. doi: 10.1093/cvr/cvt263. [DOI] [PubMed] [Google Scholar]
- 18.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206(1):233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206(12):2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motallebzadeh R, Rehakova S, Conlon TM, Win TS, Callaghan CJ, Goddard M, et al. Blocking lymphotoxin signaling abrogates the development of ectopic lymphoid tissue within cardiac allografts and inhibits effector antibody responses. FASEB J. 2012;26(1):51–62. doi: 10.1096/fj.11-186973. [DOI] [PubMed] [Google Scholar]
- 21.Kirveskari J, Paavonen T, Hayry P, Renkonen R. De novo induction of endothelial L-selectin ligands during kidney allograft rejection. J Am Soc Nephrol. 2000;11(12):2358–2365. doi: 10.1681/ASN.V11122358. [DOI] [PubMed] [Google Scholar]
- 22.Toppila S, Paavonen T, Nieminen MS, Hayry P, Renkonen R. Endothelial L-selectin ligands are likely to recruit lymphocytes into rejecting human heart transplants. Am J Pathol. 1999;155(4):1303–1310. doi: 10.1016/S0002-9440(10)65232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102(41):14723–14728. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thaunat O, Patey N, Caligiuri G, Gautreau C, Mamani-Matsuda M, Mekki Y, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185(1):717–728. doi: 10.4049/jimmunol.0903589. [DOI] [PubMed] [Google Scholar]
- 25.Huibers MM, Gareau AJ, Vink A, Kruit R, Feringa H, Beerthuijzen JM, et al. The composition of ectopic lymphoid structures suggests involvement of a local immune response in cardiac allograft vasculopathy. J Heart Lung Transplant. 2015;34(5):734–745. doi: 10.1016/j.healun.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5(3):510–516. doi: 10.1111/j.1600-6143.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 27.Sato M, Hirayama S, Hwang DM, Lara-Guerra H, Wagnetz D, Waddell TK, et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol. 2009;182(11):7307–7316. doi: 10.4049/jimmunol.0803606. [DOI] [PubMed] [Google Scholar]
- 28.Wagnetz D, Sato M, Hirayama S, Matsuda Y, Juvet SC, Yeung JC, et al. Rejection of tracheal allograft by intrapulmonary lymphoid neogenesis in the absence of secondary lymphoid organs. Transplantation. 2012;93(12):1212–1220. doi: 10.1097/TP.0b013e318250fbf5. [DOI] [PubMed] [Google Scholar]
- 29.Prop J, Wildevuur CR, Nieuwenhuis P. Lung allograft rejection in the rat. II. Specific immunological properties of lung grafts. Transplantation. 1985;40(2):126–131. doi: 10.1097/00007890-198508000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Hautz T, Zelger BG, Nasr IW, Mundinger GS, Barth RN, Rodriguez ED, et al. Lymphoid neogenesis in skin of human hand, nonhuman primate, and rat vascularized composite allografts. Transpl Int. 2014;27(9):966–976. doi: 10.1111/tri.12358. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa T, Iacono A, Yousem SA. The significance of bronchus-associated lymphoid tissue in human lung transplantation: is there an association with acute and chronic rejection? Transplantation. 1999;67(3):381–385. doi: 10.1097/00007890-199902150-00007. [DOI] [PubMed] [Google Scholar]
- 32.Hruban RH, Beschorner WE, Baumgartner WA, Achuff SC, Traill TA, Digennaro KA, et al. Depletion of bronchus-associated lymphoid tissue associated with lung allograft rejection. Am J Pathol. 1988;132(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, Bribriesco AC, Nava RG, Brescia AA, Ibricevic A, Spahn JH, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5(5):544–554. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown K, Sacks SH, Wong W. Tertiary lymphoid organs in renal allografts can be associated with donor-specific tolerance rather than rejection. Eur J Immunol. 2011;41(1):89–96. doi: 10.1002/eji.201040759. [DOI] [PubMed] [Google Scholar]
- 35.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178(4):1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 37.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 38.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity. 2015;43(3):579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 40.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328(5979):749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 42.Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, et al. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin beta Receptors. Immunity. 2015;42(6):1100–1115. doi: 10.1016/j.immuni.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]