Abstract
Understanding how exercise affects communication across the brain in overweight/obese individuals may provide insight into mechanisms of weight loss and maintenance. In the current study, we examined the effects of a 6-month exercise program in 11 overweight/obese individuals (mean BMI: 33.6±1.4 mg/kg2; mean age: 38.2±3.2 years) on integrative brain “hubs,” which are areas with high levels of connectivity to multiple large-scale networks thought to play an important role in multimodal integration among brain regions. These integrative hubs were identified with a recently developed between-network connectivity (BNC) metric, using functional magnetic resonance imaging (fMRI). BNC utilizes a multiple regression analysis approach to assess relationships between the time series of large-scale functionally-connected brain networks (identified using independent components analysis) and the time series of each individual voxel in the brain. This approach identifies brain regions with high between-network interaction, i.e., areas with high levels of connectivity to many large-scale networks. Changes in BNC following exercise were determined using paired t-tests, with results considered significant at a whole-brain level if they exceeded a voxel-wise threshold of p < 0.01 and cluster-level family-wise error (FWE) correction for multiple comparisons of p < 0.05. Following the intervention, BNC in the posterior cingulate cortex (PCC) was significantly reduced (p < 0.001). The changes driving the observed effects were explored using Granger causality, finding significant reductions in both outgoing causal flow from the PCC to a number of networks (p < 0.05; language network, visual network, sensorimotor network, left executive control network, basal ganglia network, posterior default mode network), in addition to reductions in ingoing causal flow to the PCC from a number of networks (p < 0.05; ventral default mode network, language network, sensorimotor network, basal ganglia network). Change in BNC was related to changes in aerobic fitness level (VO2 max; p = 0.008) and perceived hunger (Three Factor Eating Questionnaire; p = 0.040). Overall, the impact of exercise on communication between large-scale networks may contribute to individual responsivity to exercise.
Keywords: exercise, obesity, posterior cingulate cortex, default mode network, between-network connectivity, fMRI
1. Introduction
Exercise confers numerous health benefits, including reduced risk of cardiovascular disease, type 2 diabetes, and cancer [1, 2]. The effects of exercise on weight loss and obesity prevention are, however, variable [3-7]. A potentially key factor involved in successful weight loss and maintenance with exercise is the effect of exercise on the brain. Exercise has been associated with alterations in brain structure [8-10] and neuronal responses to food cues in brain regions important in food reward [11-13]. Furthermore, a strong link has been established between physical fitness and/or exercise and improved cognitive performance [9, 14-16]. These effects may also relate to weight loss and maintenance, as numerous studies have suggested links between obesity and reduced cognitive control [17-20]. However, the mechanisms through which exercise exerts beneficial neuronal effects are unclear.
Previous studies have investigated exercise effects in discrete brain regions or networks. For example, following chronic exercise interventions, a small number of studies have observed alterations in functional connectivity within large-scale brain networks, such as the default mode network [21, 22], cognitive control networks [21, 22], and motor networks [22]. Specifically, in normal weight older adults, exercise has been associated with increased functional connectivity within the default mode and cognitive networks [21]. In overweight children, exercise has been associated with decreased synchrony within default, cognitive control, and motor networks, suggesting increased network efficiency [22]. As our ability to study brain function has improved, it has become apparent that a more complete understanding of the neurobiology of exercise requires consideration of neuronal activity not only within these large-scale brain networks, but also the transfer of information between them. Much of this information transfer occurs at integrative brain “hubs,” which are areas with high levels of connectivity to multiple large-scale networks that are thought to play an important role in multimodal sensory and cognitive integration [23-25]. Identifying how exercise influences communication between large-scale networks at these hubs may be a key part of understanding the neurobiology of exercise effects. Based on previous findings of network-specific alterations in functional connectivity following exercise, the current study tested the hypothesis that chronic exercise would result in overall altered information flow between large-scale functionally-connected networks at key integrative hubs in the brain.
To examine the effects of a 6-month exercise program on integrative brain hubs, the current study used a novel between-network connectivity (BNC) metric [26]. This data-driven approach allowed an examination of the degree to which key integrative hubs simultaneously interact with all identified large-scale networks, rather than simply focusing on relationships between specific networks and a priori regions of interest. This technique, which measures the amount of integration between large-scale networks at particular voxels, has the advantage of capitalizing upon the full spatial resolution provided by functional magnetic resonance imaging (fMRI), allowing examination of connectivity within cortical subregions not included in current anatomical atlases. Following identification of these key integrative areas, the effect of exercise on the areas was examined. The specificity and directionality of hub information flow to and from specific networks driving connectivity effects was then evaluated. Finally, the relationship between neuronal effects and behavioral measures of exercise effects was examined.
2. Methods
2.1. Participants
A subgroup of eleven individuals participating in a larger study evaluating exercise effects on total daily energy expenditure were recruited for the current study. One participant only completed fMRI measures at baseline, so complete data for ten overweight/obese adults were analyzed (five women, five men; mean body mass index (BMI) 33.6±1.4 mg/kg2; mean age 38.2±3.2 years). Participants were free of metabolic and psychiatric disease and eating disorders and were not actively dieting. Participants provided written informed consent and all procedures were in accordance with and approved by the Colorado Multiple Institutional Review Board.
2.2. Experimental design
Participants were recruited from the larger study, as has been described previously [11, 27]. Participants performed a 6-month supervised treadmill-walking program that gradually increased in intensity (60% to 75%) and duration (~15-20 min/day to 40-60 min/day) to achieve a target workload (500 kcal/day at 75% of VO2 max) by week 18. Individualized exercise prescriptions targeted 2500 kcal per week. Prescriptions were calculated from a maximal aerobic capacity test at baseline and updated according to submaximal tests every 6 weeks. Participants were required to attend more than 75% of the scheduled exercise sessions. Behavioral, hormonal and body composition measures were completed before and after the intervention, following an overnight fast and no exercise for 24 h, on a separate day from fMRI measures.
2.3. Behavioral, hormonal and body composition measures
Body composition was assessed by dual-energy X-ray absorptiometry (DPX whole-body scanner, Lunar Radiation Corp.). Resting metabolic rate (RMR) was measured by standard hood indirect calorimetry (TrueOne 2400 metabolic cart, Parvometrics). Fasting blood sampling was performed and analyzed for leptin concentration as determined by radioimmunoassay (Linco Research, Inc.). Participants completed the Three Factor Eating Inventory (TFEQ [28]), Power of Food Scale (PFS [29]), Craving and Mood Questionnaire (CMQ [30]), and Food Craving Inventory (FCI [31]). Hunger, satiety, and prospective food consumption ratings were assessed by visual analog scale (VAS) before and every 30 min for 180 min following a test meal breakfast. The test meal was served at 7:30 AM and provided 30% of daily energy intake (50% carbohydrate, 35% fat, 15% protein), estimated using baseline RMR and lean body mass plus an activity factor of 1.4. The entire meal was required to be consumed and was prepared by the University of Colorado Clinical Translational Research Center kitchen.
Maximal aerobic capacity (VO2 max) was measured on a motor-driven treadmill, on which participants walked to volitional exhaustion using the Balke treadmill protocol [32], performed at a constant speed but increasing grade. Expired gasses were collected and analyzed throughout the test using standard indirect calorimetry. Heart rate and rhythm were monitored continually using a 12-lead electrocardiogram. The test was considered successful if oxygen consumption plateaued or the participant met three of the following criteria, as per American College of Sports Medicine (ACSM) guidelines [32]: (1) maximum heart rate within 20 beats/min of the age-predicted maximal heart rate (220-age); (2) perceived exertion rating greater than 17; (3) respiratory exchange ratio greater than 1.15, and (4) volitional exhaustion. Maximal oxygen consumption was determined as the highest observed value during the test.
2.4. fMRI data acquisition
Within a week of behavioral, hormonal, and body composition measures, participants completed fMRI the morning after an overnight fast (approximately 8:00 AM; asked to not consume any food after 10:00 PM the night before). Fasting VAS appetite measures were performed prior to scanning. fMRI was performed using a GE 3.0T MR scanner. A high-resolution, T1-weighted 3D anatomical scan was acquired for each participant, after which functional images were acquired with an echo-planar gradient-echo T2* blood oxygenation level dependent (BOLD) imaging contrast technique, with TR = 2000 ms, TE = 30 ms, 642 matrix, 240 mm2 FOV, 27 axial slices angled parallel to the planum sphenoidale, 2.6 mm thick, 1.4 mm gap. An inversion-recovery echo-planar image (IR-EPI; TI = 505 ms) volume was acquired to improve coregistration between the echo-planar images and gray matter templates used in preprocessing. Participants completed fMRI during 10 minutes of rest with eyes open.
2.5. Data analysis
2.5.1. fMRI preprocessing
fMRI data were preprocessed and analyzed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London, UK). Functional data were realigned to the first echo-planar image, normalized to the Montreal Neurological Institute (MNI) EPI template, using the gray-matter-segmented IR-EPI as an intermediate to improve registration, and smoothed with an 8 mm full width at half maximum (FWHM) Gaussian kernel.
2.5.2. Between-network connectivity (BNC)
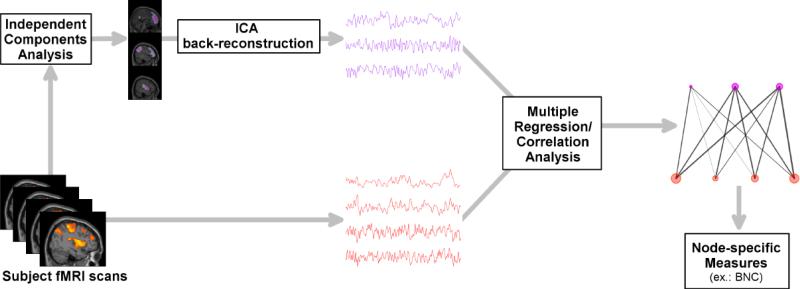
The method used to assess between-network connectivity (BNC) has been explained in detail previously [26]. Briefly, this technique measures the amount and diversity of connectivity between all large-scale networks occurring at a single voxel. BNC is calculated using simple and multiple correlation coefficients, along with each network's time series estimated for each subject (Figure 1). A voxel with high BNC indicates that the voxel is strongly connected (i.e., correlated) to many networks, suggesting a high degree of information flow between the networks at that voxel. In contrast, low BNC could indicate either that a voxel's connections to many intercommunicating resting-state networks are negligible, or that the voxel's connections are to networks that are not communicating with one another. Either case would result in a low BNC, indicating that this voxel would not be considered an integration hub.
Figure 1.
Between-network connectivity (BNC) analysis pathway. fMRI: functional magnetic resonance imaging; ICA: independent components analysis; BNC: between-network connectivity.
Large-scale networks were identified using group independent components analysis (ICA) within the GIFT toolbox (http://icatb.sourceforge.net). Data for each session (pre- and post-exercise intervention) were processed jointly to identify networks present in both sessions. The dimensionality of the data from each subject was reduced to 70 components using principle component analysis and concatenated into an aggregate dataset for input into ICA. Fifty independent components were estimated using information-theoretic criteria [33], extracted using the infomax algorithm [34]. Individual subject components and time series were back-reconstructed using GICA3 [35]. Fourteen artifactual components corresponding to white matter, cerebral spinal fluid or head movement were identified based on spatial distributions and frequency characteristics, and excluded from further analysis.
Voxel time series were processed to remove sources of noise and minimize the influence of movement. Time series were detrended and band-pass filtered between 0.1 and 0.01 Hz. Signals for white matter, cerebral spinal fluid, and six movement parameters were regressed out. Additional movement control was provided by removing volumes with excessive framewise displacement [36].
BNC was calculated using the resulting non-artifactual ICA time series and denoised voxel time series. For each voxel, BNC was measured by subtracting the total amount of variance explained by the ICA component time series (the multiple correlation coefficient) from the sum of individual squared correlations with the ICA time series. All resulting raw BNC values were standardized to z-scores to facilitate comparison between subjects, by subtracting the whole-brain mean for each subject and dividing by the whole-brain standard deviation. The distribution of BNC throughout the entire brain was examined using a one-sample t-test in SPM8. Pre- and post-intervention conditions were compared using a paired t-test. Results were considered significant at a whole-brain level if they exceeded a voxel-wise threshold of p < 0.01 and cluster-level family-wise error (FWE) correction for multiple comparisons of p < 0.05.
2.5.3. Granger causality
Effective connectivity of local peaks of high BNC was analyzed using time-delayed Granger causality (GC) implemented in the Granger Causality Connectivity Analysis toolbox [37]. ICA time series were centered to a mean of zero, linear trends were removed, and time series were concatenated across subjects within each group. After concatenation, model order was determined using Bayesian information criteria. Conditions were compared with a random permutation test, using the assumption of exchangeability of conditions under the null hypothesis. Condition labels were randomized for all subjects, the resulting time series were concatenated, and GC was calculated as above, for 1000 permutations in total. Significance level was set at alpha < 0.05.
2.5.4. Behavioral, hormonal, and body composition measures
Analyses for behavioral, hormonal, and body composition measures were performed with SPSS 22.0 (IBM Corp.). Total area under the curve for appetite VAS ratings using all post-meal time points was used. Effects of exercise as compared to baseline were compared with a paired t-test (alpha of 0.05). For correlations between fMRI and behavioral data, regression analyses were performed in SPSS using standardized BNC z-scores reflecting network activity extracted from SPM at the local maxima within the significant cluster. To explore relationships between individual ICA network connectivity at BNC hubs and behavioral data, voxel-to-network connectivity between the peak voxel of each integrative hub of interest and each ICA network was first calculated as a Pearson's correlation between these time series. Connectivity at this voxel-to-network level was then correlated with behavioral data.
3. Results
3.1. Behavioral, hormonal, and body composition measures
Percent body fat was significantly reduced in this group of participants following the exercise intervention (mean±SEM: 36.4±2.3 to 34.5±2.3%, t = 2.50, p = 0.034), with a trend towards a reduction in fat mass (35.6±3.1 to 33.1±3.6 kg, t = 1.85, p = 0.098) and no significant reduction in body weight (98.4±5.6 to 95.9±6.4 kg, p > 0.05). There were significant reductions in fasting blood leptin concentration following the intervention (33.0±6.9 to 21.7±4.9 ng/ml, t = 2.72, p = 0.026). Additionally, a significant increase in VO2 max was observed after the exercise intervention (27.02±1.24 to 32.03±1.61 ml/kg/min, t = 3.87, p = 0.004). No changes were observed in behavioral measures (Table 1).
Table 1.
Eating behaviors and appetite measures, at baseline and post-exercise intervention.
| Baseline | Post-Exercise | |||
|---|---|---|---|---|
| Measure | Mean | SEM | Mean | SEM |
| TFEQ: Restraint | 9.1 | 1.2 | 9.0 | 1.4 |
| TFEQ: Disinhibition | 8.8 | 1.3 | 8.0 | 1.1 |
| TFEQ: Hunger | 5.9 | 1.0 | 4.9 | 1.0 |
| CMQ | 37.6 | 8.1 | 34.1 | 7.0 |
| FCI | 40.8 | 4.4 | 35.4 | 4.7 |
| PFS | 51.6 | 7.5 | 41.7 | 6.4 |
| Hunger AUC | 133.9 | 16.4 | 187.4 | 44.1 |
| Satiety AUC | 441.0 | 44.1 | 399.4 | 53.8 |
| PFC AUC | 192.4 | 24.5 | 236.4 | 40.7 |
TFEQ: Three Factor Eating Questionnaire; CMQ: Craving and Mood Questionnaire; FCI: Food Craving Inventory; PFS: Power of Food Scale; AUC: area under the curve in response to a meal; PFC: prospective food consumption.
3.2. fMRI
3.2.1. Between-network connectivity (BNC)
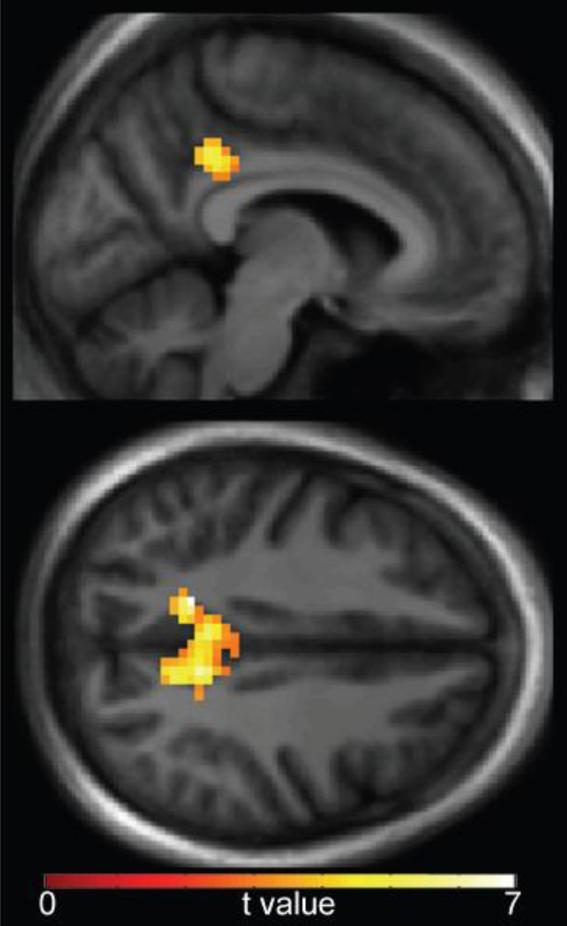
At baseline, high BNC was observed in a number of regions, including the posterior cingulate cortex, insula, precentral gyrus, postcentral gyrus, cuneus, superior temporal gyrus, and lingual gyrus (Table 2). These findings are consistent with prior observations [26]. Following the 6-month exercise intervention, BNC in the posterior cingulate cortex (PCC; x = −3, y = −43, z = 40) was significantly reduced, t = 6.34, p < 0.001 (Figure 2).
Table 2.
Between-network connectivity (BNC) peaks at baseline.
| Local maxima coordinatesa | ||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | t valueb | cluster size | p valuec |
| Precentral gyrus (R) | 42 | −19 | 43 | 8.08 | 4168 | <0.001 |
| Postcentral gyrus (R) | 54 | −16 | 49 | 6.90 | ||
| Superior frontal gyrus (L) | −24 | −7 | 58 | 6.35 | ||
| Posterior cingulate cortex | 0 | −28 | 31 | 5.35 | ||
| Anterior insula (R) | 36 | 17 | 4 | 9.18 | 315 | <0.001 |
| Posterior insula (R) | 33 | −13 | 10 | 3.44 | ||
| Anterior insula (L) | −36 | 8 | 4 | 5.80 | 209 | 0.004 |
| Insula (L) | −36 | −1 | 7 | 5.03 | ||
| Insula (L) | −36 | −7 | −8 | 3.16 | ||
| Cuneus (L) | −12 | −79 | 31 | 4.89 | 1435 | <0.001 |
| Cuneus (R) | 9 | −82 | 28 | 4.74 | ||
| Lingual gyrus (L) | −15 | −70 | −11 | 4.62 | ||
| Parietal operculum (R) | 48 | −19 | 16 | 5.21 | 190 | 0.006 |
| Superior temporal gyrus (R) | 66 | −22 | 7 | 3.98 | ||
| Superior temporal gyrus (R) | 63 | −34 | 16 | 3.77 | ||
Stereotactic coordinates in MNI space.
t values reported for local maxima within clusters.
p values for significant clusters, corrected for multiple comparisons across the entire brain volume.
Figure 2.
Reduction in posterior cingulate cortex (PCC) between-network connectivity (BNC) post-intervention, compared to baseline. Data are thresholded at a voxel-wise threshold of p < 0.01 and a cluster-extent threshold of p < 0.05 (family-wise error-corrected for multiple comparisons), and overlaid onto a group-average anatomical image for visualization.
3.2.2. Granger causality
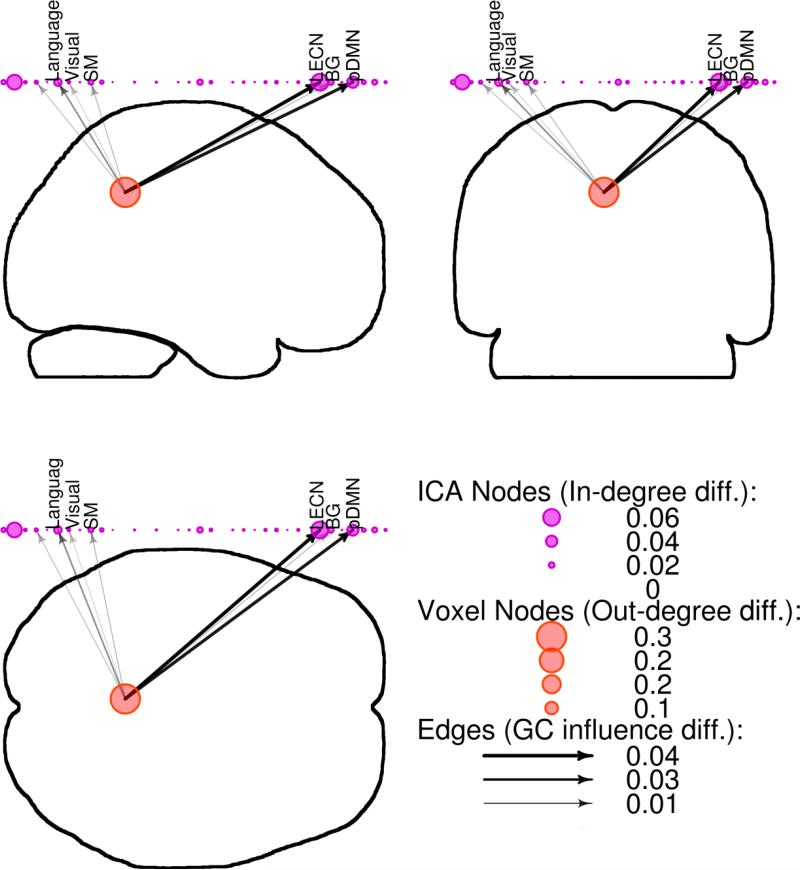
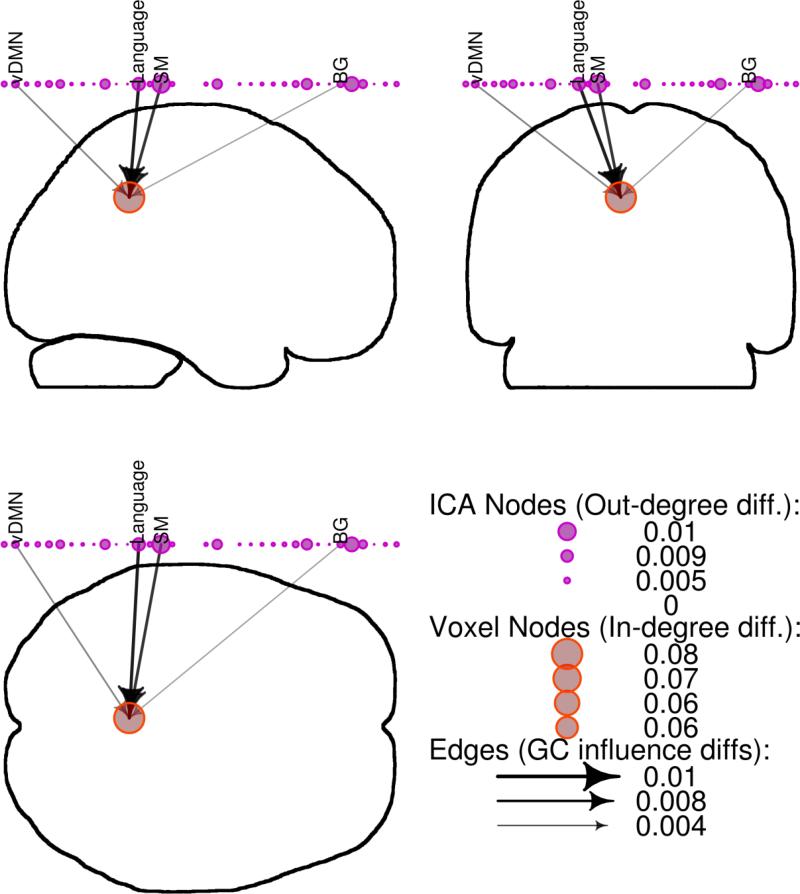
Significant changes in outgoing causal flow from the PCC following exercise were identified (change in weighted out-degree = 0.22, significant at p < 0.05 by permutation testing; Figure 3). This was driven by reduced connection strength in a number of networks, including a language network, visual network, sensorimotor network, left executive control network, basal ganglia network, and posterior default mode network (DMN). Ingoing causal flow to PCC was also altered post-intervention (change in weighted in-degree = 0.07, significant at p < 0.05 by permutation testing; Figure 4), driven by reductions in causal influence from a ventral default mode network, language network, sensorimotor network, and basal ganglia network.
Figure 3.
Reductions in outgoing causal flow at the posterior cingulate cortex (PCC) following exercise. Granger causality (GC) results were displayed on whole-brain silhouettes (“glass brain”) from three viewpoints, with PCC voxels represented by red circles, independent components analysis (ICA) networks represented by purple circles above the brain with descriptive labels, and GC strength and direction represented by black arrows. Language: language network; Visual: visual network; SM: sensorimotor network; LECN: left executive control network; BG: basal ganglia network; pDMN: posterior default mode network.
Figure 4.
Reductions in ingoing causal flow at the posterior cingulate cortex (PCC) following exercise. Granger causality (GC) results were displayed on whole-brain silhouettes with PCC voxels represented by red circles, independent components analysis (ICA) networks represented by purple circles above the brain with descriptive labels, and GC strength and direction represented by black arrows. vDMN: ventral default mode network; Language: language network; SM: sensorimotor network; BG: basal ganglia network.
3.2.3. Correlations between fMRI and behavioral measures
Baseline BNC was not significantly correlated with any baseline body composition, hormonal, or behavioral measures, p > 0.05. Post-intervention change in VO2 max was significantly correlated with post-intervention change in PCC BNC, r = 0.67, p = 0.035. Further exploration using voxel-to-network connectivity suggests that connectivity between PCC and a ventral DMN network contributed to this effect, as changes in PCC/ventral DMN connectivity were significantly correlated with VO2 max change, r = 0.78, p = 0.008. As the PCC is a main component of the posterior DMN, this could also be interpreted as changes between ventral and posterior components of the DMN. Post-intervention change in hunger, as measured by the TFEQ, was also significantly correlated with post-intervention change in BNC, r = −0.78, p = 0.041. Change in connectivity between PCC and a visuospatial network was associated with the change in hunger score, r = 0.91, p = 0.005.
4. Discussion
The current study utilized a novel between-network connectivity (BNC) method [26] to determine effects of an exercise intervention on whole-brain integrative information flow in overweight/obese adults. This approach identified exercise-related changes in communication between large-scale neuronal networks and integrative “hub” regions. We have previously reported reductions in activity within the default mode network following exercise in this group [27]. However, the effects of exercise on whole-brain BNC have not previously been investigated. Thus, the current study expanded upon our previous work [11, 27] to examine exercise effects on large-scale network communication with individual voxels across the brain. Following the 6-month exercise intervention, reduced BNC was observed for the posterior cingulate cortex (PCC), a region identified as having high BNC at baseline. High BNC indicates that a region is functionally connected to numerous large-scale networks, and is thus likely important in information integration [26]. Reduced BNC in the PCC following exercise suggests that the intervention impacted information flow at a whole-brain level and between multiple large-scale networks.
The PCC is a prominent integrative hub and a key region of the default mode network (DMN) [38-44]. The DMN is a network of brain regions thought to reflect a baseline state of brain function most active when people are focused on internal mental states, such as in self-referential thinking and autobiographical memory, and during external unfocused attention (i.e., monitoring the environment) [39-41, 43-45]. Previous studies by our group and others have found that PCC DMN activity is increased in obese and overweight individuals [46, 47], and that activity in the region is reduced following chronic exercise [27]. Because these previous studies only examined activity within the DMN, the interpretation of the findings were necessarily limited to the primary functions of the network. As such, it was suggested that increased PCC DMN activity may reflect increased attention to internal states, such as appetite, an effect that may be lessened by exercise. The current study, however, suggests that exercise effects extend far beyond a single network.
The observed extensive interactions between the PCC, DMN and other brain networks is perhaps not surprising, given the extensive connections to and from this region. The PCC is the only node that directly interacts with all other DMN nodes, supporting this region's central role in integration of intrinsic activity [39, 40, 43, 44]. It also has structural and functional connections with myriad other non-DMN brain regions [42, 48-50]. The region has been implicated in a large number of processes requiring coordinated integration, including self-related processing [40, 41, 51], autobiographical memory [41, 52, 53], mind wandering [54, 55], emotional stimulus processing [56], evaluating personal significance [40, 41], reward outcome monitoring [57], and allocating neuronal resources to salient stimuli [38, 44, 50]. Likely as a component of these types of processes, PCC involvement has been observed in a number of processes related to food-intake behaviors and/or weight regulation. For example, PCC activation has been observed during presentation of visual food cues [58-60] and during food tasting [61, 62]. Furthermore, previous studies have identified a relationship between body mass index (BMI) and PCC responsivity to food cues [63], as well as altered PCC response to food cues in obese, compared to lean, individuals [64, 65].
To better understand the effects driving the observed BNC differences, an exploratory Granger causality analysis was performed to examine alterations in connection strength in both outgoing and ingoing causal information flow between the PCC and large-scale networks. These findings suggest that exercise may refine the flow of information between the PCC and a subset of large-scale networks. Observed reductions in outgoing connectivity from the PCC to other components of the DMN, such as the precuneus, and networks involved in various aspects of sensory processing (sensorimotor network, visual network) could relate to the role of the PCC in monitoring the external environment and accordingly allocating neuronal resources to salient stimuli [38, 44, 50]. The observed reduction in incoming information flow to the PCC from receptive components of language and sensorimotor networks could reflect a similar refinement in monitoring incoming sensory information. Changes in outgoing information flow from the PCC to the left executive control network could relate to improvements in cognitive function associated with exercise and physical fitness [9, 14-16]. Supporting this, exercise has been associated with altered neuronal responses during cognitive task performance in overweight children (decreased activation in precentral gyrus and posterior parietal cortex; increased activation in anterior cingulate and superior frontal gyrus) [66]. Furthermore, in older adults, exercise-associated alterations in functional connectivity have been associated with increases in brain-derived neurotrophic factor (BDNF) and other growth factors involved in synaptic plasticity, neuroprotection, and neurogenesis [67]. Specifically, increased connectivity within the DMN (between parahippocampal gyrus and middle temporal gyrus) following 12 months of exercise in older adults was associated with increased blood serum levels of BDNF, insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF).
Change in aerobic fitness, as measured by VO2 max, was related to change in PCC BNC following the exercise intervention, such that greater improvements in fitness were associated with greater reductions in BNC. This effect was driven by changes in connectivity between the PCC and other components of the DMN. Voss et al. previously reported a link between VO2 max and DMN connectivity, including evidence that the relationship between aerobic fitness and cognitive performance may be mediated by functional connectivity among various DMN regions [68]. The observed relationship between BNC change and VO2 max change in the current study further supports this hypothesis. As Voss et al. observed increased connectivity within the DMN to be associated with aerobic fitness and improved cognitive function, the present finding could suggest that exercise exerts differential effects on within-network connectivity versus between-network connectivity. Previous studies have posited that network refinement results from both increased within-network connectivity and decreased between-network connectivity [22, 69, 70].
Post-intervention change in perceived hunger, as measured by the TFEQ, was also associated with change in BNC, with less BNC reduction related to greater decreases in hunger. This was driven by changes between the PCC and a visuospatial network, possibly indicating altered salience of external visual cues post-intervention. Although there was no significant change in hunger scores following the intervention, the relationship between BNC change and hunger change could potentially relate to observed variability in appetite changes and compensatory energy intake following chronic exercise [71].
A potential limitation of the current study is sample size. Even with this limitation, however, the observed BNC changes suggest that the sample size was sufficient to detect effects. Subsequent studies with larger sample sizes may allow for detection of behavioral effects and a more in-depth examination of relationships between brain and behavioral effects. A session effect is another potential limitation, with the possibility that change in BNC could be due to scanning sessions simply being spaced 6 months apart, rather than due to exercise effects. Previous studies, however, have determined activity in large-scale brain networks, including the DMN, to be reliable and consistent across recording sessions, up to as long as 16 months between sessions [72-74]. Nonetheless, because reliability of the BNC metric used in the current study has not yet been reported, we investigated the possibility of session effects by identifying a group of individuals (N =10) who were not part of the current study, but who had completed resting-state fMRI scans spaced between 6 months to 1 year apart. As they were not included in the current study, this group of participants (mean BMI: 29.3±7.1 mg/kg2; mean age: 38.5±11.6 years) did not participate in the exercise intervention. This group did not significantly differ from the intervention group in age, BMI, or BNC at baseline (p > 0.05). No significant between-session effects on BNC were observed in this group, suggesting that effects observed in the current study were not simply due to time between sessions. While this control group is helpful for examining potential session effects, future studies should include a group that participates in a control intervention for the same length of time as the exercise intervention, such as a diet or stretching group.
In conclusion, the exercise-associated reduction in between-network connectivity observed in the current study suggests an impact of exercise on information flow across the brain. Identifying the influence of exercise on communication between large-scale neuronal networks could be important for understanding how exercise causes neuronal and behavioral change. The current study represents an initial step in establishing these effects, by suggesting an impact of exercise on whole-brain communication involving the PCC, a critical integrative hub, in overweight/obese individuals. Future studies are warranted to determine if exercise effects on BNC are similar in lean individuals, and if effects on BNC may be related to the variability of weight loss observed in response to exercise. Given that changes in obesity-related measures (e.g., BMI, percent body fat) were not related to BNC change, and that baseline BNC was not related to these measures at baseline, it seems likely that these effects could be generalizable to a non-obese population. Supporting this, our results are consistent with previous studies, focusing on within-network connectivity, that suggest exercise can improve network refinement and efficiency in populations different from the overweight/obese adults in the current study, specifically lean older adults [21, 68] and overweight children [22]. It is also possible that the impact of exercise on large-scale network communication at integrative hubs may contribute to individual responsivity to exercise, an effect that should be examined in future studies.
Highlights.
-Between-network connectivity (BNC) assessed exercise effects on brain connectivity.
-BNC in the posterior cingulate cortex was reduced following chronic exercise.
-Change in BNC was related to changes in aerobic fitness level and perceived hunger.
-Exercise effects on BNC may contribute to individual responsivity to exercise.
Acknowledgments
We thank Debra Singel and Yiping Du for their assistance with fMRI, Andrea Salzberg for her work on study design and data collection, and Jamie Bechtell for her work on data collection. Funding for this study was provided by NIH Colorado CTSI grant UL1RR025780, NIH Clinical Nutrition Research Unit grant P30DK48520, and NIH grants R01MH102224 (JRT), R01DK077088 (ELM), R01DK103691 (JRT), R01DK089095 (MAC and JRT), R01DK072174 (MAC), R21DK102052 (JRT), and K01DK100445 (KTL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laaksonen DE, Lindstrom J, Lakka TA, Eriksson JG, Niskanen L, Wikstrom K, et al. Physical activity in the prevention of type 2 diabetes: The Finnish Diabetes Prevention Study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 2.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: The evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. DOI: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–1350. doi: 10.1001/archinte.163.11.1343. DOI: 10.1001/archinte.163.11.1343163/11/1343. [DOI] [PubMed] [Google Scholar]
- 4.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: Identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond) 2008;32:177–184. doi: 10.1038/sj.ijo.0803712. DOI: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 5.Barwell ND, Malkova D, Leggate M, Gill JM. Individual responsiveness to exercise-induced fat loss is associated with change in resting substrate utilization. Metabolism. 2009;58:1320–1328. doi: 10.1016/j.metabol.2009.04.016. DOI: 10.1016/j.metabol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: Increase in orexigenic drive but improvement in meal-induced satiety. Am J Clin Nutr. 2009;90:921–927. doi: 10.3945/ajcn.2009.27706. DOI: 10.3945/ajcn.2009.27706. [DOI] [PubMed] [Google Scholar]
- 7.King NA, Horner K, Hills AP, Byrne NM, Wood RE, Bryant E, et al. Exercise, appetite and weight management: Understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br J Sports Med. 2012;46:315–322. doi: 10.1136/bjsm.2010.082495. DOI: 10.1136/bjsm.2010.082495. [DOI] [PubMed] [Google Scholar]
- 8.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 9.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. PNAS. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. DOI: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffer DJ, Krafft CE, Schwarz NF, Chi L, Rodrigue AL, Pierce JE, et al. An 8-month exercise intervention alters frontotemporal white matter integrity in overweight children. Psychophysiology. 2014;51:728–733. doi: 10.1111/psyp.12227. DOI: 10.1111/psyp.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105:1028–1034. doi: 10.1016/j.physbeh.2011.11.023. DOI: 10.1016/j.physbeh.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evero N, Hackett LC, Clark RD, Phelan S, Hagobian TA. Aerobic exercise reduces neuronal responses in food reward brain regions. J Appl Physiol. 2012;112:1612–1619. doi: 10.1152/japplphysiol.01365.2011. DOI: 10.1152/japplphysiol.01365.2011. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree DR, Chambers ES, Hardwick RM, Blannin AK. The effects of high-intensity exercise on neural responses to images of food. Am J Clin Nutr. 2014;99:258–267. doi: 10.3945/ajcn.113.071381. DOI: 10.3945/ajcn.113.071381. [DOI] [PubMed] [Google Scholar]
- 14.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. PNAS. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. DOI: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159:1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. DOI: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pontifex MB, Hillman CH, Fernhall B, Thompson KM, Valentini TA. The effect of acute aerobic and resistance exercise on working memory. Med Sci Sports Exerc. 2009;41:927–934. doi: 10.1249/MSS.0b013e3181907d69. DOI: 10.1249/MSS.0b013e3181907d69. [DOI] [PubMed] [Google Scholar]
- 17.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: The Framingham Heart Study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. DOI: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 18.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. DOI: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, et al. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity. 2012;20:2406–2411. doi: 10.1038/oby.2012.112. DOI: 10.1038/oby.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes (Lond) 2014;38:494–506. doi: 10.1038/ijo.2013.142. DOI: 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00032. DOI: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krafft CE, Pierce JE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, et al. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience. 2014;256:445–455. doi: 10.1016/j.neuroscience.2013.09.052. DOI: 10.1016/j.neuroscience.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. DOI: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. DOI: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 25.van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. DOI: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Wylie KP, Kronberg E, Maharajh K, Smucny J, Cornier MA, Tregellas JR. Between-network connectivity occurs in brain regions lacking layer IV input. Neuroimage. 2015;116:50–58. doi: 10.1016/j.neuroimage.2015.05.010. DOI: 10.1016/j.neuroimage.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadden KL, Cornier MA, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport. 2013;24:866–871. doi: 10.1097/WNR.0000000000000013. DOI: 10.1097/WNR.000000000000001300001756-201310230-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 29.Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. DOI: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: A fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. DOI: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 31.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the Food-Craving Inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. DOI: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 32.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Williams and Wilkins; Baltimore, MD: 2013. [DOI] [PubMed] [Google Scholar]
- 33.Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28:1251–1266. doi: 10.1002/hbm.20359. DOI: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 35.Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. DOI: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. DOI: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seth AK. A Matlab toolbox for Granger causal connectivity analysis. J Neurosci Methods. 2010;186:262–273. doi: 10.1016/j.jneumeth.2009.11.020. DOI: 10.1016/j.jneumeth.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. DOI: 10.1073/pnas.98.2.67698/2/676 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. DOI: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 40.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. DOI: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 41.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. DOI: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. DOI: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantini D, Vanduffel W. Emerging roles of the brain's default network. The Neuroscientist. 2013;19:76–87. doi: 10.1177/1073858412446202. DOI: 10.1177/1073858412446202. [DOI] [PubMed] [Google Scholar]
- 44.Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. DOI: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stawarczyk D, Majerus S, Maquet P, D'Argembeau A. Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One. 2011;6:e16997. doi: 10.1371/journal.pone.0016997. DOI: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, et al. Altered default network activity in obesity. Obesity. 2011;19:2316–2321. doi: 10.1038/oby.2011.119. DOI: 10.1038/oby.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, et al. The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. DOI: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. DOI: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn Sci. 2011;15:143–151. doi: 10.1016/j.tics.2011.02.002. DOI: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. DOI: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Frontiers in human neuroscience. 2013;7:647. doi: 10.3389/fnhum.2013.00647. DOI: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one's own past: Neural networks involved in autobiographical memory. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 54.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. DOI: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 55.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. DOI: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. DOI: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade reward signals in posterior cingulate cortex. Neuron. 2003;40:1031–1040. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- 58.Cornier MA. The effects of overfeeding and propensity to weight gain on the neuronal responses to visual food cues. Physiol Behav. 2009;97:525–530. doi: 10.1016/j.physbeh.2009.03.019. DOI: S0031-9384(09)00128-0 [pii]10.1016/j.physbeh.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. DOI: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 60.Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. DOI: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gautier JF, Chen K, Uecker A, Bandy D, Frost J, Salbe AD, et al. Regions of the human brain affected during a liquid-meal taste perception in the fasting state: A positron emission tomography study. Am J Clin Nutr. 1999;70:806–810. doi: 10.1093/ajcn/70.5.806. [DOI] [PubMed] [Google Scholar]
- 62.DelParigi A, Chen K, Salbe AD, Reiman EM, Tataranni PA. Sensory experience of food and obesity: A positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage. 2005;24:436–443. doi: 10.1016/j.neuroimage.2004.08.035. DOI: 10.1016/j.neuroimage.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 63.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. DOI: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 64.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. DOI: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 65.Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. Normal-weight adults. Appetite. 2012;58:303–312. doi: 10.1016/j.appet.2011.10.014. DOI: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krafft CE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, Pierce JE, et al. An 8-month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity. 2014;22:232–242. doi: 10.1002/oby.20518. DOI: 10.1002/oby.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. DOI: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. DOI: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. DOI: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51:156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. DOI: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hopkins M, King NA, Blundell JE. Acute and long-term effects of exercise on appetite control: Is there any benefit for weight control? Current opinion in clinical nutrition and metabolic care. 2010;13:635–640. doi: 10.1097/MCO.0b013e32833e343b. DOI: 10.1097/MCO.0b013e32833e343b. [DOI] [PubMed] [Google Scholar]
- 72.Chen S, Ross TJ, Zhan W, Myers CS, Chuang KS, Heishman SJ, et al. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 2008;1239:141–151. doi: 10.1016/j.brainres.2008.08.028. DOI: 10.1016/j.brainres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. PNAS. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. DOI: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, et al. The resting brain: Unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. DOI: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]