Abstract
Objectives
A previous study proposed that serial full-thickness excisional biopsies of vocal fold leukoplakia therapeutically decreased dysplasia grade. The current investigation aimed to 1) analyze the pathological evolution and natural history of these lesions and 2) re-examine the role of serial excisions on dysplasia grade regression in long-term follow-up.
Study Design
Retrospective case series
Methods
Patients treated for vocal fold dysplasia (1994 – 2013) with serial full-thickness microflap-type excisions were identified and followed longitudinally. Excluded were those with one excision, invasive cancer at initial excision, or history of laryngeal cancer or radiation. Data from surgical procedures, associated pathology, and patient characteristics were recorded. Weighted repeated measures ordinal logistic regression measured associations with pathology findings.
Results
Of 55 patients [median age 65 (interquartile range 54 - 73), 89% male, 63% ever smokers, 27% alcohol users], 31 met inclusion criteria. During the study period, patients had two to 44 excisions with median time between excisions of 4.0 months. Each additional excision increased odds of higher-grade pathology by 4% (odds ratio 1.04, 95% confidence interval 1.01 – 1.06; p=0.007). A transition model demonstrated patients with moderate dysplasia, severe dysplasia, or carcinoma in situ on a prior biopsy had 2.64-, 5.64-, and 8.73-times increased odds of the same or higher pathology grade at the next excision.
Conclusions
Data does not support the hypothesis that serial full-thickness excisions decrease dysplasia grade. Progression of dysplasia appears to be non-linear, but higher-grade dysplasia is more likely to progress to malignancy.
Keywords: vocal fold leukoplakia, dysplasia, progression, biopsy, diagnosis, treatment
INTRODUCTION
Vocal fold leukoplakia is the clinical finding of an epithelial white-plaque whose prevalence in the U.S. is estimated at 10.2 and 2.1 per 100,000 in males and females, respectively.1 Hoarseness is the most common presenting complaint; however, it can be asymptomatic. Pathologic correlation is important because leukoplakia exists on a spectrum from benign hyperkeratosis to premalignant dysplasia to invasive squamous cell carcinoma. Approximately half of biopsied laryngeal leukoplakia is found to represent dysplasia or invasive carcinoma.2,3 Of premalignant dysplastic lesions, the reported malignant transformation rate in the literature range from 1% to 40%, with an apparent increased risk among lesions with higher dysplasia grades.1
As such, most surgeons are reluctant to manage newly diagnosed leukoplakia with watchful waiting, preferring instead to biopsy and obtain pathologic examination that can distinguish benign from dysplastic and malignant lesions.1 Accurate diagnosis requires that the biopsy contain all epithelial layers including the basement membrane in order to determine both degree of differentiation and depth of invasion. Leukoplakia found on biopsy to represent hyperkeratosis or non-malignant epithelial changes are at low risk for malignant transformation and often only require surveillance and reassurance.4 When biopsy results are consistent with invasive malignancy, treatment options consist primarily of surgical excision or radiation.5
As stated, there is little controversy over the treatment of non-dysplastic benign lesions or invasive carcinoma. In contrast, the management of intervening grades of dysplasia (i.e. mild, moderate, severe) and carcinoma in situ (CIS) is less clear. Dysplastic leukoplakia typically recurs after excisional biopsy(s), presumably due to field cancerization,6 and without histological examination, there is no means to assess whether transformation to worse degrees of dysplasia or to frank malignancy has occurred. Moreover, there is a lack of evidence surrounding the natural history of laryngeal dysplasia and this uncertainty begets practice variability. Specifically, most surgeons offer patients varying degrees and frequency of surveillance and have differing thresholds for repeat biopsy. Others defer serial biopsy opting instead for repeated ablation of dysplastic leukoplakia (e.g., potassium-titanyl-phosphate [KTP] laser) or use a combination of excisional and ablative approaches.
A prior study suggested that serial full-thickness excision has a therapeutic effect on dysplastic leukoplakia. Specifically, data promulgating this approach derived from a case-series of 54 patients with laryngeal dysplasia or CIS who were managed with serial full-thickness excisions.7 In it, only 5% of patients with severe dysplasia or CIS developed invasive carcinoma, a markedly lower rate than 15.7% to 54.5% seen in other studies that followed severe dysplasia and CIS with different forms of intervention.5 Regression in the dysplasia grade was also observed, to which the lower rate of malignant transformation was partially attributed. It concluded that serial full-thickness excision offers an optimal combination of disease control and preservation of function.
Careful analysis of this study, however, reveals several methodological limitations related to the study design and its non-continuous, dichotomous analysis (i.e., only comparing first to last pathology). The assumption was made that dysplasia progresses linearly; that is, mild dysplasia, if untreated, will naturally devolve into moderate, severe, CIS and, ultimately invasive carcinoma. While plausible, a different paradigm is that vocal fold dysplasia is a dynamic, time-varying process where dysplasia grade fluctuates in severity over time. If the second hypothesis is true, then comparing initial pathology to final pathology without considering the intervening pathologic grades may misrepresent the disease process and the therapeutic effect of serial excisional biopsy. To this end, a cohort of patients managed over a 19-year period using identical methodology as in the aforementioned study was identified. Using this cohort allowed 1) analysis of the pathological evolution and natural history of these lesions and 2) the ability to re-examine the effect of serial excisions on dysplasia grade regression.
METHODS
This retrospective case series was approved by the Institutional Review Board (IRB#130408) and is in compliance with Principles of Good Clinical Practice and the Declaration of Helsinki.
Study Population
Data from all patients presenting between 1994 and 2013 with vocal fold leukoplakia diagnosed via laryngoscopy by the senior author (RHO) were captured. To be included in the study, patients must have undergone greater than one biopsy during this period and had pathologically confirmed laryngeal dysplasia as defined by the World Health Organization classification (WHO)8 within at least one specimen. Excluded were patients with invasive laryngeal cancer at initial excision, a history of laryngeal cancer, prior radiation therapy to the head or neck, a history of vocal fold surgery (e.g., cordectomy, endoscopic partial laryngectomy), or any type of open neck surgery.
Variables
Data were extracted from charts of patients meeting inclusion and exclusion criteria and included patient age at diagnosis, gender (male/female), cigarette smoking history (yes/no); alcohol use in the last six months (yes/no), date of initial excisional biopsy (“Time 0”), interval between biopsies (months), highest pathologic dysplasia grade from each surgery (none, mild, moderate, severe, carcinoma in situ, invasive), and laterality of leukoplakia over the study period (unilateral, bilateral). The highest pathologic grade from each surgical date was used because patients often had more than one biopsy performed during a single microdirect laryngoscopy.
Surveillance Algorithm
As part of initial evaluation, all patients underwent videostroboscopic laryngeal examination. Direct microlaryngoscopy with full-thickness excision or biopsy was performed on all with leukoplakia. Patients with pathologically confirmed dysplasia were placed under serial laryngeal surveillance with videostroboscopy at three-month intervals. Changes in color, vibratory parameters, lesion size, ulceration, or thickness triggered return repeat excisional biopsy in the operating theater. Changes in vocal fold leukoplakia characteristics were considered in the context of the previous pathology with higher-grade dysplasia (severe dysplasia or CIS) prompting a lower threshold for repeated biopsy.
All biopsies were performed using a standardized and consistent approach involving a full-thickness, microflap-type excisional technique described previously.9 In brief, this process involves direct microlaryngoscopy with a sickle knife incision lateral to the lesion, elevation of the affected epithelium beneath the basement membrane using a laryngeal microflap elevator, and excision of the lesion with micro-scissors. If multiple or bilateral vocal fold lesions were identified, each was biopsied using this method.
Analysis
Descriptive data on patient and disease characteristics were summarized using median, 25th and 75th percentile (interquartile range [IQR]) for continuous variables, and percentages for categorical variables. Pathology was assessed multiple times per patient, and we estimated within-subject changes in in dysplasia grade (none, mild, moderate, severe, CIS, invasive) over time using ordinal logistic regression. The ordinal regression model was weighted to give each subject equal influence, and the robust sandwich estimator clustering on subject identifier was used to account for repeated observations on the same patient. Separate univariable models were fit to estimate odds of higher dysplasia grades from time from first biopsy (Time 0), number of surgeries, whether the next dysplasia grade was conditional on the previous pathology, and whether the final grade was conditional on the baseline dysplasia grade.
Multivariate models were pre-specified to adjust for smoking status and age at presentation. Patient-level and cumulative trends in dysplasia grade were depicted graphically with 95% confidence intervals. We also considered a transition model to test if pathology from the prior excisional biopsy was associated with pathology from the subsequent biopsy. Specifically, the odds of the same or worsened relative to a lower pathologic grade at the next excision were estimated. Results are reported as odds ratios (OR) with 95% confidence intervals (CI).
RESULTS
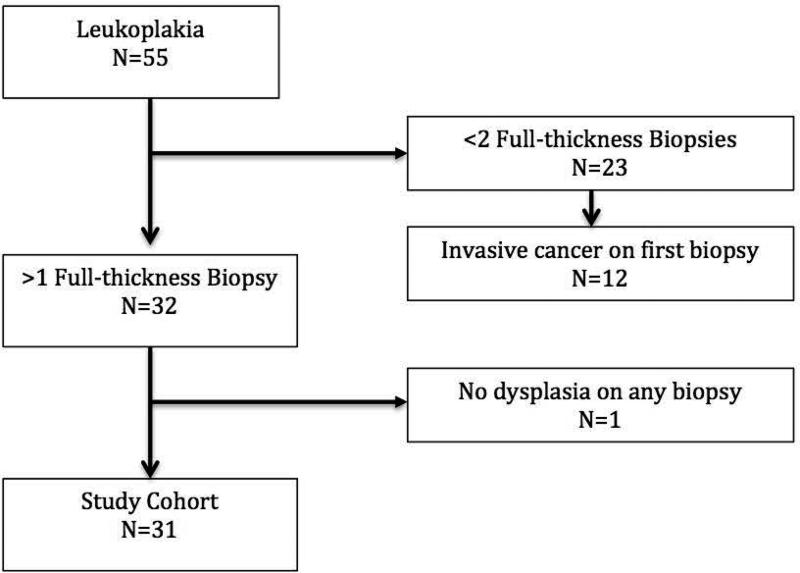
Of 55 patients who underwent microflap excisional biopsy for leukoplakia during this time interval [median age at presentation 65 years (interquartile range 54 - 73), 89% male, 64% ever smokers, 27% alcohol users], 31 met inclusion criteria (Figure 1). There was no significant difference in characteristics between included and excluded patients (Table 1). Reason for exclusions were: 1) only one excisional biopsy [CIS or invasive disease with subsequent treatment (e.g. radiation therapy)] (n=14), 2) loss to follow up (n=1), 3) non-dysplastic leukoplakia with no further biopsies (n=4), 4) were diagnosed with oropharyngeal cancer (treated with radiation) (n=1), 5) dysplastic leukoplakia with no evidence of disease on clinic follow-up (n=3), or 6) no dysplasia within the leukoplakia on any excisional biopsy (n=1). Several patients met greater than one of the exclusion criteria.
Figure 1.
Study flow diagram with exclusions
Table 1.
Comparison of patient characteristics among included and excluded patients
| Characteristics | Included (n=31) | Excluded (n=24) | P | |
|---|---|---|---|---|
| Age, median years (IQR) | 61 (52 – 72) | 72 (57 – 76.5) | 0.07 | |
| Gender | Male | 83.9% | 95.8% | 0.16 |
| Female | 16.1% | 4.2% | ||
| Ever smokers | Yes | 61.3% | 66.7% | 0.68 |
| No | 38.7% | 33.3% | ||
| Alcohol use | Yes | 19.4% | 33.3% | 0.49 |
| No | 74.2% | 62.5% | ||
| NR* | 6.4% | 4.2% | ||
NR: not recorded
Included patients had between two and 44 excisions during the study period with a median time between excisions of 4.0 months (IQR 2.2 – 8.3). Overall, 13 patients had unilateral vocal fold dysplasia (i.e. present on the same vocal fold throughout follow-up), whereas 18 patients initially had or developed bilateral dysplasia (i.e. originating from either unilateral or bilateral dysplasia at initial biopsy).
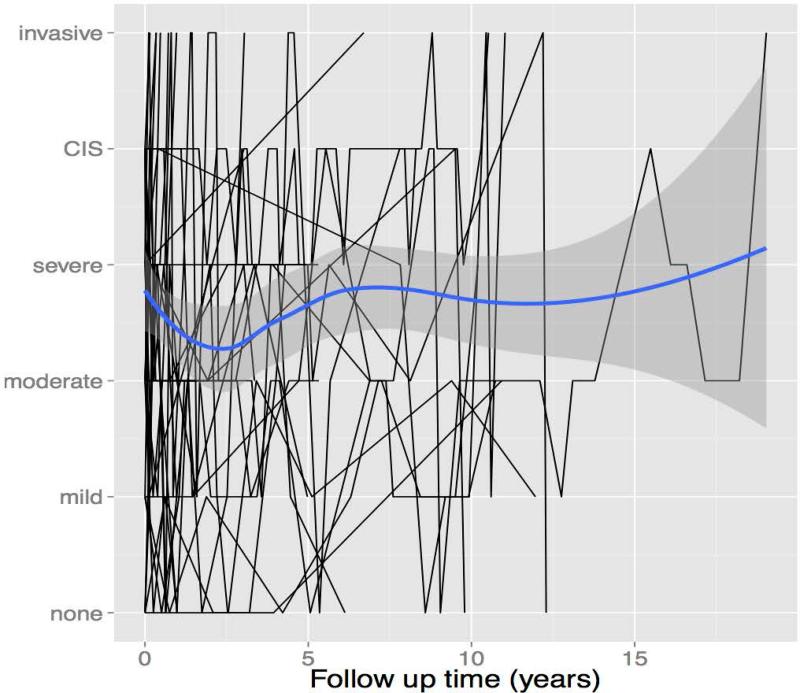
Serial pathologic dysplasia grades from surgical biopsies for the 31 included patients are shown in Figure 2. This illustrates that trends in dysplasia grade widely fluctuate within an individual, and overall, generally worsen over time. In all, four had no dysplasia on initial biopsy (12.9%), while the remainder had varying degrees of dysplasia [mild: 4 (12.9%), moderate: 6 (19.4%), severe: 6 (19.4%), and CIS: 11 (35.5%)]. Overall, eleven had dysplastic lesions that transformed into invasive cancer (35.5%): two from an initial biopsy showing no dysplasia, one from mild dysplasia, two from moderate dysplasia, three from severe dysplasia, and three from CIS. Three patient's leukoplakia regressed to no dysplasia (9.7%): one from mild dysplasia and two from CIS. One patient had no dysplasia at initial and last pathologic evaluation.
Figure 2.
Overall (blue line) and individual patient (black lines) trends in dysplasia grades by follow-up time (Grey area represents 95% confidence interval of overall trend line)
Regression Analyses
Two independent regression analytic models were used to examine 1) the affect of follow-up time and 2) surgical sequence/number of surgeries performed, in order to determine whether these factors affected dysplasia grade (Table 2). Longer follow-up time was significantly associated with greater odds of worse dysplasia (OR 1.09, CI 1.05 – 1.14, p<0.001). However, in stratified analysis, patients followed for 1 – 3 years had 41% reduced odds of dysplasia progression compared to those followed for less than 1 year (OR 0.59, CI 0.43 – 0.83, p=0.002).
Table 2.
Odds of higher pathologic grade of dysplasia based on follow-up time and number of surgeries performed, adjusted for age and smoking status.
| Adjusted Odds Ratio | 95% CI | P-value | |
|---|---|---|---|
| Time (years) | 1.09 | 1.05 – 1.14 | <0.001 |
| Surgeries | 1.04 | 1.01 – 1.06 | 0.007 |
The analysis using surgical sequence in lieu of follow-up time demonstrated similar results. More surgeries were associated with increased odds of disease progression (OR 1.04, CI 1.01 – 1.06, p=0.007). However, when compared to those who had 0 – 5 surgeries, those who had 5 – 10 had 40% decreased odds of worsened dysplasia (OR 0.60, CI 0.43 – 0.84, p=0.003). In contrast, those who underwent greater than 10 surgeries had 77% increased odds of having a worsened degree of dysplasia (OR 1.77, CI 1.04 – 2.99, p=0.034).
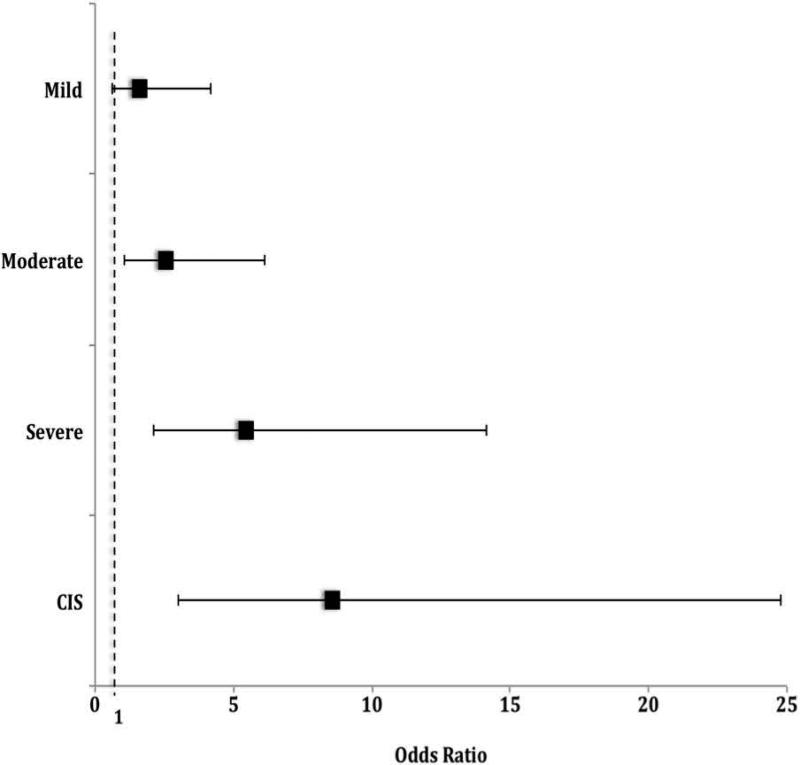
A transition model was used to test if previous pathology outcome predicted the subsequent biopsy pathological outcome. It found patients with moderate dysplasia, severe dysplasia, or CIS on a prior biopsy had 2.64-, 5.64-, and 8.73-increased odds of the same or worse status after the following surgery (p<0.05) (Figure 3).
Figure 3.
Odds of the same or worse pathology on subsequent biopsy based on the previous pathology outcome (dashed line indicates no effect)
DISCUSSION
The pathologic evolution and natural history of laryngeal dysplasia is not well understood. It may present as vocal fold leukoplakia, which is traditionally biopsied to determine its significance. Management is varied among surgeons when pathology reports dysplasia.5 This study took advantage of a 19-year longitudinal experience treating patients with vocal fold dysplastic leukoplakia who were managed consistently with serial full-thickness mucosal excisional biopsies. A previous study suggested that such serial biopsies were associated with dysplasia regression, thus implying a possible therapeutic effect. The present study sought to re-examine the effectiveness of serial excision on dysplasia grade regression and to capture the pathological evolution and natural history of dysplastic vocal fold leukoplakia.
Serial Excision Did Not Reverse Dysplasia Progression
This study did not find evidence that serial excision reduced vocal fold dysplasia grade. To the contrary, it found that longer duration of follow-up and more excisions were both associated with worsening grades of dysplasia. Specifically, for every additional year of follow-up, odds of worsened dysplasia increased by 9%. In fact, each additional excision was associated with 4% increased odds of worsened dysplasia. These analyses were highly correlated because of the interdependence of follow-up time and number of surgeries. Nonetheless, the analysis indicates that repeated excisions do not appear to reverse dysplasia progression. Available data is not sufficient to determine whether excision slows the natural history of these lesions. However, the results herein directly contrast with those from the previous study on serial excision that reported a therapeutic regression of dysplasia with this approach.7
A transition model was also used to examine whether the previous pathology was predictive of the pathologic grade of the next subsequent excision. Odds of having the same or worse pathologic grade at the next excision were significantly increased when the prior pathology was moderate or severe dysplasia, or CIS. Moreover, odds increased incrementally with the previous pathology grade: 2.64- 5.64- and 8.73-fold for moderate and severe dysplasia and CIS, respectively. This analysis further emphasizes that serial excision does not appear to have the therapeutic effect of decreasing vocal fold dysplasia grade. It does, however, argue for increased vigilance of lesions as dysplasia grade increases, since there is increased likelihood that it will worsen toward malignant transformation. These findings add to the literature that worse degrees of dysplasia have higher rates of malignant transformation.2,3,10
The results generally show that moderate to higher grades of dysplasia tends to progress. In fact, 6 of 17 (35%) patients initially diagnosed with severe dysplasia or CIS progressed to invasive cancer over time and repeated excisions. This rate is within the range reported by previous studies (15.7 – 54%), which had different timeframes and types of intervention.11-13 It is also higher than the 5% rate of progression to invasive disease from severe dysplasia or CIS noted in the study by Schweinfurth et al. that used a similar cohort of patients as the present study.7 Discrepancy in transformation rate is likely the result longer follow-up, and the use of a different analytic approach that incorporated all longitudinal pathology results from each patient rather than only the first and last pathologies.
Dysplasia May Not Be Linear Process
It was also observed from Figure 2 that dysplasia progression is not a linear process. The grade of dysplasia worsened and improved longitudinally in many patients. While it is possible that excisions affected these fluctuations, it is also possible that these are the natural history of dysplasia. There is evidence from other fields that dysplasia may resolve spontaneously without treatment, which is in contradistinction to our understanding that the process of malignant transformation is linear, progressing from mild to severe dysplasia, CIS and ultimately to invasive cancer. For example, 50% and 62% of patients with cervical cytologically diagnosed mild and moderate dysplasia respectively have been observed to undergo spontaneous regression without major treatment.14,15 Barrett's esophagus has also been observed to spontaneously regress in some patients without the need for antireflux surgery.16
Another possible explanation for the non-linear progression of dysplasia could relate to variability in the reading pathologists or sampling error. In this study, all specimens were examined by pathologists specialized in head and neck diseases; however, even within this group there is likely some inter-rater variability. In fact, 30 different pathologists contributed reports in the 19-year period of this study. While inter-rater reliability of reading pathologists was not within the scope of the study, a post hoc analysis did not demonstrate intra-rater propensity toward reporting more or less malignant transformation or any particular pathologic grade. Fleskens et al. have suggested it may be advisable for more than one pathologist to evaluate suspect cases of dysplasia or carcinoma.17 This practice should be given careful consideration before recommending more invasive surgery or radiation therapy.
Limitations
This study harnesses 19-years of experience treating patients with vocal fold dysplasia with serial full-thickness excision and provides interesting insights into the disease process. It does have limitations beyond those inherent in retrospective reviews. In particular, there was not a control group, which limits the ability to compare this serial excisional approach to any other treatment modality. Therefore, the comparative effectiveness of this technique for treatment of dysplasia cannot be assessed. Furthermore, insights into the natural history of this condition can only be made if the excisional biopsies did not alter the disease process. While assumed, this cannot be definitely confirmed without inclusion of a watchful waiting (i.e. no treatment) or natural history comparison group. Finally, current smoking status and intensity during this period was inconsistently documented; therefore, smoking status was dichotomized in ever/never smokers for analysis. Despite these issues, the present study does provide a window into the longitudinal disease course in this unique patient population and into the role the role of serial full-thickness excision of vocal fold dysplasia.
CONCLUSION
Vocal fold dysplasic leukoplakia is often recurrent even after excision. Data does not support the hypothesis that serial full-thickness excisions decreases dysplasia grade. Vocal fold dysplasia appears to be a dynamic, time-varying process where dysplasia grade fluctuates in severity over time. Lesions may regress or progress to higher pathologic states. Higher-grade dysplasia is more likely to undergo malignant transformation and should be closely surveilled. Full-thickness epithelial biopsies are an important diagnostic modality in the surveillance of dysplastic leukoplakia.
ACKNOWLEDGEMENTS
Dr. Francis is supported by NIH NIDCD K23DC013559. The project described was supported by CTSA award No UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflict of Interest: None by any author
Financial Disclosures: None by any author
This research was presented during the Triological Society Meeting at the Combined Otolaryngology Spring Meeting in Boston, MA, USA on April 24, 2015.
REFERENCES
- 1.Bouquot JE, Gnepp DR. Laryngeal precancer: a review of the literature, commentary, and comparison with oral leukoplakia. Head Neck. 1991;13:488–497. doi: 10.1002/hed.2880130604. [DOI] [PubMed] [Google Scholar]
- 2.Gallo A, de Vincentiis M, Della Rocca C, et al. Evolution of precancerous laryngeal lesions: A clinicopathologic study with long-term follow-up on 259 patients. Head Neck. 2001;23:42–47. [PubMed] [Google Scholar]
- 3.Ricci G, Molini E, Faralli M, Simoncelli C. Retrospective study on precancerous laryngeal lesions: long-term follow-up. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2003;23:362–367. [PubMed] [Google Scholar]
- 4.Zeitels SM, Casiano RR, Gardner GM, et al. Management of common voice problems: Committee report. Otolaryngol Head Neck Surg. 2002;126:333–348. doi: 10.1067/mhn.2002.123546. [DOI] [PubMed] [Google Scholar]
- 5.Sadri M, McMahon J, Parker A. Management of laryngeal dysplasia: a review. Eur Arch Otorhinolaryngol. 2006;263:843–852. doi: 10.1007/s00405-006-0078-y. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Schweinfurth JM, Powitzky E, Ossoff RH. Regression of laryngeal dysplasia after serial microflap excision. Ann Otol Rhinol Laryngol. 2001;110:811–814. doi: 10.1177/000348940111000902. [DOI] [PubMed] [Google Scholar]
- 8.Barnes LE,J, Reichard P, Sidransky D. Pathology and Genetics of Head and Neck Tumours (IARC WHO Classification of Tumours). Lyon. 2005 [Google Scholar]
- 9.Courey MS, Garrett CG, Ossoff RH. Medial microflap for excision of benign vocal fold lesions. Laryngoscope. 1997;107:340–344. doi: 10.1097/00005537-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Isenberg JS, Crozier DL, Dailey SH. Institutional and comprehensive review of laryngeal leukoplakia. Ann Otol Rhinol Laryngol. 2008;117:74–79. doi: 10.1177/000348940811700114. [DOI] [PubMed] [Google Scholar]
- 11.Miller AH, Fisher HR. Clues to the life history of carcinoma in situ of the larynx. Laryngoscope. 1971;81:1475–1480. doi: 10.1288/00005537-197109000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Stenersen TC, Hoel PS, Boysen M. Carcinoma in situ of the larynx: an evaluation of its natural clinical course. Clinical otolaryngology and allied sciences. 1991;16:358–363. doi: 10.1111/j.1365-2273.1991.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 13.Plch J, Par I, Navratilova I, Blahova M, Zavadil M. Long term follow-up study of laryngeal precancer. Auris, nasus, larynx. 1998;25:407–412. doi: 10.1016/s0385-8146(98)00041-8. [DOI] [PubMed] [Google Scholar]
- 14.Nasiell K, Roger V, Nasiell M. Behavior of mild cervical dysplasia during long-term follow-up. Obstetrics and gynecology. 1986;67:665–669. doi: 10.1097/00006250-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Nasiell K, Nasiell M, Vaclavinkova V. Behavior of moderate cervical dysplasia during long-term follow-up. Obstetrics and gynecology. 1983;61:609–614. [PubMed] [Google Scholar]
- 16.Weston AP, Badr AS, Hassanein RS. Prospective multivariate analysis of factors predictive of complete regression of Barrett's esophagus. The American journal of gastroenterology. 1999;94:3420–3426. doi: 10.1111/j.1572-0241.1999.01603.x. [DOI] [PubMed] [Google Scholar]
- 17.Fleskens SA, Bergshoeff VE, Voogd AC, et al. Interobserver variability of laryngeal mucosal premalignant lesions: a histopathological evaluation. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:892–898. doi: 10.1038/modpathol.2011.50. [DOI] [PubMed] [Google Scholar]