Abstract
Cockroach sensitization is an important risk factor for the development of asthma. However, its underlying immune mechanisms and the genetic etiology for differences in allergic responses remain unclear. Cockroach allergens identification and their expression as biologically active recombinant proteins has provided a basis for studying the mechanisms regarding cockroach allergens induced allergic sensitization and asthma. Glycans in allergens may play a crucial role in the immunogenicity of allergic diseases. Protease-activated receptor (PAR)-2, Toll-like receptor (TLR), and C-type lectin receptors have been suggested to be important for the penetration of cockroach allergens through epithelial cells to mediate allergen uptake, dendritic cell maturation, antigen presenting cell (APC) function in T cell polarization, and cytokine production. Environmental pollutants, which often co-exist with the allergen, could synergistically elicit allergic inflammation, and aryl hydrocarbon receptor (AhR) activation and signaling may serve as a link between these two elements. Genetic factors may also play an important role in conferring the susceptibility to cockroach sensitization. Several genes have been associated with cockroach sensitization and asthma-related phenotypes. In this review, we will discuss the epidemiological evidence for cockroach allergen-induced asthma, cockroach allergens, the mechanisms regarding cockroach allergens induced innate immune responses, and the genetic basis for cockroach sensitization.
Keywords: asthma, cockroach allergens, glycan, aryl hydrocarbon receptor (AhR), genetics
Introduction
Asthma is the most prevalent chronic illness in children with both increasing clinical and public health concerns (1). The prevalence of asthma in the United States has increased from 7.3% in 2001 to 8.4% in 2010 (2). Currently, asthma affects over 300 million people and one out of every 250 deaths worldwide is attributed to this disease. However, the reason for this increased prevalence remains poorly understood. It has become apparent that interaction between environmental factors early in life and specific genetic factors is important in the development of asthma. Despite the numerous efforts to discover genes associated with asthma and allergy through various approaches, including genome-wide association studies (GWAS) and investigation of gene-environment interactions, so far, there has been no “genetic variants or specific genes” that are generally recognized. Although, genetic heterogeneity and ethnic differences may contribute to the failure in discovering these genetic factors for asthma, environmental factors, on the other hand, may be critical for the development of asthma in genetically susceptible individuals. Indeed, allergens trigger asthma attacks in 60-90% of children and in 50% of adults, and 75-85% of patients with asthma have positive skin tests. Exposure to indoor environmental allergens such as cockroach, house dust mite (HDM), pet dander, pollen, and mold can induce allergic asthma and atopy (3, 4). House dust mite and cockroach antigens are common, and exposure and sensitization have been shown to increase asthma morbidity. In particular, exposure to cockroach allergens appears to have a greater effect on asthma morbidity than dust mite or pet allergy among inner-city children with asthma (5). In this review, we will specifically discuss about the epidemiological evidence for cockroach allergen-induced asthma, major components for immunogenicity in cockroach allergens, and mechanisms regarding the cockroach allergens induced innate immune responses.
Cockroach allergen exposure and risk of asthma
Cockroach exposure has been linked to cockroach sensitization and allergic respiratory symptoms. In fact, sensitization to cockroach allergens has been identified as one of the strongest risk factors for the development of asthma in low-income urban populations (5). In studies comprising children and adults, the prevalence of cockroach allergy ranges from 17-41% in the United States (6, 7). Cockroach allergens are detected in 85% of inner-city US homes and 60–80% of inner-city children with asthma are sensitized to cockroach based on the skin prick testing (8). The levels of cockroach allergens measure in the home of these children are strongly associated with a greater risk for the development of cockroach sensitization (9, 10). This finding was supported by studies from the National Cooperative Inner City Asthma Study (NCICAS) and demonstrated a clear relationship between cockroach allergen exposures and sensitization in asthmatic children living in Baltimore inner cities (4). This suggests that cockroach sensitization is a specific and major contributor to asthma morbidity for individuals who are exposed to high levels of cockroach allergens. Indeed, studies in the New York City Neighborhood Asthma and Allergy Study (NAAS) have found that cockroach allergens were more prevalent in the bed dust from homes in neighborhoods with high prevalence of asthma compared to ones with low asthma prevalence (10). Further convincing evidence was provided from a recent meta-analysis study summarizing research findings published from 2000 to 2013 on indoor exposures and exacerbation of asthma through PubMed suggests a causal relationship between cockroach allergens exposure and exacerbation of asthma, especially in adults who are sensitized to cockroach (11). Moreover, cockroach-sensitized patients exposed to higher levels of cockroach allergens in their homes have greater asthma morbidity, particularly among children in the United States inner cities as compared to non-sensitized or non-exposed children. The rate of hospitalization for asthma was 3.4 times higher among children who are skin test positive for cockroach allergens and whose bedrooms had high levels of cockroach allergens (12). These children also had 78% more visits to hospitals, significantly more wheezing, and missed more school days compared to children who were skin test negative to cockroach allergens (13).
Cockroach allergens exposures have also been reported in European urban communities, such as those found in the public housing units of Strasbourg, France (14). In Poland, approximately 25% of asthmatic children are sensitized to cockroaches and most of their homes have detectable levels of cockroach allergens (15). Among these children, there is an association between cockroach sensitization and a more severe asthma. In Asia, cockroach allergens are found in 11% to 98% of dust samples collected from 9 cities across the southern and tropical regions of China (16). In Taiwan, 58% of asthmatic patients are allergic to the cockroach allergen, Per a 2 (17). These studies implicated that cockroach allergens exposure and sensitization are clinically relevant across multiple continents.
Home-based environmental interventions to decrease the exposure to indoor cockroach allergen has led to a reduction in asthma-associated morbidity (19, 20). These findings demonstrated the relevance of cockroach allergen exposure and sensitization in the contribution to the higher asthma prevalence observed in the United States. The studies also support the necessity of cockroach testing and consideration of this allergen in the treatment of allergic respiratory diseases.
Cockroach testing, immunotherapy, and current limitations
Cockroach allergy is diagnosed with cockroach crude extract via skin testing and/or by measurement of specific IgE to cockroach allergens. Cockroach allergen-specific IgE levels have been shown to be correlated with allergens exposure among sensitized participants and a range of inflammatory, physiologic, and clinical markers (20). However, the lack of immunodominant allergen(s) and complex patterns of IgE responses to multiple cockroach extracts have made it difficult to produce standardized cockroach allergenic extract with content that would promote high efficacy for those heterogeneously sensitized patients (21). Thus, it is essential to have the standardized cockroach extracts with reliable potency and contents for the diagnosis of cockroach allergy. Similarly, cockroach immunotherapy for cockroach allergy holds promise as a treatment strategy with immune-modulatory and clinical effects in a limited number of trials (22-25), but the lack of standardized cockroach extracts limit their potential to provide optimal clinical efficacy to patients. Thus, further works are needed to identify major allergenic components in cockroach and clone immunodominant cockroach allergens in order to provide therapeutic cockroach allergen products with enhanced clinical efficacy.
Cockroach allergens
Over 4,000 cockroach species have been identified. However, only a few species live in people's homes and have been the focus for cockroach allergen-related research. These cockroaches include American cockroach (Periplaneta americana), German cockroach (Blattella germanica), oriental cockroach (Blatta orientalis), brown-banded cockroach (Supella longipalpa) and smoky brown cockroach (Periplaneta fulliginosa). In particular, both German and American cockroaches are the predominant species that infest human dwellings (23). Predominant sources of environment cockroach allergens include cockroach saliva, fecal matter, spermatophore, shredded skin, and desiccated remains of the insect. Inhalation of these cockroach allergens can cause allergic responses in human. Thus, it is essential to identify major determinants in cockroach that induce allergic responses leading to asthma. In the past decade, many cockroach allergens have been identified, sequenced, purified, and produced as biologically active recombinant proteins from German and American cockroaches. There have been 9 German cockroach allergens (Bla g 1-8 and Bla g 11) and 9 American cockroach allergens (Per a 1-3, Per a 6-7, and Per a 9-12) (www.allergen.org) (29, 30) identified thus far as listed in Table 1. For example, the recombinant cockroach allergen Bla g 1 has been expressed in both E. coli and P. pastoris (31, 32), while Bla g 4 (33), Bla g 5 (34-36), Per a 1 (37), and Per a 3 (38) have been produced in Escherichia coli, and Bla g 2 (39), Bla g 4 (40), Per a 1 (41), and Per a 7 (38) were made in the yeast Pichia pastoris, thus providing a way of controlled cockroach allergy diagnosis and experimentation compared to traditional methods that relied on the use of heterogeneous cockroach extract. A novel allergen, a chymotrypsin-like serine protease, has been recently identified from the German cockroach. The amino acid sequence of the novel allergen showed 32.7 to 43.1% identity with mite trypsin and chymotrypsin allergens, and serum from 28.6% German cockroach allergic subjects showed IgE binding to the recombinant protein (42). It is worth noting that there is a substantial degree of homology and variable IgE cross-reactivity between recognized German cockroach allergens and some homologous groups from the American cockroach allergens. Additionally, individual IgE responses vary in regards to multiple conformational allergic epitopes contained within a single allergen such as Bla g 2 (40, 43). In addition to IgE-mediated allergic responses, these purified allergens can induce allergic inflammations that are not mediated by IgE interactions. For instance, the human beta-defensin 3 has been shown to bind Bla g 2 and could modify the ability of Bla g 2 to induce localized allergic inflammatory and mucosal responses (44, 45). Bla g 7 can potently induce T cell immunoglobulin mucin domain (TIM) 4 expression on dendritic cells (46), which plays a critical role in T cell proliferation and Th2 cell development (47, 48). Per a 7 has been demonstrated to up-regulate the expression of protease-activated receptors, thereby, triggering Th2 cytokines secretion (e.g., IL-4 and IL-13) (49). Additionally, Per a 7 can reduce the production of IL-12 and the expression of the Toll-like receptor (TLRs) 9 (50). At present, these newly-characterized cockroach allergens have led an improvement in knowledge of the structure and function of cockroach allergens, and are crucial for the development of novel strategies for diagnosis and therapy.
Table 1.
Characteristics and functions of allergens from German and American cockroaches
| Allergen | M.W.(*) | Function/Homology | IgE Prevalence | Major Linear IgE Epitopes | GeneBank Accession # |
|---|---|---|---|---|---|
| Bla g 1 | 46 | • Lipids-associated and/or binding protein (118) (i.e. palmitic, oleic, and steric acids) • Nonspecific transport of lipid molecules in cockroach • Non-enzymatically active aspartic protease (34, 40, 119) |
20-40% | a.a. 1-111, 289-403, and 394-491 (32) |
AF072219 AF072221 L47595 AF072220 |
| Bla g 2 | 36 | • Glycoprotein, decorated glycans indicated to be important for IgE binding (55, 73) • Binds to human beta-defensin 3 (44) |
40-70% | a.a. 1-75 and 146-225 (45) | U28863 |
| Bla g 3 | 79(*) | • Homologous to hemocyanin and American cockroach allergen Per a 3 (120) | n.r. | n.r. | GU086323 |
| Bla g 4 | 21 | • Ligand binding protein, members of the calycin protein family (121) | 17-40% | a.a. 34-73, 78-113, and 118-152 (34) | U40767 |
| Bla g 5 | 23 | • Sigma class glutathione S-transferase (35, 36, 122) | 35-68% | a.a. 176-200 (37) | U92412 |
| Bla g 6 | 21 | • Homologous to muscle protein troponin C with four calcium-binding domains (35) | 14% | Dependent upon calcium level, a.a. 96-151 (123) |
DQ279092 DQ279093 DQ279094 |
| Bla g 7 | 31 | • German cockroach tropomyosin (124) • Can induce TIM4, CD80, and CD86 and increased IL-13 secretion in human DCs (125) • Potential involvement in DCs-induced Th2 polarization (47) |
18% | n.r. | AF260897 |
| Bla g 8 | n.r. | • Calcium binding protein • Myosin light chain (47) |
n.r. | n.r. | DQ389157 |
| Bla g 11 | 57 | • α-amylase |
DQ355516 KC207403 |
||
| Per a 1 | 45 | • Homologous to the mosquito precursor protein, ANG12, which may be involved in digestion (123) | 9-100% | a.a. 358-446 (38) |
AF072222 U78970 U69957 U69261 U69260 |
| Per a 2 | 42 | • Inactive aspartic protease-like (126) • 42-44% homology to Bla g 2 |
81% | a.a. 57-86, 200-211, and 299-309 (17) | GU188391 |
| Per a 3 | 72 | • Homologous to insect hemolymph proteins, arylphorin/hemocyanin (127) | 26-95% | a.a. 400-409, 466-471, 580-595, and 595-605 (39) |
L40818 L40820 L40819 L40821 |
| Per a 5(**) | 25 | • Glutathione S-transferase (128) | 25% | n.r. | AY563004 |
| Per a 6 | 17 | • Homologous to insect troponin Cs and vertebrate calmodulins (129) | 14% | n.r. | AY792950 |
| Per a 7 | 33 | • Tropomyosin (123) • Induce reduction of IL-12 production and expression of TLR9 in P815 mastocytoma cells (130) |
13-54% | n.r. |
Y14854 AF106961 |
| Per a 9 | 43 | • Arginine kinase (51) | 80-100% | p. LTPCRNK | AY563004 |
| Per a 10 | 28 | • Serine protease and insect trypsins (131) | 82% | n.r. | AY792954 |
| Per a 11 | 55 | • α-amylase (132) | 83% | n.r. | n.r. |
| Per a 12 | 45 | • Chitinase (133) | 64% | n.r. | n.r. |
Mass determination by mass spectrometry
Has not been officially reported in the official site for Allergen Nomenclature
Glycans in cockroach allergens may be a major determinant for immunogenicity
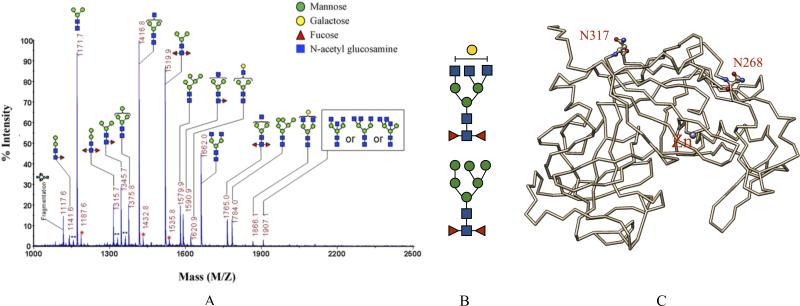
Despite the rapid increase in knowledge about the action of these purified and biological active cockroach allergens, the contribution of other potential virulence factors (i.e., macromolecules such as lipids and carbohydrates) that could exist in cockroach excrete and cockroach allergens and its clinical relevance to the development of human asthma remains unknown. Glycans are sugar modification attached to glycoproteins and glycolipids. They are generally complex heteropolymers, in contrast to the storage homopolymers that made up glycogen and amylose. More recently, intravenous and subcutaneous immunoglobulin preparations contain carbohydrate-specific IgG antibodies to microbial antigens, tumor-associated carbohydrate antigens, and host glycans (51), implicating the importance of these modifying macromolecules in human health and disease. Furthermore, polysaccharides, widely concerned as bioactive macromolecules in recent centuries, have been proved to benefit the intestinal health. Dietary polysaccharides can regulate the intestinal microenvironment and stimulate the macrophages or lymphocytes in gut tissues to fight against diseases like cancer (52). A recent study by Shade et al. had revealed that the ability of IgE to trigger an allergic reaction through its interaction with mast cells is dependent on a single site of antibody glycosylation (53), suggesting a possible route to the selective disruption of IgE glycosylation sites for the treatment of allergic diseases. Importantly, it has been suggested that glycan may be crucial in cockroach allergen-induced allergic asthma. For example, surface epitopes mapped from a murine monoclonal antibody against Bla g 2, 4C3, was found to contain a carbohydrate moiety (54, 55) and the prevention of Bla g 2 glycosylation by nucleotide point mutation significantly reduces IgE binding, Th2 cytokine IL-13 production, and increased IL-10 (32, 44). Our recent studies have also demonstrated that Bla g 2 specific IgE from patients with cockroach allergy may be, at least partially, due to glycans (unpublished data). In addition, profiling of N-linked glycans from Bla g 2 using matrix-assisted laser desorption/ionization-mass spectrometry revealed a predominance of tri-antennary N-linked core di-fucose modified glycans with mannose-, galactose-, and/or N-acetyl glucosamine- (GlcNAc) terminated moiety (Figure 1). Thus, we suspected that these glycan determinants are of insect (i.e., cockroach) due to the fact that di-fucosylation of the innermost GlcNAc moiety is commonly present in insects (56). These studies give a glimpse into the potential association between immunogenicity and particular structural features of glycans and their possible contributions to allergic diseases. Furthermore, recent progress has made it possible to gain a precise understanding of structure-function relationships with a series of the systematic synthesis of high-mannose-type glycans (57).
Figure 1.
Glycans in cockroach allergens. Surface epitopes mapped from a murine monoclonal antibody against the cockroach allergen Bla g 2 was found to contain a carbohydrate moiety (references 33, 44) and the prevention of glycosylation significantly reduces IgE binding to Bla g 2 (references 21). (A) MALDI-TOF mass spectrum of N-linked glycans prepared from purified natural Bla g2 glycoprotein (adopted from Tsai et al, 2013) demonstrated a predominance of (B) tri-antennary core di-fucose modified glycans with mannose-, galactose-, and/or N-acetyl glucosamine- (GlcNAc) terminated moiety. (C) These tri-antennary core di-fucose modified glycans are predicted to decorate Bla g 2 (1YG9) at asparagine (ball-and-stick) 268 and 317 (N268, N317). Glycan compositions were assigned based on the measured m/z values with the m/z values of the putative composition of permethylated glycans. **is undermethylated glycans. *is unknown peaks.
Mechanisms underlying the cockroach allergen-induced allergic inflammation
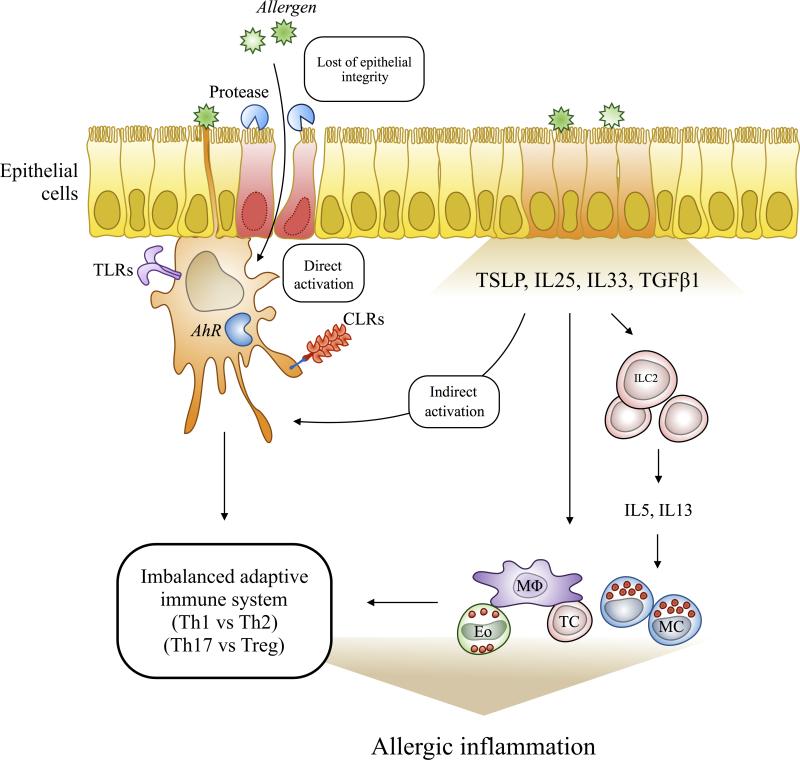
Allergic inflammation is widely accepted as the fundamental driving force in the development of allergen sensitization that can ultimately leads to asthma. Like many other indoor/outdoor allergens (e.g., house dust mite, fungi, pollen, and animal dander), cockroach excrete particles can gain access to the lungs by lodging across the nasal/oral cavities, where it can provoke allergic type inflammation by allergen-induced epithelial damage or direct contact with cells of the epithelium (58, 59). Although much more is known about the allergen-triggered early immune events, little is known about the allergen-derived signals that initiate allergic inflammation. Here, we summarized the cockroach allergen-triggered signaling events that may contribute to the development of allergic inflammation. As illustrated in Figure 2, cockroach allergens can directly activate epithelial cells and induce the production of epithelial cells derived cytokines and chemokines (e.g., TSLP, IL25, IL33, and TGFβ1), which recruit inflammatory cells to the allergen-damaged airway for repairing and inflammation suppression. On the other hand, cockroach can disturb airway epithelial integrity through proteinase-activated receptor-2 (PAR-2), which would lead to an increased penetration of allergens, resulting in activation of innate immune cells [e.g. dendritic cells (DCs) and mesenchymal stem cells (MSCs)], through C-type lectin receptors (CLRs), Toll-like receptors (TLRs), and aryl hydrocarbon receptor (AhR) (60-63). These activated innate immune cells will lead to an imbalance of the adaptive immune system toward a more Th2 and Th17 phenotypes. Furthermore, the epithelial cell-derived cytokines TSLP, IL25 and IL33 can interact with their respective receptors expressed in innate lymphoid cell type 2 (ILC2), leading to the secretion of IL5 and IL13 and subsequently allergic inflammation (64). In addition, it is well known that genetic or epigenetic factors are also major determinants for the development of cockroach allergy (65). Thus, it is likely that these genetic determinants may modify or convey the susceptibility to cockroach allergens-induced allergic responses in patients with cockroach allergy and asthma.
Figure 2.
Schematic diagram of the proposed mechanisms underlying the cockroach allergen-induced allergic inflammation. Cockroach allergens gain access to the lungs by lodging across the nasal and oral cavity, where it can directly activate epithelial cells and induce the production of epithelial cells derived cytokines and chemokines. These epithelial derived cytokines and chemokine can activate innate immune cells leading to an imbalanced adaptive immune response and the development of cockroach sensitization and allergic asthma. Proteases in cockroach extract can damage the epithelium leading to an increased penetration of allergens and activation of innate immune cells via TLRs, AhR, and CLRs. MC: mast cell, TC: T-cell, MΦ: macrophage, Eo: Eosinophil, ILC2: Innate lymphoid class 2 cell, CLRs: C-type lectin receptors, and TLRs: Toll-like receptors.
Proteinase-activated receptor-2 (PAR-2) and environmental allergen-induced asthma
Like many airborne allergens from house dust mite Dermatophagoides pteronyssinus and Aspergillus fumigatus, cockroach allergens also contain protease activity (12, 66). Indeed, measurement of protease activity in German cockroach frass and whole body extract confirmed the presence of serine protease activity, which can induce pro-inflammatory cytokines production, especially TNF-α and IL-8, from challenged airway epithelial cells via PAR-2 (67). PAR-2, a major member in a family of proteolytically activated G-coupled receptors, is expressed on a variety of cell types located throughout the airways (67, 68), and PAR-2 activation is a critical early step in the development of allergic asthma. This was well-supported by studies on proteases from Alternaria alternata that PAR-2 plays a role in proteases-induced rapid [Ca2+] increases in human airway epithelial in vitro and cell recruitment in vivo (69). However, the mechanisms regarding the PAR-2-mediated cockroach allergen-induced allergic response have not been fully elucidated. Recent studies suggest that the dual oxidase-2 (DUOX2)-ROS pathway in airway epithelial cells plays a crucial role in mediating the activation of PAR-2-stimulated airway reactivity, inflammation, oxidative stress and apoptosis in cockroach allergen-induced mouse model of asthma (70). These data suggest that proteases may link the innate and adaptive immune responses via PAR-2 activation and signaling. However, although Bla g 2 shares sequence homology with the aspartic proteinase family of proteolytic enzymes, it lacks proteolytic activity in a standard milk-clotting and hemoglobin assay using casein as a substrate (40, 71, 72), suggesting that Bla g 2 is enzymatically inactive and perhaps other factors, beside proteolytic activity, may play a crucial role in allergen-induced immunological responses.
Toll-like receptors (TLRs) mediate allergen-induced sensitization and inflammation
TLRs are transmembrane proteins highly expressed in DCs and the recognition of molecular signatures of potential pathogens via TLRs plays an important role in mediating allergen induced innate and adaptive immune responses (73). For instance, exposure to exogenous pathogen components could activate DCs through TLRs, which then can tailor the adaptive immune response to the nature of the pathogen. Conversely, antigen presentation by DCs lacking of TLRs generally leads to tolerance (74). TLR4 activation in airway epithelial cells by house dust mite (75) has been demonstrated to be sufficient in promoting allergic sensitization via the release of innate cytokines such as IL-25, IL-33, and TSLP. Together, these findings implicate that TLR signaling is critical in mediating antigen-induced immune responses. Studies have also demonstrated a role for TLRs in mediating cockroach allergen-induced allergic responses. For example, German cockroach frass contains a TLR2 ligand that can directly activate cells of the innate immune system, leading to the release of MMP-9 and decreased acute allergic responses in experimentally induced asthma in mice (76). Studies from our group on gene expression profiles in cockroach allergens treated DCs demonstrated that, among all TLR genes included for array analysis, both TLR2 and TLR8 were up-regulated in patients with cockroach allergy in comparison to healthy individuals (Manuscript in preparation). Thus, it would be of interest to extensively investigate the role and possible mechanisms of TLRs in mediating cockroach allergen-induced allergic responses.
C-type lectin receptors (CLRs) recognize glycans in allergens
C-type lectin receptors (CLRs) are pattern recognition receptor with at least one carbohydrate recognition domain (CRD) (77). The CRD that contains the conversed residue motifs determines the carbohydrate specificity, thus, CLRs are implicated to be crucial in the recognition allergenic glycans present on allergens and have been evolved to facilitate the endocytosis and presentation of pathogens (78, 79). In fact, signaling through CLRs has been shown to induce T-cell activation, tolerance and modification of cellular responses via cross-regulation of the TLR-mediated effect (80). This has been clearly illustrated by DC-SIGN (dendritic cell-specific, CD209) (80) and MRC1 (81). Thus, the expression of distinct CLRs on immune cells (i.e., dendritic cells and macrophages) may broaden the pathogen recognition profile to induce tolerance or activate immunity.
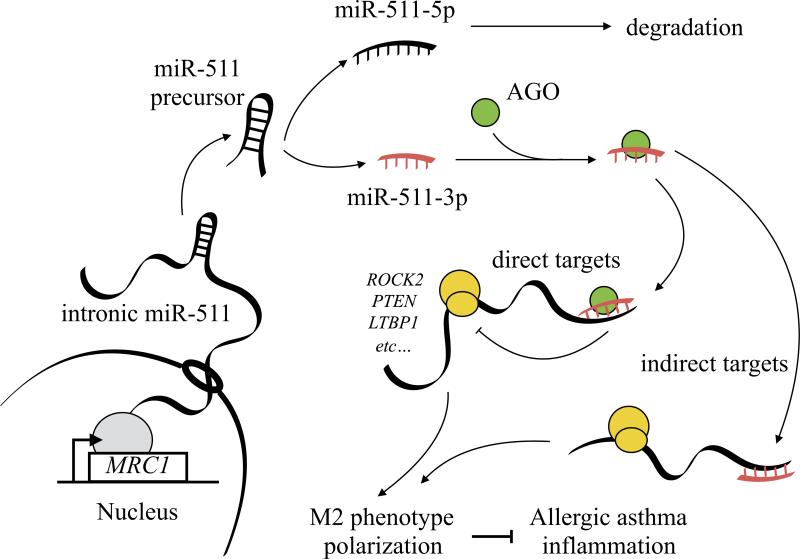
The fact that most allergens contain complex glycan modifications raises the possibility that allergenic glycan-CLR signaling may be important for allergenic immune responses. For instance, study on peanut allergens suggests that Ara h 1 (82), a major peanut allergens, is able to polarize Th2 response by interacting with DC-SIGN on monocyte-derived DCs (83). Bovine serum albumin (BSA) coupled with a common glycoform (fucosylated glycan lacking the alpha1,3-linked mannose) of allergens, including BG60 (Cyn dBG-60; Bermuda grass pollen) and Der p2 (house dust mite) showed significant bindings to DC-SIGN and its receptor, L-SIGN (84). The interaction between BG60 and DC-SIGN activated Raf-1 and ERK kinases leading to an increased expression of TNF-α (85). MRC1, encoding the mannose receptor, has been shown to mediate the uptake of diverse native allergens by DCs and determines allergen-induced T cell polarization through the modulation of indoleamine 2,3 dioxygenase (IDO) activity (85). Furthermore, MRC1 is able to mediate cat allergen Fel d 1-induced allergic responses (86). While the direct interaction between allergenic glycan and CLR has not been demonstrated, we previously reported a functional interaction for MRC1 and cockroach allergens in antigen binding, antigen recognition and downstream immune responses (87). Our ongoing study has led to a novel observation that the deletion of MRC1 in mice (MR−/−) may exacerbate cockroach allergen-induced lung inflammation and play a role in regulating allergen-induced macrophage polarization (unpublished). However, MRC1 lacks a signaling motif and the mechanisms underlying MRC1-mediated macrophage function remains unknown. Interestingly, a very recent study suggests that miRNAs may have the ability to shape the balance of M1 and M2 macrophage polarization (e.g., miR-155, miR-146) and skewing the immune response (88, 89). miR-511-3p, the functional strand of miR-511, is an intronic miRNA encoded within the MRC1 gene correlates with MRC1 expression in both tissue-resident and tumor-associated macrophages (90). As illustrated in Figure 3 an intronic miRNA encoded within MRC1 is processed by the miRNA machinery to generate the mature miR-511-3p sequence. miR-511-3p then directly targets several genes and indirectly modulates the expression of several genes and regulate macrophage polarization, subsequently leading to allergic diseases and asthma. Indeed, miR-511-3p has been shown to directly target Rho-associated coiled-coil containing protein kinase 2 (ROCK2), a serine-threonine kinase that regulates the cell cytoskeleton contractility (91). ROCK2 can phosphorylate IRF4 and promote alternative activation of macrophages (92). These studies provide evidence for future more in-depth studies on the mechanisms with the focus on these identified miR-511-3p targets to identify their association with macrophage polarization, function and macrophage-driven lung inflammation in asthma.
Figure 3.
Schematic diagram of the proposed mechanisms for miR-511-3p in modulating allergic inflammation in asthma. MRC1 transcribes the primary intronic miR-511, and followed by the miRNA machinery to generate the mature miR-511-3p sequence. The miR-511-3p can directly targets several genes (e.g., ROCK2, PTEN, and LTBP1) and shape the balance of M1 and M2 macrophage polarization and skew the immune response. In addition, miR511-3p may also modulate the expression of several indirect targets. MRC1, macrophage receptor, AGO, Argonaut.
Air pollution boosts cockroach allergy and asthma
Cockroach allergens are a major contributor to the increased risk in developing allergic asthma, particularly in urban areas. Exposure air pollution, particularly diesel exhaust and other combustion-related byproducts, can increase the likelihood of developing cockroach allergy (93, 94). This was further supported by studies showing that prenatal exposure to either diesel exhaust particulates (DEP) or polycyclic aromatic hydrocarbons (PAHs), is associated with a greater risk for the development of allergic sensitization, early childhood wheeze, and asthma (95, 96). Particularly, prenatal exposure to cockroach allergen was associated with a greater risk of allergic sensitization and this risk was increased by exposure to nonvolatile PAHs (97). Similarly, recent studies demonstrated that exposure to traffic-related air pollutants during childhood (i.e., PAH) is associated with the development and exacerbation of asthma with increasing likelihood of sensitization to cockroach allergens in urban inner-city children (98). Together, these findings suggest that exposure to environmental pollutants may exacerbate the allergen-induced allergic sensitization and asthma. However, the underlying molecular mechanism remains unknown.
Aryl hydrocarbon receptor mediates allergen-induced exacerbation of asthma
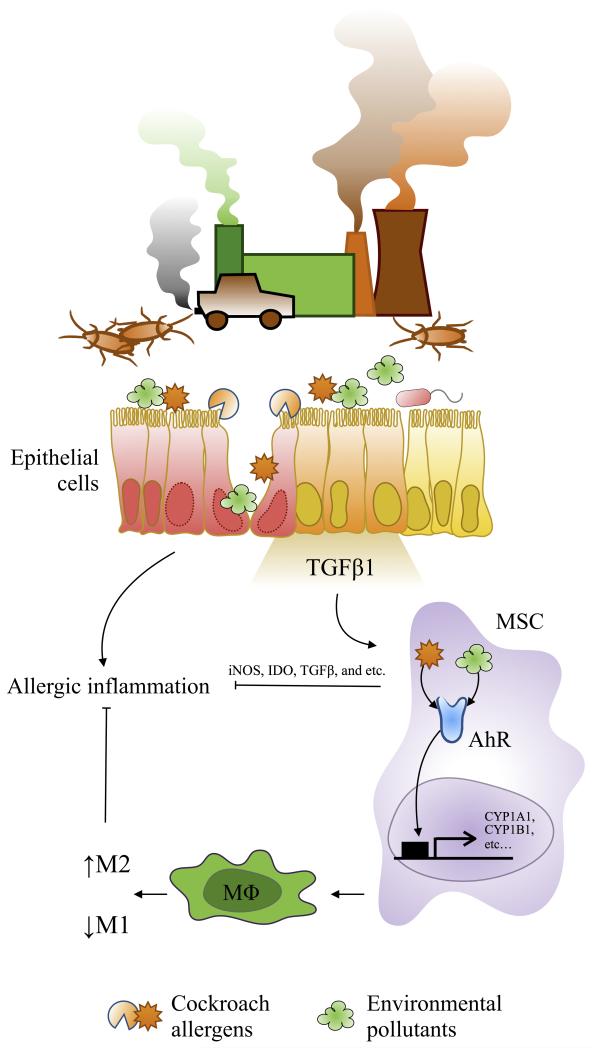
Aryl hydrocarbon receptor (AhR) is a multifunctional regulator that senses and responds to environmental stimuli and has been shown to play a role in normal cell development and immune regulation (99-101). Environmental pollutants such as DEP and PAH can activate AhR signaling leading to changes in target gene transcription (e.g., cytochrome P450 cyp1a1, cyp1b1) and a variety of immunotoxicological effects (102-107). Recent studies found that bacterial compounds can also act as potential AhR ligands and that recognition of these virulence factors by AhR contributes to host defense against invading microbial pathogens (64, 100, 106). Therefore, AhR not only elicits protection against environmental toxic molecules, but also serve as a sensor for invading pathogenic microbes (AhR-microbiome). Studies from our own group have suggested a critical role of AhR in controlling mast cell differentiation, growth, and function (108), and cockroach allergens induced immune responses (107). Furthermore, AhR deficiency led to exacerbation of lung inflammation when exposed to cockroach allergen in our well-established asthma mouse model (Xu et al. J Immunol 2015, In Press). Especially, cockroach allergens can directly induce activation of AhR signaling in bone marrow-derived MSCs, and AhR regulates MSC migration and allergic immune suppression. These findings suggest that AhR protects lungs from cockroach allergen-induced inflammation though MSCs and, therefore, is a potential target for the treatment of allergic asthma. In addition, AhR was increased in patients with allergic asthma compared to healthy controls (64), and plays a critical role in mediating the pro-inflammatory effects of traffic-related particulate matter (PM) (109). Together, these studies suggest a critical role for AhR in environmental pollutant and allergen-induced allergic inflammation and asthma. AhR may serve as a link between environmental pollutants and cockroach allergens contributing to the increased risk of developing allergic diseases like asthma.
Genetic determinants in the development of cockroach allergy
It has been suggested that there is a genetic basis for allergen-induced sensitization (110, 111). However, only a handful of studies have been performed to identify genetic factors specifically for cockroach sensitization. A genome wide screen demonstrated linkage between the HLA-linked marker DRB1*0101 and DRB1*0102 in the Hutterites, a white founder population, and African American population, respectively. Genome wide quantitative-trait loci (QTL) analysis of 533 Chinese families with asthma, provided evidence of linkage at a possible QTL D4S1647 for skin reactivity to cockroach defined by skin prick tests (SPT) (112). Furthermore, evidence of linkage between IgE and cockroach sensitization was found on chromosome 5q23 where TSLP is located (113). Single-nucleotide polymorphisms (SNPs) in several genes including mannose-binding lectin (MBL), IL-12A, TLR6, C11orf30, STAT6, SLC25A46, HLA-DQB1, IL1RL1, LPP, MYC, IL2 and HLA-B were associated with cockroach allergy (114-116). We performed analysis for 895 single nucleotide polymorphisms (SNPs) in 179 candidate genes in a total of 631 children from Boston Birth Cohort and identified several genes that are associated with cockroach sensitization including JAK1, JAK3, IL5RA, FCER1A, and ADAM33 with the strongest association for FCER1A(117). Interestingly, when analysis was performed for allergic sensitization to house dust mite, JAK2, MAML1, and NOD1 showed significant association to HMD but not cockroach, suggesting that sensitization to different allergens may be determined by their unique loci. Furthermore, environmental exposure has been suggested to play a critical role in asthma by interacting with genetic factors in genetically susceptible individuals. Thus, it is essential to assess the gene-environment interactions to determine if the associations for cockroach sensitization are modified by cockroach allergens exposure in the future.
Conclusion
Cockroach sensitization has been established as an important risk factor for the development of asthma. The identification of cockroach allergens from cockroach excrete and their molecular cloning and expression as biologically active recombinant proteins has allowed for a better our understanding in the mechanisms of cockroach allergens causes allergic disease, like asthma. There has been a potential association between immunogenicity and particular structural features of glycans. Glycans in cockroach allergens may be major determinants for immunogenicity. Several receptors (PAR-2, TLRs, and CLRs) and their signaling pathways have been found to be important in the penetration of cockroach allergens through epithelial cells, mediating allergen uptake, and signaling T cells to activate inappropriate immune responses. Environmental pollutants, which often co-exist with allergen, could synergistically elicit allergic inflammation that leads to asthma. Recent studies suggest that cockroach allergens can active AhR signaling, which may be crucial in environmental pollutant promoted cockroach allergen-induced allergic diseases. Genetic factors play a crucial role in allergic sensitization. SNPs in or near several genes have been associated with allergic sensitization (e.g., TLR6, STAT6, HLA-DQB1, IL1RL1, IL2 and HLA-B) and cockroach sensitization (e.g., TSLP, MBL2, CD14, IL-12A, JAK1, JAK3, IL5RA, FCER1A, and ADAM33). We believe that continuous studies on characterizing cockroach allergens and exploring the mechanisms regarding allergen- induced immunity and gene-environment interaction will add value to the existing research investment. These studies will offer novel insights into the molecular mechanisms that cause cockroach sensitization and subsequently asthma. Findings from these studies will contribute to the development of novel therapeutics and diagnostics of cockroach allergy that could ultimately lead to the prevention and treatment of allergic asthma.
Figure 4.
Proposed model in the role of AhR in modulating environmental pollutant and allergen-induced allergic inflammation. Airway epithelial cells can be damaged after exposure to environmental pollutants and cockroach allergens and release cytokines and chemokines (e.g., TGFβ1), which can recruit MSCs and some other inflammatory cells to the epithelial damaged sites for tissue repairing/inflammation. The recruited MSCs activated through AhR by environmental pollutants or cockroach allergens or both synergistically release anti-inflammatory factors (e.g., iNOS, IDO, and TGFβ1) and suppress airway inflammation. On the other hand, activated MSCs may modulate macrophage differentiation through AhR and inhibit airway inflammation. MSC: mesenchymal stem cell, AhR: aryl hydrocarbon receptor, MΦ: macrophage, M1: classically activated macrophage, M2: alternative activated macrophage, IDO: indoleamine 2,3-dioxygenase.
Acknowledgements
We thank Dr. Faoud T. Ishmael for his insightful review of the manuscript.
Funding: This research was supported by National Institutes of Health (NIH) grants RO1ES021739 (to P. Gao) and R21 AI109062 (to P. Gao).
Footnotes
Author contribution
All authors have contributed to the development, writing of the manuscript and approved the final version.
Conflict of interest
All authors stated there is no conflict of interest.
References
- 1.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65(2):152–167. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 2.Gergen PJ, Togias A. Inner city asthma. Immunol Allergy Clin North Am. 2015;35(1):101–114. doi: 10.1016/j.iac.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahn U, Lau S, Bergmann R, Kulig M, Forster J, Bergmann K, et al. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol. 1997;99(6 Pt 1):763–769. doi: 10.1016/s0091-6749(97)80009-7. [DOI] [PubMed] [Google Scholar]
- 4.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102(4 Pt 1):563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 5.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125(3):540–544. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147(3):573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 8.Sohn MH, Kim KE. The cockroach and allergic diseases. Allergy Asthma Immunol Res. 2012;4(5):264–269. doi: 10.4168/aair.2012.4.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew GL, Perzanowski MS, Canfield SM, Goldstein IF, Mellins RB, Hoepner LA, et al. Cockroach allergen levels and associations with cockroach-specific IgE. J Allergy Clin Immunol. 2008;121(1):240–245. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Olmedo O, Goldstein IF, Acosta L, Divjan A, Rundle AG, Chew GL, et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol. 2011;128(2):284–292. e287. doi: 10.1016/j.jaci.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect. 2015;123(1):6–20. doi: 10.1289/ehp.1307922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao P. Sensitization to cockroach allergen: immune regulation and genetic determinants. Clin Dev Immunol. 2012;2012:563760. doi: 10.1155/2012/563760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portnoy J, Chew GL, Phipatanakul W, Williams PB, Grimes C, Kennedy K, et al. Environmental assessment and exposure reduction of cockroaches: a practice parameter. J Allergy Clin Immunol. 2013;132(4):802–808. e801–825. doi: 10.1016/j.jaci.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Blay F, Sanchez J, Hedelin G, Perez-Infante A, Verot A, Chapman M, et al. Dust and airborne exposure to allergens derived from cockroach (Blattella germanica) in low-cost public housing in Strasbourg (France). J Allergy Clin Immunol. 1997;99(1 Pt 1):107–112. doi: 10.1016/s0091-6749(97)70307-5. [DOI] [PubMed] [Google Scholar]
- 15.Stelmach I, Jerzynska J, Stelmach W, Majak P, Chew G, Gorski P, et al. Cockroach allergy and exposure to cockroach allergen in Polish children with asthma. Allergy. 2002;57(8):701–705. doi: 10.1034/j.1398-9995.2002.23561.x. [DOI] [PubMed] [Google Scholar]
- 16.Zheng YW, Lai XX, Zhao de Y, Zhang CQ, Chen JJ, Zhang L, et al. Indoor Allergen Levels and Household Distributions in Nine Cities Across China. Biomed Environ Sci. 2015;28(10):709–717. doi: 10.3967/bes2015.101. [DOI] [PubMed] [Google Scholar]
- 17.Lee MF, Song PP, Hwang GY, Lin SJ, Chen YH. Sensitization to Per a 2 of the American cockroach correlates with more clinical severity among airway allergic patients in Taiwan. Ann Allergy Asthma Immunol. 2012;108(4):243–248. doi: 10.1016/j.anai.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 18..
- 19.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 20.Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–524. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 21.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65(11):1414–1422. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassirpour G, Zoratti E. Cockroach allergy and allergen-specific immunotherapy in asthma: potential and pitfalls. Curr Opin Allergy Clin Immunol. 2014;14(6):535–541. doi: 10.1097/ACI.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood RA, Togias A, Wildfire J, Visness CM, Matsui EC, Gruchalla R, et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol. 2014;133(3):846–852. e846. doi: 10.1016/j.jaci.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang BC, Johnson J, Morgan C, Chang JL. The role of immunotherapy in cockroach asthma. J Asthma. 1988;25(4):205–218. doi: 10.3109/02770908809071367. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava D, Gaur SN, Arora N, Singh BP. Clinico-immunological changes post-immunotherapy with Periplaneta americana. Eur J Clin Invest. 2011;41(8):879–888. doi: 10.1111/j.1365-2362.2011.02480.x. [DOI] [PubMed] [Google Scholar]
- 26.Arruda LK, Barbosa MC, Santos AB, Moreno AS, Chapman MD, Pomes A. Recombinant allergens for diagnosis of cockroach allergy. Curr Allergy Asthma Rep. 2014;14(4):428. doi: 10.1007/s11882-014-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbes SJ, Jr., Sever M, Archer J, Long EH, Gore JC, Schal C, et al. Abatement of cockroach allergen (Bla g 1) in low-income, urban housing: A randomized controlled trial. J Allergy Clin Immunol. 2003;112(2):339–345. doi: 10.1067/mai.2003.1597. [DOI] [PubMed] [Google Scholar]
- 28.Wilkerson RR. A multifaceted, home based, environmental intervention reduced asthma related morbidity in children with atopic asthma. Evid Based Nurs. 2005;8(2):43. doi: 10.1136/ebn.8.2.43. [DOI] [PubMed] [Google Scholar]
- 29.Ledford DK. Indoor allergens. J Allergy Clin Immunol. 1994;94(2 Pt 2):327–334. [PubMed] [Google Scholar]
- 30.Marsh DG, Goodfriend L, King TP, Lowenstein H, Platts-Mills TA. Allergen nomenclature. Bull World Health Organ. 1986;64(5):767–774. [PMC free article] [PubMed] [Google Scholar]
- 31.Radauer C, Nandy A, Ferreira F, Goodman RE, Larsen JN, Lidholm J, et al. Update of the WHO/IUIS Allergen Nomenclature Database based on analysis of allergen sequences. Allergy. 2014;69(4):413–419. doi: 10.1111/all.12348. [DOI] [PubMed] [Google Scholar]
- 32.Mueller GA, Pedersen LC, Lih FB, Glesner J, Moon AF, Chapman MD, et al. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol. 2013;132(6):1420–1426. doi: 10.1016/j.jaci.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomes A, Vailes LD, Helm RM, Chapman MD. IgE reactivity of tandem repeats derived from cockroach allergen, Bla g 1. Eur J Biochem. 2002;269(12):3086–3092. doi: 10.1046/j.1432-1033.2002.02990.x. [DOI] [PubMed] [Google Scholar]
- 34.Arruda LK, Vailes LD, Hayden ML, Benjamin DC, Chapman MD. Cloning of cockroach allergen, Bla g 4, identifies ligand binding proteins (or calycins) as a cause of IgE antibody responses. J Biol Chem. 1995;270(52):31196–31201. doi: 10.1074/jbc.270.52.31196. [DOI] [PubMed] [Google Scholar]
- 35.Jeong KJ, Jeong KY, Kim CR, Yong TS. IgE-binding epitope analysis of Bla g 5, the German cockroach allergen. Protein Pept Lett. 2010;17(5):573–577. doi: 10.2174/092986610791112765. [DOI] [PubMed] [Google Scholar]
- 36.Mueller GA, Pedersen LC, Glesner J, Edwards LL, Zakzuk J, London RE, et al. Analysis of glutathione S-transferase allergen cross-reactivity in a North American population: Relevance for molecular diagnosis. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arruda LK, Vailes LD, Platts-Mills TA, Hayden ML, Chapman MD. Induction of IgE antibody responses by glutathione S-transferase from the German cockroach (Blattella germanica). J Biol Chem. 1997;272(33):20907–20912. doi: 10.1074/jbc.272.33.20907. [DOI] [PubMed] [Google Scholar]
- 38.Melen E, Pomes A, Vailes LD, Arruda LK, Chapman MD. Molecular cloning of Per a 1 and definition of the cross-reactive Group 1 cockroach allergens. J Allergy Clin Immunol. 1999;103(5 Pt 1):859–864. doi: 10.1016/s0091-6749(99)70430-6. [DOI] [PubMed] [Google Scholar]
- 39.Wu CH, Lee MF, Wang NM, Luo SF. Sequencing and immunochemical characterization of the American cockroach per a 3 (Cr-PI) isoallergenic variants. Mol Immunol. 1997;34(1):1–8. doi: 10.1016/s0161-5890(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 40.Pomes A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V. Cockroach allergen Bla g 2: structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med. 2002;165(3):391–397. doi: 10.1164/ajrccm.165.3.2104027. [DOI] [PubMed] [Google Scholar]
- 41.Vailes LD, Kinter MT, Arruda LK, Chapman MD. High-level expression of cockroach allergen, Bla g 4, in Pichia pastoris. J Allergy Clin Immunol. 1998;101(2 Pt 1):274–280. doi: 10.1016/S0091-6749(98)70393-8. [DOI] [PubMed] [Google Scholar]
- 42.Sookrung N, Indrawattana N, Tungtrongchitr A, Bunnag C, Tantilipikorn P, Kwangsri S, et al. Allergenicity of native/recombinant tropomyosin, per a 7, of American cockroach (CR), Periplaneta americana, among CR allergic Thais. Asian Pac J Allergy Immunol. 2009;27(1):9–17. [PubMed] [Google Scholar]
- 43.Jeong KY, Son M, Lee JH, Hong CS, Park JW. Allergenic characterization of a novel allergen, homologous to chymotrypsin, from german cockroach. Allergy Asthma Immunol Res. 2015;7(3):283–289. doi: 10.4168/aair.2015.7.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Gustchina A, Glesner J, Wunschmann S, Vailes LD, Chapman MD, et al. Carbohydrates contribute to the interactions between cockroach allergen Bla g 2 and a monoclonal antibody. J Immunol. 2011;186(1):333–340. doi: 10.4049/jimmunol.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dietrich DE, Martin AD, Brogden KA. Human beta-defensin HBD3 binds to immobilized Bla g2 from the German cockroach (Blattella germanica). Peptides. 2014;53:265–269. doi: 10.1016/j.peptides.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dietrich DE, Xiao X, Dawson DV, Belanger M, Xie H, Progulske-Fox A, et al. Human alpha- and beta-defensins bind to immobilized adhesins from Porphyromonas gingivalis. Infect Immun. 2008;76(12):5714–5720. doi: 10.1128/IAI.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L, Zhang M, Ma W, Jin S, Song W, He S. Cockroach allergen Bla g 7 promotes TIM4 expression in dendritic cells leading to Th2 polarization. Mediators Inflamm. 2013;2013:983149. doi: 10.1155/2013/983149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6(5):455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 49.Liu T, He SH, Zheng PY, Zhang TY, Wang BQ, Yang PC. Staphylococcal enterotoxin B increases TIM4 expression in human dendritic cells that drives naive CD4 T cells to differentiate into Th2 cells. Mol Immunol. 2007;44(14):3580–3587. doi: 10.1016/j.molimm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Zhang H, Yang H, Zhang L, Chen X, Zheng X, et al. Induction of T-helper type 2 cytokine release and up-regulated expression of protease-activated receptors on mast cells by recombinant American cockroach allergen Per a 7. Clin Exp Allergy. 2008;38(7):1160–1167. doi: 10.1111/j.1365-2222.2008.02991.x. [DOI] [PubMed] [Google Scholar]
- 51.Yang H, Kong X, Wei J, Liu C, Song W, Zhang W, et al. Cockroach allergen Per a 7 down-regulates expression of Toll-like receptor 9 and IL-12 release from P815 cells through PI3K and MAPK signaling pathways. Cell Physiol Biochem. 2012;29(3-4):561–570. doi: 10.1159/000338510. [DOI] [PubMed] [Google Scholar]
- 52.Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med. 2015;7(269):269ra261. doi: 10.1126/scitranslmed.3010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X, Nie S, Xie M. Interaction between Gut Immunity and Polysaccharides. Crit Rev Food Sci Nutr. 2015:0. doi: 10.1080/10408398.2015.1079165. [DOI] [PubMed] [Google Scholar]
- 54.Shade KT, Platzer B, Washburn N, Mani V, Bartsch YC, Conroy M, et al. A single glycan on IgE is indispensable for initiation of anaphylaxis. J Exp Med. 2015;212(4):457–467. doi: 10.1084/jem.20142182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glesner J, Wunschmann S, Li M, Gustchina A, Wlodawer A, Himly M, et al. Mechanisms of allergen-antibody interaction of cockroach allergen Bla g 2 with monoclonal antibodies that inhibit IgE antibody binding. PLoS One. 2011;6(7):e22223. doi: 10.1371/journal.pone.0022223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woodfolk JA, Glesner J, Wright PW, Kepley CL, Li M, Himly M, et al. Antigenic determinants of the bilobal cockroach allergen Bla g 2. J Biol Chem. 2015 doi: 10.1074/jbc.M115.702324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 58.Fujikawa K, Seko A, Takeda Y, Ito Y. Approaches toward High-Mannose-Type Glycan Libraries. Chem Rec. 2015 doi: 10.1002/tcr.201500222. [DOI] [PubMed] [Google Scholar]
- 59.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108(5):797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 60.Page K, Lierl KM, Herman N, Wills-Karp M. Differences in susceptibility to German cockroach frass and its associated proteases in induced allergic inflammation in mice. Respir Res. 2007;8:91. doi: 10.1186/1465-9921-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. J Allergy Clin Immunol. 2003;112(6):1112–1118. doi: 10.1016/j.jaci.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 62.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183(2):1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Page K, Ledford JR, Zhou P, Dienger K, Wills-Karp M. Mucosal sensitization to German cockroach involves protease-activated receptor-2. Respir Res. 2010;11:62. doi: 10.1186/1465-9921-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, Mirza S, Xu T, Tripathi P, Plunkett B, Myers A, et al. Aryl hydrocarbon receptor (AhR) modulates cockroach allergen-induced immune responses through active TGFbeta1 release. Mediators Inflamm. 2014;2014:591479. doi: 10.1155/2014/591479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 66.King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161(7):3645–3651. [PubMed] [Google Scholar]
- 67.Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy. 2003;33(1):35–42. doi: 10.1046/j.1365-2222.2002.01481.x. [DOI] [PubMed] [Google Scholar]
- 68.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91(20):9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bohm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, et al. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J. 1996;314(Pt 3):1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ubl JJ, Grishina ZV, Sukhomlin TK, Welte T, Sedehizade F, Reiser G. Human bronchial epithelial cells express PAR-2 with different sensitivity to thermolysin. Am J Physiol Lung Cell Mol Physiol. 2002;282(6):L1339–1348. doi: 10.1152/ajplung.00392.2001. [DOI] [PubMed] [Google Scholar]
- 71.Nadeem A, Alharbi NO, Vliagoftis H, Tyagi M, Ahmad SF, Sayed-Ahmed MM. Proteinase activated receptor-2-mediated dual oxidase-2 up-regulation is involved in enhanced airway reactivity and inflammation in a mouse model of allergic asthma. Immunology. 2015;145(3):391–403. doi: 10.1111/imm.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arruda LK, Vailes LD, Mann BJ, Shannon J, Fox JW, Vedvick TS, et al. Molecular cloning of a major cockroach (Blattella germanica) allergen, Bla g 2. Sequence homology to the aspartic proteases. J Biol Chem. 1995;270(33):19563–19568. doi: 10.1074/jbc.270.33.19563. [DOI] [PubMed] [Google Scholar]
- 73.Wunschmann S, Gustchina A, Chapman MD, Pomes A. Cockroach allergen Bla g 2: an unusual aspartic proteinase. J Allergy Clin Immunol. 2005;116(1):140–145. doi: 10.1016/j.jaci.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 74.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7(301):301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 75.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6(2):163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 76.Ullah MA, Loh Z, Gan WJ, Zhang V, Yang H, Li JH, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014;134(2):440–450. doi: 10.1016/j.jaci.2013.12.1035. [DOI] [PubMed] [Google Scholar]
- 77.Page K, Ledford JR, Zhou P, Wills-Karp M. A TLR2 agonist in German cockroach frass activates MMP-9 release and is protective against allergic inflammation in mice. J Immunol. 2009;183(5):3400–3408. doi: 10.4049/jimmunol.0900838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272(24):6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 79.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3(9):697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 81.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197(1):7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Tachado SD, Patel N, Zhu J, Imrich A, Manfruelli P, et al. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J Leukoc Biol. 2005;78(3):665–674. doi: 10.1189/jlb.1204699. [DOI] [PubMed] [Google Scholar]
- 83.Burks AW, Cockrell G, Stanley JS, Helm RM, Bannon GA. Recombinant peanut allergen Ara h I expression and IgE binding in patients with peanut hypersensitivity. J Clin Invest. 1995;96(4):1715–1721. doi: 10.1172/JCI118216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177(6):3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 85.Hsu SC, Chen CH, Tsai SH, Kawasaki H, Hung CH, Chu YT, et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J Biol Chem. 2010;285(11):7903–7910. doi: 10.1074/jbc.M109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Royer PJ, Emara M, Yang C, Al-Ghouleh A, Tighe P, Jones N, et al. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185(3):1522–1531. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 87.Emara M, Royer PJ, Abbas Z, Sewell HF, Mohamed GG, Singh S, et al. Recognition of the major cat allergen Fel d 1 through the cysteine-rich domain of the mannose receptor determines its allergenicity. J Biol Chem. 2011;286(15):13033–13040. doi: 10.1074/jbc.M111.220657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsai YM, Hsu SC, Zhang J, Zhou YF, Plunkett B, Huang SK, et al. Functional interaction of cockroach allergens and mannose receptor (CD206) in human circulating fibrocytes. PLoS One. 2013;8(5):e64105. doi: 10.1371/journal.pone.0064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182(8):4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 2012;287(26):21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, et al. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell Rep. 2012;1(2):141–154. doi: 10.1016/j.celrep.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67(9):545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120(9):3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Acciani TH, Brandt EB, Khurana Hershey GK, Le Cras TD. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin Exp Allergy. 2013;43(12):1406–1418. doi: 10.1111/cea.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70(3):245–256. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- 96.Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133(3):713–722. e714. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finkelman FD. Diesel exhaust particle exposure during pregnancy promotes development of asthma and atopy. J Allergy Clin Immunol. 2014;134(1):73–74. doi: 10.1016/j.jaci.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 98.Perzanowski MS, Chew GL, Divjan A, Jung KH, Ridder R, Tang D, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131(3):886–893. doi: 10.1016/j.jaci.2012.12.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung KH, Lovinsky-Desir S, Perzanowski M, Liu X, Maher C, Gil E, et al. Repeatedly high polycyclic aromatic hydrocarbon exposure and cockroach sensitization among inner-city children. Environ Res. 2015;140:649–656. doi: 10.1016/j.envres.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512(7515):387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- 101.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511(7508):184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang SK, Zhang Q, Qiu Z, Chung KF. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J Thorac Dis. 2015;7(1):23–33. doi: 10.3978/j.issn.2072-1439.2014.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 104.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 105.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182(11):6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 106.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107(46):19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, Tung HY, Tsai YM, Hsu SC, Chang HW, Kawasaki H, et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood. 2013;121(16):3195–3204. doi: 10.1182/blood-2012-08-453597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Memari B, Bouttier M, Dimitrov V, Ouellette M, Behr MA, Fritz JH, et al. Engagement of the Aryl Hydrocarbon Receptor in Mycobacterium tuberculosis-Infected Macrophages Has Pleiotropic Effects on Innate Immune Signaling. J Immunol. 2015;195(9):4479–4491. doi: 10.4049/jimmunol.1501141. [DOI] [PubMed] [Google Scholar]
- 109.Zhu J, Cao Y, Li K, Wang Z, Zuo P, Xiong W, et al. Increased expression of aryl hydrocarbon receptor and interleukin 22 in patients with allergic asthma. Asian Pac J Allergy Immunol. 2011;29(3):266–272. [PubMed] [Google Scholar]
- 110.Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crain EF, Walter M, O'Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect. 2002;110(9):939–945. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amr S, Bollinger ME, Myers M, Hamilton RG, Weiss SR, Rossman M, et al. Environmental allergens and asthma in urban elementary schools. Ann Allergy Asthma Immunol. 2003;90(1):34–40. doi: 10.1016/S1081-1206(10)63611-3. [DOI] [PubMed] [Google Scholar]
- 113.Xu X, Fang Z, Wang B, Chen C, Guang W, Jin Y, et al. A genomewide search for quantitative-trait loci underlying asthma. Am J Hum Genet. 2001;69(6):1271–1277. doi: 10.1086/324650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hunninghake GM, Lasky-Su J, Soto-Quiros ME, Avila L, Liang C, Lake SL, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177(8):830–836. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao PS, Shimizu K, Grant AV, Rafaels N, Zhou LF, Hudson SA, et al. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 2010;18(6):713–719. doi: 10.1038/ejhg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pistiner M, Hunninghake GM, Soto-Quiros ME, Avila L, Murphy A, Lasky-Su J, et al. Polymorphisms in IL12A and cockroach allergy in children with asthma. Clin Mol Allergy. 2008;6:6. doi: 10.1186/1476-7961-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bonnelykke K, Matheson MC, Pers TH, Granell R, Strachan DP, Alves AC, et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat Genet. 2013;45(8):902–906. doi: 10.1038/ng.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tripathi P, Hong X, Caruso D, Gao P, Wang X. Genetic determinants in the development of sensitization to environmental allergens in early childhood. Immun Inflamm Dis. 2014;2(3):193–204. doi: 10.1002/iid3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yi MH, Jeong KY, Kim CR, Yong TS. IgE-binding reactivity of peptide fragments of Bla g 1.02, a major German cockroach allergen. Asian Pac J Allergy Immunol. 2009;27(2-3):121–129. [PubMed] [Google Scholar]
- 120.Lee H, Jeong KY, Shin KH, Yi MH, Gantulaga D, Hong CS, et al. Reactivity of German cockroach allergen, Bla g 2, peptide fragments to IgE antibodies in patients' sera. Korean J Parasitol. 2008;46(4):243–246. doi: 10.3347/kjp.2008.46.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khurana T, Collison M, Chew FT, Slater JE. Bla g 3: a novel allergen of German cockroach identified using cockroach-specific avian single-chain variable fragment antibody. Ann Allergy Asthma Immunol. 2014;112(2):140–145. e141. doi: 10.1016/j.anai.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 122.Shin KH, Jeong KY, Hong CS, Yong TS. IgE binding reactivity of peptide fragments of Bla g 4, a major German cockroach allergen. Korean J Parasitol. 2009;47(1):31–36. doi: 10.3347/kjp.2009.47.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hindley J, Wunschmann S, Satinover SM, Woodfolk JA, Chew FT, Chapman MD, et al. Bla g 6: a troponin C allergen from Blattella germanica with IgE binding calcium dependence. J Allergy Clin Immunol. 2006;117(6):1389–1395. doi: 10.1016/j.jaci.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 124.Un S, Jeong KY, Yi MH, Kim CR, Yong TS. IgE binding epitopes of Bla g 6 from German cockroach. Protein Pept Lett. 2010;17(9):1170–1176. doi: 10.2174/092986610791760432. [DOI] [PubMed] [Google Scholar]
- 125.Jeong KY, Lee J, Lee IY, Ree HI, Hong CS, Yong TS. Allergenicity of recombinant Bla g 7, German cockroach tropomyosin. Allergy. 2003;58(10):1059–1063. doi: 10.1034/j.1398-9995.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- 126.Wang NM, Lee MF, Wu CH. Immunologic characterization of a recombinant American cockroach (Periplaneta americana) Per a 1 (Cr-PII) allergen. Allergy. 1999;54(2):119–127. doi: 10.1034/j.1398-9995.1999.00902.x. [DOI] [PubMed] [Google Scholar]
- 127.Lee MF, Chang CW, Song PP, Hwang GY, Lin SJ, Chen YH. IgE-Binding Epitope Mapping and Tissue Localization of the Major American Cockroach Allergen Per a 2. Allergy Asthma Immunol Res. 2015;7(4):376–383. doi: 10.4168/aair.2015.7.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu CH, Lee MF, Tseng CY. IgE-binding epitopes of the American cockroach Per a 3 allergen. Allergy. 2003;58(10):986–992. doi: 10.1034/j.1398-9995.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 129.Wei JF, Yang H, Li D, Gao P, He S. Preparation and identification of Per a 5 as a novel American cockroach allergen. Mediators Inflamm. 2014;2014:591468. doi: 10.1155/2014/591468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Satinover SM, Reefer AJ, Pomes A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. 2005;115(4):803–809. doi: 10.1016/j.jaci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 131.Sookrung N, Chaicumpa W, Tungtrongchitr A, Vichyanond P, Bunnag C, Ramasoota P, et al. Periplaneta americana arginine kinase as a major cockroach allergen among Thai patients with major cockroach allergies. Environ Health Perspect. 2006;114(6):875–880. doi: 10.1289/ehp.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sudha VT, Arora N, Gaur SN, Pasha S, Singh BP. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy. 2008;63(6):768–776. doi: 10.1111/j.1398-9995.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 133.Fang Y, Long C, Bai X, Liu W, Rong M, Lai R, et al. Two new types of allergens from the cockroach, Periplaneta americana. Allergy. 2015 doi: 10.1111/all.12766. [DOI] [PubMed] [Google Scholar]