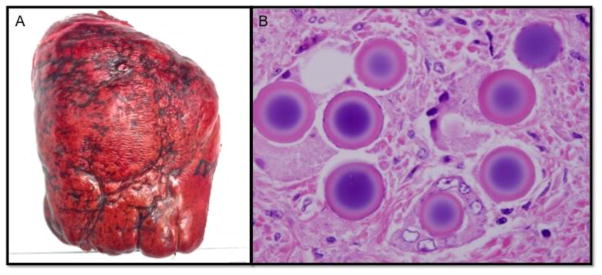
An obese 55-year-old woman with nonalcoholic fatty liver disease presented seven years after resection of a T3N1 ileal carcinoid tumor with an elevated chromogranin A, multifocal metastatic disease to the liver, and carcinoid syndrome. She underwent right hepatic artery Yttrium-90 (Y90) radioembolization, followed a month later by selective Y-90 treatment to segment IV. She then presented to our clinic 10 months later remaining symptomatic with flushing, diarrhea, anxiety, myalgia, pain, and persistent night sweats despite sandostatin administration. At least eleven tumors were identified in the right lobe of the liver and three in segment IV on liver specific imaging. These lesions were stable over a year with no new lesions. At exploration, there was marked hypertrophy of the left lateral segment due to the yttrium-90 treatment of segments IV–VIII, corresponding with preoperative volumetrics predicting a functional liver remnant (FLR) of 40% after extended right hepatectomy.1 The right lobe and segment IV were fibrotic, hard, and visibly damaged (Panel A). The gland had a thick, fibrotic capsule and the parenchyma was dense, inflexible, and difficult to dissect, consistent with the previously reported morbidity of these operations.2 Extended right hepatectomy was performed. Final pathology demonstrated 15 foci of metastatic well-differentiated neuroendocrine carcinoma that were negative for necrosis, as was expected given her continued symptoms despite radioembolization. Numerous amorphous spheres, frequently in clusters, were present in segments IV–VIII in vessels and approximating tumors consistent with prior Y90 radioembolization (panel B). The patient had an uneventful postoperative recovery and remains symptom free on follow-up.
Treatment options for metastatic tumors to the liver have increased in recent years and currently include radioembolization in selected patients.3 Surgical cytoreduction and complete metastasectomy continue to offer improvement in symptoms, quality of life, and survival in patients with neuroendocrine liver metastases, however, hepatectomy after radioembolization is unique and carries increased morbidity/mortality,2 likely due to Y90-induced liver fibrosis. We demonstrate images of fibrotic yttrium-90 radiation affected liver (A), and histological sections of radioembolic microbeads in blood vessels and distributed around resected tumors (B).
Figure.
(A) Resected liver segments IV–VIII demonstrate significant Y90-induced radiation fibrosis and damage that is unique to post radioembolized liver and complicates resection. (B) Histopathologic sections revealed amorphous microspheres ranging from 25 to 55 microns in diameter generally within vascular spaces and usually in clusters. Oil immersion revealed the microspheres to demonstrate a refractile appearance and to have a basophilic center that often was surrounded by a white halo with an eosinophilic periphery.
References
- 1.Teo JY, Allen JC, Jr, Ng DC, Choo SP, Tai DW, Chang JP, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2015 doi: 10.1111/hpb.12490. [DOI] [PubMed] [Google Scholar]
- 2.Henry LR, Hostetter RB, Ressler B, Bowser I, Yan M, Vaghefi H, et al. Liver resection for metastatic disease after y90 radioembolization: a case series with long-term follow-up. Annals of surgical oncology. 2015;22(2):467–74. doi: 10.1245/s10434-014-4012-z. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell JE, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery. 2016;159(1):320–35. doi: 10.1016/j.surg.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]