Abstract
Hemorrhage is one of the leading causes of death in patients with trauma. We recently demonstrated that resveratrol can improve cardiac function and prolong life following severe hemorrhagic injury (HI) in a rat model. The present work is focused on determining changes in NF-κb dependent gene expression in the heart and the systemic cytokine milieu following HI and the effect of resveratrol treatment. The results indicate an increase in phosphorylated NF-κb in the heart with a concomitant increase in the expression of NF-κb dependent genes following HI. There was also a significant increase of systemic cytokine levels, both pro and anti-inflammatory, following HI and resolution when treated with resveratrol. This study demonstrates the potential role NF-κb has in the physiological response to HI and the effectiveness of resveratrol in reducing immune activation.
Keywords: NFκb, trauma, hemorrhage, inflammation, shock
INTRODUCTION
Trauma is the number one cause of death in the United States, in the age group of 1 – 46 years and third cause of death worldwide [1]. Hemorrhage accounts for approximately 40% death associated with trauma [2]. Understanding the mechanisms following hemorrhagic shock is critical in reversing compromised molecular pathways and to prolonging survival.
Hemorrhagic shock is characterized by a strong inflammatory response that conceivably may be critical for survival following the insult [3]. However a sustained and exacerbated inflammatory response may be deleterious to the outcome following hemorrhagic injury (HI). NF-κb plays a major role in promoting transcription of a number of inflammatory genes [4].
Recent studies from our laboratory and other laboratories have demonstrated a profound salutary effect of resveratrol on organ function in experimental models of HI [5–8]. Resveratrol is a naturally occurring polyphenol found in various plants and fruits, including grapes. Resveratrol is an antioxidant and has been shown to improve mitochondrial function and reduce inflammation. Studies have shown that resveratrol activates SIRT1, a sirtuin family of proteins, which deacetylates a number of critical proteins including NF-κb [9, 10]. Phosphorylation and acetylation of the p65 subunit are known to regulate the function of NF-κb [10].
Our continued studies demonstrated that resveratrol can improve survival and prolong life following HI even in the absence of resuscitation fluid [11]. However the role of resveratrol on the inflammatory response following HI or the role of NF-κb regulated genes is not well defined. In this study we determined the cytokine expression changes in the plasma and heart following HI, and with resveratrol treatment.
METHODS
Animals
Male Sprague Dawley rats (250–350g) were purchased from Charles River Laboratory (Wilmington, MA, USA) and housed in GRU animal facility. HI procedure and hemodynamics were described before [11]. Briefly, HI was induced by bleeding 60% of the circulating blood volume in 45 minutes and maintaining the animals at low blood pressure (40+5 mm Hg) for another 45 minutes followed by resuscitation as described below. Resveratrol (10 mg/Kg body weight) or vehicle (DMSO) was administered 10 minutes after the start of resuscitation, intravenously. All the experiments were in accordance with Institutional Animal Care and Use Committee at Georgia Regents University.
2-hour study
In this group of animals, following HI, resuscitation was carried out with Ringers Lactate, two times the volume of shed blood. After resuscitation, the animals were observed for two hours, left ventricular function was measured, sacrificed and heart tissue and blood samples collected for molecular analysis.
3-hour study
The animals in this group were resuscitated with Ringers Lactate, two times the volume of shed blood. However, after resuscitation, the animals were observed for 3 hours, sacrificed and heart tissue and blood were collected for further study.
Real Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from heart tissue using Total RNA isolation mini kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s protocol (Qiagen Inc, Valencia, CA) and cDNA synthesized (Agilent Technologies, CA). The sequence of the primers used were: NFkB p65: Forward: CCTCATCTTTCCCTCAGAGC, Reverse: CGCACTTGTAACGGAAACGC 3”; IL-2: Forward: ACTTCAAGCCCTGCAAAGGA, Reverse: GTTTCAATTCTGTGGCCTGCTT; IL-6: Forward: GAGCCCACCAGGAACGAAA, Reverse: AACTGGCTGGAAGTCTCTTGC; IL-10: Forward: TGCGACGCTGTCATCGATTT, Reverse: GTAGATGCCGGGTGGTTCAA; MIP-1α, Forward: CTGCCAAGTAGCCACATCCA, Reverse: GGAATGTGCCCTGAGGTCTT; TNF-α: Forward: ACGTCGTAGCAAACCACCAA; Reverse: GCAGCCTTGTCCCTTGAAGA; β-actin: Forward: AGTACCCCATTGAACACG; Reverse: AATGCCAGTGGTACGACC. Quantitative SYBR green (Biorad, MA) real time PCR was performed using Stratagene Mx3000P (Agilent Technologies) real time PCR instrument with primer sets for each gene and normalized to beta actin. The results are expressed after normalizing to the values obtained for samples in sham group.
Western Blot Analysis
The heart tissues were homogenized and the proteins resolved on a 10% SDS polyacrylamide gel, transferred to PVDF membrane, blocked using 5% (w/v) non-fat dried milk and then incubated with respective antibodies overnight at 4°C or for 1 h at room temperature (RT). The membranes were probed with antibodies to NF-κb (Cat # 8242; Cell Signaling, MA), and p-NF-κb (Ca t# 3033; Cell Signaling Technology, Danvers, MA). The membranes were subsequently washed and incubated with horseradish peroxidase conjugated secondary antibody for 1h at RT and developed using enhanced chemilumiscence (Cat# NEL113001EA, Perkin Elmer, MA). Protein bands developed on X-ray films were quantified using the ImageJ software (Wayne Rasband, NIH).
Plasma Cytokine Analysis
Cytokine levels in plasma were quantified by Rat Cytokine I Array (Aushon Biosystem, Billerica, MA, USA) by a multiplex method.
Statistics
Statistical analysis were performed by applying Mann-Whitney U test (plasma) or non-parametric t-test with Welch’s correction using GraphPad software (La Jolla, CA).
RESULTS AND DISCUSION
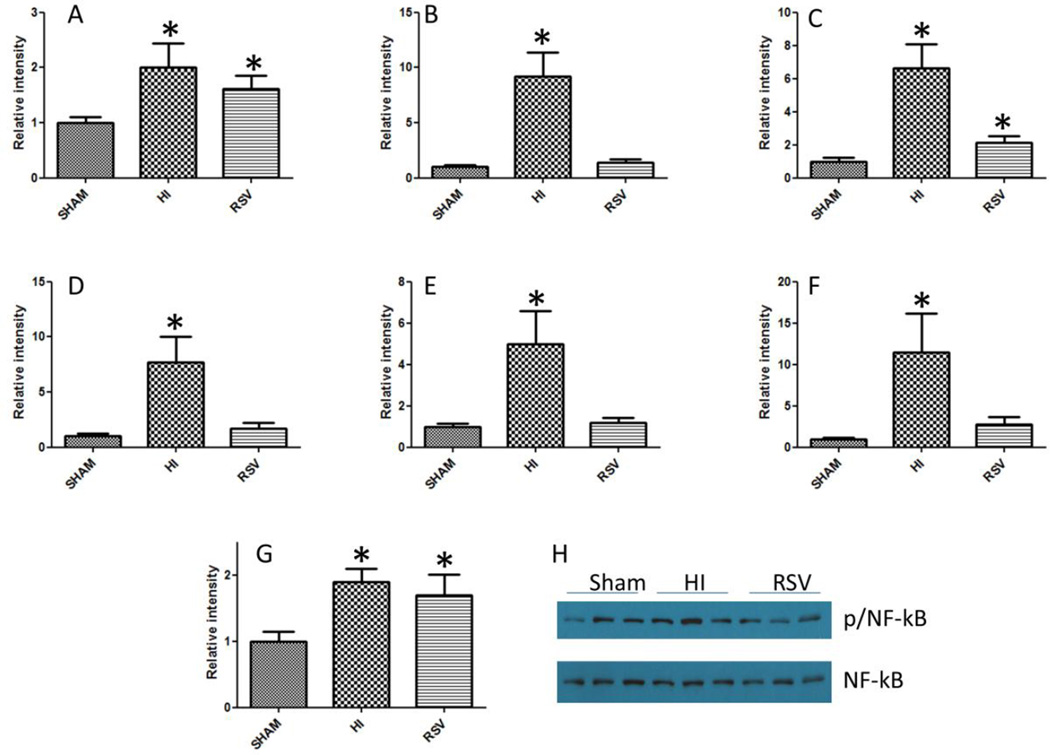
HI leads to declined organ function and mortality which are significantly improved by resuscitation in the presence of small molecule activators of SIRT1, resveratrol and SRT1720 [5, 11]. In order to understand the events leading to declined cardiac function, we tested the gene expression level of NF-κb, a transcription factor that promotes the expression of inflammatory genes and a panel of NF-κb-regulated cytokines in the heart following HI and resuscitation, by real time PCR (Figure 1). NF-κb gene expression was significantly increased following HI whereas the reduction following treatment with resveratrol was not significant. The ratio of phosphorylated p65 subunit of NF-κb (p-NF-κb) to the unphosphorylated form showed a trend similar to the p65 gene expression (Figure 1G and H). All the cytokines tested, IL-2, IL-6, IL-10, TNF-α and the chemokine MIP-1α demonstrated several fold increase in expression following HI and a sharp decline in resveratrol group (Fig 1B-F).
Figure 1. NF-κb and NF-κb regulated cytokine expression in the heart following HI.
NF-κb and NF-κb dependent cytokine gene expression changes following HI and with resveratrol treatment were determined by SYBR green real time PCR amplification of NF-κb p65 (A), IL-2 (B), IL-6 (C), IL-10 (D), TNF-α (E), and MIP-α (F). Panel G shows densitometric data on the ratio of band intensities of p-NF-κb/NF-κb as obtained by Western blot (Panel H). All quantitative results are averages of three sets of experiments. n=5-7; *=p < 0.05; bars indicate mean±SEM.
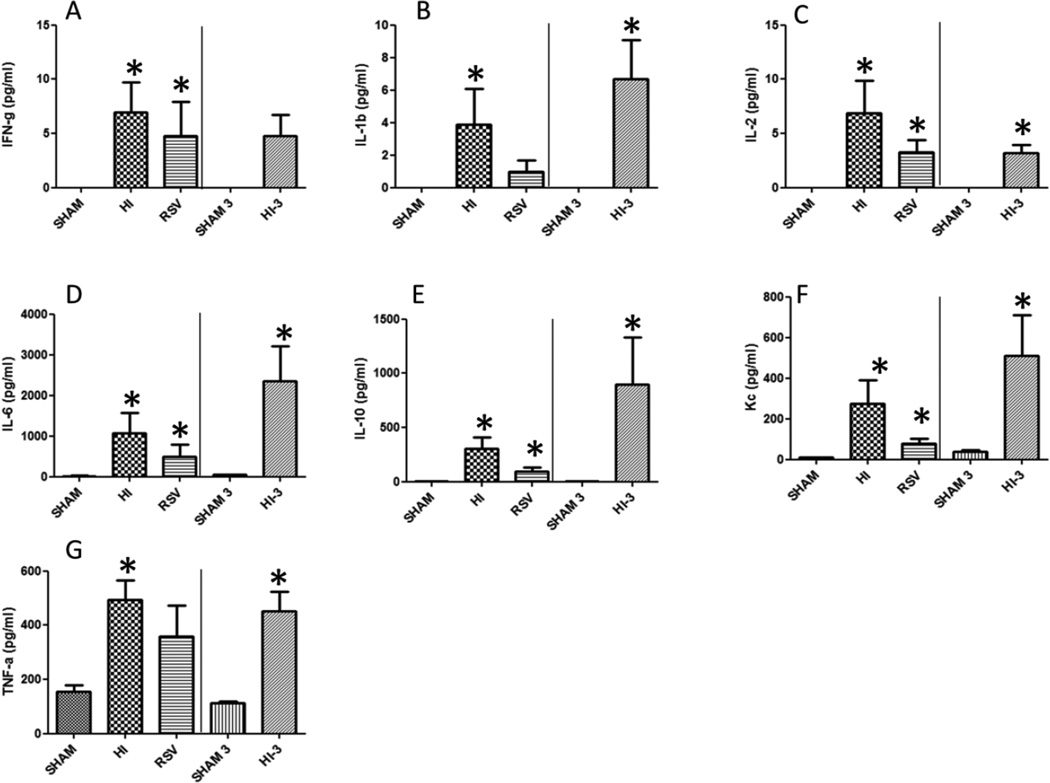
We further tested the plasma levels for a battery of inflammatory and non-inflammatory cytokines using a multiplex cytokine array. These cytokines were IFN-γ, IL-1β, IL-2, IL-6, IL-10, KC and TNF-α. HI induced a significant increase in the plasma levels of all the tested cytokines (Fig. 2A-G). As there was a rapid increase in plasma cytokines following HI at 2 hours, we tested these cytokines in a subset of animals at 3 hours following HI procedure to determine whether the cytokine levels had plateaued or continue to increase. The rapid increase observed by 2 hours was not continued in a similar trajectory, instead some cytokines demonstrated a plateau or decrease at 3 hours demonstrating that testing cytokine parameters at two hours following hemorrhagic injury is optimal.
Figure 2. Plasma cytokine profile following HI.
Plasma cytokine profiles were determined using a multiplex method in sham, HI and RSV groups at 2 hours following HI and resuscitation; or sham and HI (sham-3 and HI-3) at 3 hours following HI and resuscitation. Values represent average of duplicates. n=6 in each group; *=p < 0.05; bars indicate mean±SEM.
In the plasma, IFN-γ was undetectable in sham, and following HI the levels rose to 6.9 and 4.8 pg/ml at 2 hours and 3 hours respectively. However, resveratrol administration did not bring down the elevated IFN-γ levels (Fig. 2A) significantly. Plasma levels of IL-1β (Fig. 2B) and IL-2 (Fig. 2C) were also undetectable in sham. IL-1β increased significantly in 2 hour and 3 hour plasma samples (3.89 vs 6.68pg/ml). Resveratrol treatment reduced IL-1β levels to 0.97 pg/mL (2 hour). IL-2 levels increased significantly in both 2 hour (6.9 pg/ml) and 3 hour (3.17 pg/ml) samples. Resveratrol treatment reduced the 2 hour plasma concentration of IL-2 to 3.24 pg/ml (Fig. 2C). HI induced a 73 fold (14.5 to 1064 pg/ml) increase in the level of plasma IL-6 after 2 hours and 46 fold (50 to 2360 pg/ml) increase after 3 hours compared to sham. RSV treatment was able to diminish the 2 hour plasma level to 34 fold (493 pg/ml) (Fig. 2D). Anti-inflammatory IL-10 was almost undetectable in the sham animals and HI resulted in very high systemic levels at both 2 and 3 hours (300 and 895 pg/ml, respectively) and was significantly reduced in resveratrol treated animals (300 vs 92 pg/ml) (Fig. 2E). In a similar manner, keratinocyte-derived cytokine (KC) levels showed significant increase in plasma levels at 2 and 3 hours compared to sham. RSV treatment resulted in a substantial decrease in KC levels (Fig. 2F). TNF-α levels were also markedly elevated at 2 and 3 hours following HI. Though resveratrol treatment reduced plasma TNF-α levels, in this experiment the reduction did not achieve significance (Fig. 2G).
HI is associated with production of a number of inflammatory cytokines [12, 13]. In the current study we found a sustained increase in the plasma levels of IFN-γ, IL-1β, IL-2, IL-6, IL- 10 and TNF-α following HI. Sperry et al have shown that increase in IL-6 levels in HI correlates with multiple organ failure (MOF); male gender with higher IL-6 serum levels being more prone to MOF [14]. In our study in male SD rats, IL-6 level in plasma remained high at 2h and 3h post-resuscitation and was reduced with RSV administration consistent with the better outcome after injury [11]. This is consistent with a previous study that showed increased hepatic myeloperoxidase activity, cytokine-induced neutrophil chemoattractant (CINC)-1, CINC-3, ICAM-1, and IL-6 levels [6].
In our study, the plasma levels of IL-1β and IL-2 were elevated following HI and showed a significant reduction with RSV suggesting an attenuated pro-inflammatory response. Immunomodulatory cytokine IL-10 displayed differential effects in lung and liver with its deficiency augmenting acute lung but not liver injury following hemorrhagic shock [15]. However in our study the plasma samples of HI rats were presented with higher IL-10 levels when compared to sham.
The alluring finding in the study is that although we observed an increased level of NF-κb p65 gene expression as well as the ratio of the phosphorylated transactivator subunit p65 with a concomitant increase in the expression of cytokines that are promoted by NF-κb, the reduction in the expression of cytokines with resveratrol treatment was not followed by similar change in NF-κb. At least two different reasons may be attributed to this, either that resveratrol mediated reduction in inflammatory response is NF-κb independent or resveratrol-activated SIRT1 deacetylates the p65 subunit and therefore phosphorylation alone is not a marker for its activation. These possibilities are being currently addressed in our laboratory. Additionally both proinflammatory and anti-inflammatory cytokines showed similar trend following hemorrhagic shock. In conclusion, a significant increase in NF-κb and NF-κb-dependent cytokines were observed following hemorrhagic shock and resveratrol treatment resulted in significant reduction of the tested cytokines.
Highlights.
-
-
Increased expression and phosphorylation of NF-kb p65 subunit was observed following hemorrhagic shock in a rat model.
-
-
Severe hemorrhage leads to exacerbated systemic immune response.
-
-
The study also demonstrated increased expression of NF-kb dependent cytokine genes in the heart following hemorrhagic shock.
-
-
Resveratrol treatment attenuated upregulation of inflammatory cytokines following hemorrhagic injury.
Acknowledgments
RR acknowledges financial support from the National Institute of General Medical Sciences (R01 GM 101927) and laboratory start up assistance from the Georgia Regents University, Augusta, GA.
Abbreviations
- HI
hemorrhagic injury
- MOF
multiple organ failure
- RSV
resveratrol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention NCfIPaC. Web-based Injury Statistics Query and Reporting System (WISQARS) 2015 [Google Scholar]
- 2.Curry N, Hopewell S, Doree C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Critical care (London, England) 2011;15:R92. doi: 10.1186/cc10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namas R, Ghuma A, Hermus L, Zamora R, Okonkwo DO, Billiar TR, et al. The acute inflammatory response in trauma / hemorrhage and traumatic brain injury: current state and emerging prospects. Libyan J Med. 2009;4:97–103. doi: 10.4176/090325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartuzi P, Hofker MH, van de Sluis B. Tuning NF-kappaB activity: a touch of COMMD proteins. Biochimica et biophysica acta. 2013;1832:2315–2321. doi: 10.1016/j.bbadis.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Jian B, Yang S, Chaudry IH, Raju R. Resveratrol improves cardiac contractility following trauma-hemorrhage by modulating Sirt1. Molecular medicine (Cambridge, Mass) 2012;18:209–214. doi: 10.2119/molmed.2011.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu HP, Yang SC, Lau YT, Hwang TL. Role of Akt-dependent up-regulation of hemeoxygenase-1 in resveratrol-mediated attenuation of hepatic injury after trauma hemorrhage. Surgery. 2010;148:103–109. doi: 10.1016/j.surg.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Guan Y, Widlund AL, Becker LB, Baur JA, Reilly PM, et al. Resveratrol ameliorates mitochondrial dysfunction but increases the risk of hypoglycemia following hemorrhagic shock. The journal of trauma and acute care surgery. 2014;77:926–233. doi: 10.1097/TA.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell RD, Swet JH, Kennedy KL, Huynh TT, Murphy MP, McKillop IH, et al. MitoQ modulates oxidative stress and decreases inflammation following hemorrhage. The journal of trauma and acute care surgery. 2015;78:573–579. doi: 10.1097/TA.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 9.Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochimica et biophysica acta. 2015 doi: 10.1016/j.bbadis.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni SS, Canto C. The molecular targets of resveratrol. Biochimica et biophysica acta. 2015;1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Ayub A, Poulose N, Raju R. Resveratrol Improves Survival and Prolongs Life Following Hemorrhagic Shock. Molecular medicine (Cambridge, Mass) 2015;21:305–312. doi: 10.2119/molmed.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brochner AC, Toft P. Pathophysiology of the systemic inflammatory response after major accidental trauma. Scand J Trauma Resusc Emerg Med. 2009;17:43. doi: 10.1186/1757-7241-17-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziraldo C, Vodovotz Y, Namas RA, Almahmoud K, Tapias V, Mi Q, et al. Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies. PloS one. 2013;8:e79804. doi: 10.1371/journal.pone.0079804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperry JL, Friese RS, Frankel HL, West MA, Cuschieri J, Moore EE, et al. Male gender is associated with excessive IL-6 expression following severe injury. The Journal of trauma. 2008;64:572–578. doi: 10.1097/TA.0b013e3181650fdf. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 15.Kobbe P, Lichte P, Schreiber H, Reiss LK, Uhlig S, Pape HC, et al. Inhalative IL-10 attenuates pulmonary inflammation following hemorrhagic shock without major alterations of the systemic inflammatory response. Mediators Inflamm. 2012;2012:512974. doi: 10.1155/2012/512974. [DOI] [PMC free article] [PubMed] [Google Scholar]