Abstract
Background
There is increasing recognition of the role of B cell dysfunction in HIV pathogenesis, but little is known about how these perturbations may influence responses to vaccinations.
Methods
Healthy controls (n = 16) and antiretroviral therapy (ART)-treated aviremic HIV-infected subjects (n = 26) receiving standard-of-care annual influenza vaccinations were enrolled in the present study. Total bacterial 16S rDNA levels were assessed by quantitative polymerase chain reactions in plasma. Serologic responses were characterized by ELISA, hemagglutination inhibition assay (HI), and microneutralization, and cell-mediated responses were assessed by ELISPOT (antigen-specific IgG+ antibody-secreting cells (ASCs)) and flow cytometry at pre-vaccination (D0), day 7-10 (D7) and day 14-21 (D14) post-vaccination.
Results
Decreased peripheral CD4+ T cell absolute counts and increased frequencies of cycling and apoptotic B cells were found at baseline in HIV-infected subjects relative to healthy controls. In healthy controls, post-vaccination neutralizing activities were related to the frequencies of vaccine-mediated apoptosis and cycling of B cells, but not to CD4+ T cell counts. In patients, both baseline and post-vaccination neutralizing activities were directly correlated with plasma level of bacterial 16S rDNA. However, overall vaccine responses including antibody titers and fold changes were comparable or greater in HIV-infected subjects relative to healthy controls.
Conclusion
B cell function correlates with measures of recall humoral immunity in response to seasonal influenza vaccination in healthy controls but not in ART-treated patients.
Keywords: HIV disease, B cells, antibody responses, influenza vaccine
Introduction
The progressive depletion of peripheral CD4+ T cells is the hallmark of immunodeficiency in HIV disease [1]. Loss of CD4+ T cells has been linked to persistent immune activation [2] and less so to the magnitude of HIV replication [3]. HIV infection and chronic immune activation also drive B cell perturbations characterized by loss of B cells especially memory B cells, B cell polyclonal activation, and impaired responses to some vaccines [4-6].
Seasonal influenza vaccination is the most effective method of preventing influenza infection, however, the efficacy of influenza vaccination is different in each individual. Assessing the predictors of immunogenicity of vaccines is difficult but essential in designing novel vaccines. The multiplicity of phenotypes and functions for each immune compartment accounts for the difficulties of defining the predictors of vaccination. Age [7], sex [8], activation-induced cytidine deaminase (AID) response [9], percentage of switched memory B cells [9], IL-21R expression on B cells [10,11], IL-28B [12], genes related to B cell proliferation and immunoglobulin production [13], and innate immune activation [14] have all been shown to correlate with the strength of vaccine responses. The exact determinants of seasonal influenza vaccine are not fully understood.
HIV infection is associated with a high incidence of influenza infection [15]. Annual seasonal influenza vaccination is recommended for all HIV-1-infected individuals to reduce influenza-related morbidity and mortality [16]. In HIV-infected patients, responses to influenza vaccination are frequently impaired [5,17-19]. CD4+ T cells, especially T follicular helper (Tfh) cells, B cells, innate immunity, sex, age, and the magnitude of viral replication have been shown to correlate with responses to influenza vaccination in HIV-infected patients [5,8,10,12-14,17,20-30]. In contrast, a study conducted by Siegrist et al. has shown that HIV-infected patients have higher memory responses following pandemic influenza vaccination [31]. It is clear that the influenza vaccine response is impaired in antiretroviral therapy (ART)-naïve and viremic patients [5,20]. However, whether aviremic ART-treated patients have reduced recall responses following serial annual vaccinations is not clear.
Our previous studies have shown that heightened microbial translocation plays a role in T cell and B cell activation in HIV disease [33][43]; therefore the level of microbial translocation was accessed in the current study. In addition, we performed comprehensive analyses of immune activation and potential correlates of immune responsiveness before and after influenza vaccination to determine whether serial annual vaccinations would uncover functional cellular or humoral differences between HIV-infected patients receiving successful ART and healthy HIV-negative volunteers.
Materials and Methods
Study subjects
This study was approved by the Institutional Review Board at Medical University of South Carolina. All participants provided written informed consents. In the present study, 16 healthy volunteers and 26 HIV+ ART-treated aviremic patients enrolled in the study (plasma HIV RNA < 50 copies/mL). The clinical characteristics of participants are shown in Table 1. All participants had received influenza vaccines the previous year (2012-2013); vaccination prior to that was not assessed. Participants who enrolled received the 2013-2014 influenza vaccine and blood was collected on day 0 (D0) prior to vaccination, days 7-10 (D7), and days 14-21 (D14) post-vaccination.
Table 1. Clinical characteristics and baseline immune parameters.
| HIV-(n = 16) | HIV+/ART treated (n = 26) |
P value | |
|---|---|---|---|
| Age | 38 (33-55) | 43 (32-51) | 0.87 |
| Sex (male/female) | 5/16 (31.3%) | 17/26 (65.4%) | 0.07 |
| CD4+ T cell counts | 771 (544-977) | 540 (366-722) | 0.03 |
| %annexin V+ CD4 | 19.0 (13.0-40.0) | 30.3 (19.8-38.5) | 0.2 |
| %CD38+ mCD4 | 14.3 (10.1-16.8) | 18.4 (11.3-22.9) | 0.33 |
| %B cells in PBMC | 7.7 (6.2-9.5) | 9.3 (6.9-11.3) | 0.25 |
| 16S rDNA (copie/μL) | 5.3 (0-34.7) | 18.2 (9.4-25.2) | 0.18 |
| Co-infection with HCV | 2/26 | ||
| Infection duration | |||
| 2-5 years | 5/26 | ||
| 5-10 years | 3/26 | ||
| > 10 years | 18/26 | ||
| AIDS-defining events | |||
| HIV associated malignancy | 2/26 | ||
| Opportunistic infections | 4/26 | ||
| Nadir CD4+ T cell counts | 294 (193-458) | ||
| NNRTI based therapy | 16/26 | ||
| PI based therapy | 10/26 |
Data are medians (interquartile ranges)
P value: comparisons of P value between controls and patients on D0
Vaccination
All subjects received one dose of the inactivated influenza vaccine trivalent type A and B (Fluvirin, Novartis Vaccines and Diagnostics Limited, Speke, Liverpool, US). The strains for the 2013-2014 season include two A subtype virus: A/Christchurch/16/2010 (H1N1); A/Texas/50/2012 (H3N2); and one B subtype virus, B/Massachusetts/2/2012. Vaccination was given intramuscularly to the participants during June 2013 and March 2014.
Flow cytometry
Plasma was separated by the centrifugation of EDTA-contained blood, and it was then aliquoted and stored at -80°C. Peripheral blood mononuclear cells (PBMCs) were isolated over a Ficoll-Hypaque cushion. PBMCs were used for annexin V assays. Blood samples were used for all other assays except annexin V assays. For surface staining, antibodies (Abs) were incubated with blood or PBMCs at room temperature for 10 min. After surface staining of the blood, red cells were lysed, and the cells were washed and analyzed by flow cytometry. All cell-based assays were conducted using fresh blood or PBMCs. The fluorochrome-labeled monoclonal Abs used for flow cytometry included the following: Abs against CD4-PE, IgD-PEcy7, CD19-FITC, CD27-APCcy7, CD3-Percp, CD8-APCcy, CD45RO-PEcy7, ki67-FITC, CD38-APC, annexin V-FITC, and isotype control Abs (BD Pharmingen, San Jose, CA; and Biolegend, San Diego, CA). Stained cells were analyzed with a Guava 8HT flow cytometer (Millipore, Billerica, MA).
ELISPOT
ELISPOT plates were coated with the commercial influenza vaccine (5 μg/mL) and incubated overnight at 4°C prior to the day of sample collection. Total B cells were isolated from freshly prepared PBMCs using a negative selection kit (Miltenyi Biotec, Bergisch Gladbach, Germany; purity > 95%). After blocking, freshly isolated B cells (0.5 million cells/well) were added and cultured overnight at 37°C, and the plates were incubated with a biotinylated mouse anti-human IgA, IgG or IgM Fc Ab (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 2 h. Finally, the plates were incubated with alkaline phosphatase-conjugated streptavidin and developed with substrate. Spots were enumerated with an AID reader and software (San Diego, CA).
Hemagglutination inhibition (HI assay)
HI assays were conducted using H1N1, H3N2, or B virus as Ags and human type O RBCs as indicators. Serial diluted plasma was cultured with 4 hemagglutination units of live, egg-grown virus in plates for 15 min. Plates were washed and further incubated with 0.75% human type O RBCs for an additional 60 min. The plasma minimum effective concentrations were recorded as the lowest dilution at which hemagglutination was inhibited. Abs with an HI titer ≥ 1:40 were considered to be protective [32].
ELISA
Microtiter plates were coated with a 1:20 dilution of vaccine. Biotin-labeled goat anti-human IgG, IgM, or IgA (KPL, Gaithersburg, MD) was added to the microtiter wells and cultured with diluted plasma for 60 min. Amplex Red/Horseradish peroxidase (AR-HRP) and TMB substrate solution were used to detect binding. Absorbance was measured at 405 nm, and Ab concentrations were calculated in duplicates using a standard curve (12.5-1000 pg/mL).
Microneutralization assay
A standard plaque reduction for viral microneutralization was analyzed. In brief, heat-inactivated plasma was cultured with 100 median tissue culture infective doses (TCID50) of each influenza virus in a plate at 37°C in 5% CO2 for 2 h with or without TPCK-trypsin. MDCK cells (ATCC, Manassas, VA) were added (1.5 × 104 cells/well), cultured for 18-22 h and fixed. The cells were further cultured with anti-influenza A (Bio-Rad, Hercules, CA) or B (Abcam, Cambridge, MA) nucleoprotein mAbs for 1 h and HRP-conjugated anti-mouse IgG (Affinity Biosciences Inc., Amherst, NH) for 1 h. O-phenylenediamine dihydrochloride was then added, and cells were incubated for 5 min. Absorbance was measured at 490 nm. The endpoint titer in triplicates was expressed as the reciprocal of the highest dilution of plasma with an OD value of less than X, where X = [(the average of V wells) − (the average of C wells)]/2 + (the average of C wells).
Quantitative polymerase chain reaction (PCR) for measurement of bacterial 16S rDNA
DNA was extracted from 400 μL plasma using QIAamp UCP pathogen Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. A 20 μL amplification reaction consisted of 10 μL of 2× Perfecta qPCR ToughMix (Quanta, Gaithersburg, MD), 0.3 μmol/L forward and reverse primers, 0.175 μmol/L probe (338P: 5′-FAM-GCTGCCTCCCGTAGGAGT-BHQ1− 3′), and 5 μL of the template plasma DNA. Degenerate forward (8F: 5′-AGTTTGATCCTGGCTCAG-3′) and reverse (515R: 5′-GWATTACCGCGGCKGCTG-3′) primers were used to amplify DNA templates encoding 16S rRNA. The DNA was amplified in duplicate, and mean values were calculated. A standard curve was created from serial dilutions of plasmid DNA containing known copy numbers of the template. The reaction conditions for amplification of DNA were 95°C for 5 min, followed by 40 cycles at 95°C for 15 s and at 60°C for 1 min [33].
Statistical analysis
The differences in continuous measurements between the groups were compared by Mann-Whitney's U test (unpaired) or Wilcoxon matched-paired signed-rank test (paired). To explore associations between pairs of continuous variables, Spearman's rank correlation was used. Analysis was performed using SPSS software (version 16.01, Chicago, IL, USA). All tests were 2-sided, and a P ≤ 0.05 was considered to denote statistical significance.
Results
Baseline reduced CD4+ T cell counts, increased frequencies of cycling and apoptotic B cells in patients compared with controls
We first sought to characterize baseline differences in CD4+ T cell and B cell activation and apoptosis in our patient cohort in order to provide context for understanding any differences in relative responses to vaccination. The Ab response against influenza vaccination has previously been shown to be T cell-dependent [34]; therefore, we analyzed CD4+ T cells on D0 to characterize differences between our two groups. The CD4+ T cell absolute count was lower in HIV-infected subjects compared with controls (Table 1, P = 0.03). Chronic immune activation (%CD38+ T cells) and cell apoptosis are linked to CD4+ T cell depletion in HIV disease [35,36]. Next, we analyzed the binding of annexin V to CD4+ T cells and CD38 expression on memory CD4+ T cells (CD3+CD4+CD8-CD45RO+). We found that the median frequencies of annexin V+ CD4+ T cells were 19.0 (13.0-40.0) versus 30.3 (19.8-38.5) for controls and patients respectively, and the median frequencies of CD38+ on memory CD4+ T cells were 14.3 (10.1-16.8) versus 18.4 (11.3-22.9) for controls and patients respectively, but the differences were not statistically significant (P > 0.05, Table 1). Although level of bacterial 16S rDNA tended to be higher in aviremic ART-treated patients compared with controls, the difference did not achieve significance (Table 1).
B cells were also analyzed prior to vaccination. The frequency of B cells in PBMCs was similar in controls and patients (Table 1). Theoretically, the ability to produce Abs is linked to B cell survival and Ag-mediated activation; therefore, we assessed B cell apoptosis and cycling, and found that the baseline frequencies of apoptotic B cells (P = 0.003, Fig. 1A and 1B) and cycling B cells (P = 0.03, Fig. 1A and 1C) were elevated in patients compared with controls. These data reinforces the notion that successful ART treatment may normalize B cell frequency (Table 1) but not B cell hyperactivation in treated HIV-infected patients relative to controls.
Figure 1.
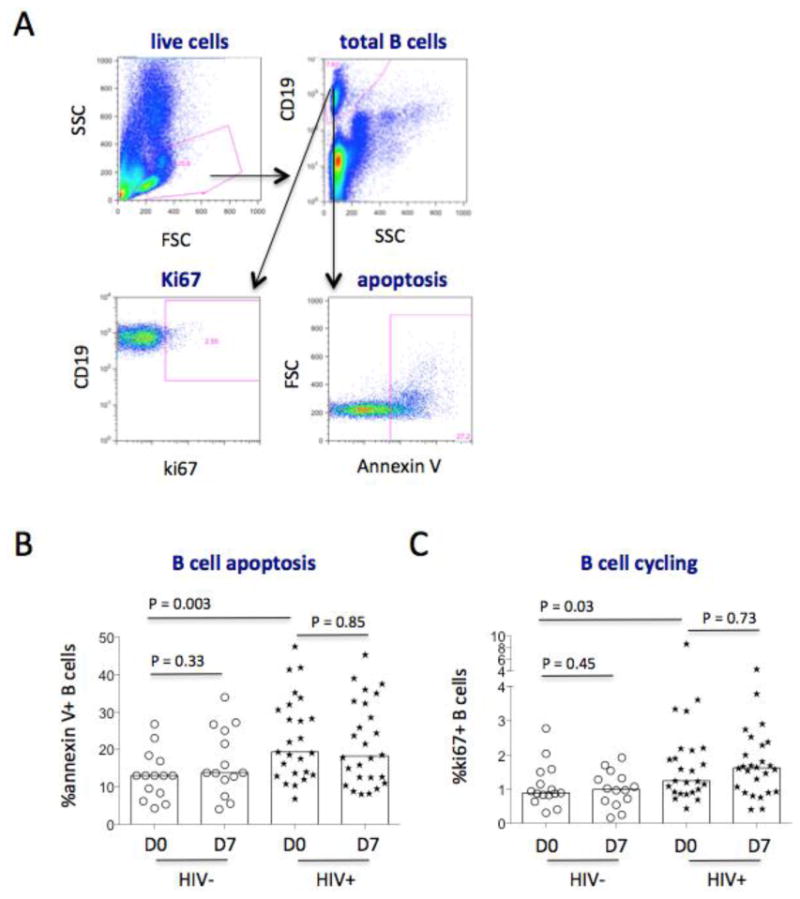
Gating strategies for B cells. Blood samples were tested for surface or intracellular staining by flow cytometry. PBMCs were tested for annexin V binding. (A) Representative dot plots, displaying the gating strategy used to assess the parameters of B cells. Live PBMCs were gated based on forward scatter (FSC) and side scatter (SSC). Next, total B cells were gated on CD19+ cells. B cell apoptosis and cycling expression were analyzed by percentages of annexin V binding and ki67+ B cells. (B) The median frequencies of annexin V binding B cells from controls (circles) and patients (stars) on D0 and D7 post-vaccination. (C) The median frequencies of ki67+ B cells from controls (circles) and patients (stars) on D0 and D7 post-vaccination.
Quantification of vaccine-specific antibodies in the plasma
To evaluate serologic Ab recall responses to vaccinations, we analyzed the levels of Ag-specific IgA, IgG, and IgM by ELISA (Fig. 2). Ag-specific IgG, IgM and IgA all increased following vaccination in both controls and patients (P < 0.05). As expected, Ag-specific IgG was consistently the predominant Ab that responded to seasonal influenza vaccination in both controls and patients compared with IgA and IgM. Unexpectedly, the plasma levels of Ag-specific IgG and IgM were higher in patients than in controls on D7 and D14 but not on D0 (Fig. 2A-2B), suggesting that patients had greater vaccine-mediated IgG and IgM Ab responses. There was no difference in the Ag-specific IgA level between patients and controls (Fig. 2C, P > 0.05). Furthermore, we found that vaccine-induced fold change (D14 versus D0) of Ag-specific IgM was higher in patients compared with those in controls, but vaccine-induced fold changes of Ag-specific IgA and IgG were similar in controls and patients (Fig. 2D).
Figure 2.
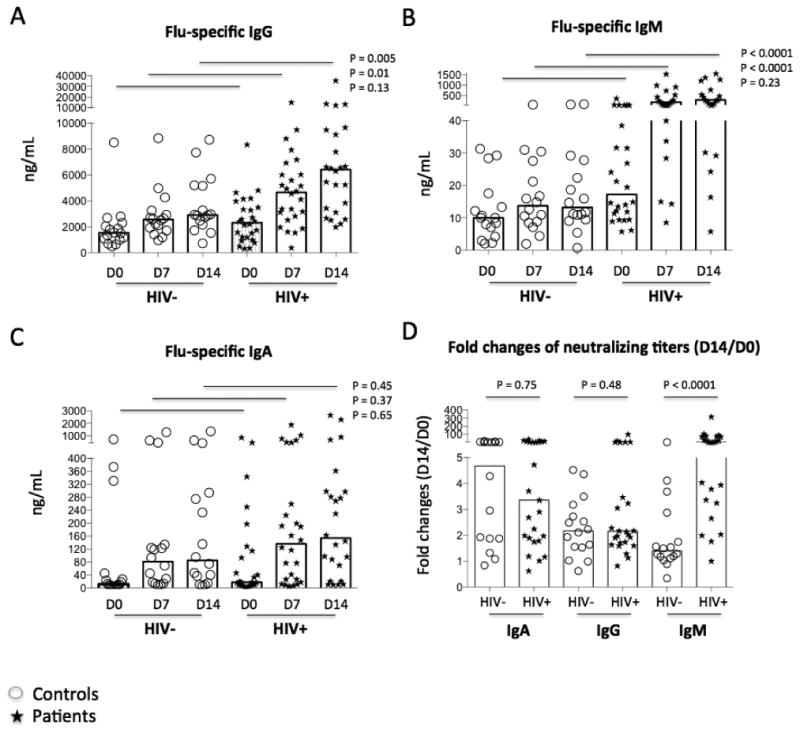
Plasma levels of antigen-specific antibody. Homologous vaccines were used as Ags to coat the plates. Plasma samples from controls (circles) and patients (stars) were diluted and tested. Median concentrations of vaccine Ag-specific IgG (A), IgM (B), and IgA (C) were shown on D0, D7 and D14 by ELISA. (D) Fold changes of Ab levels (D14 versus D0).
Antibody neutralization activities
To further determine the quality of the Ab response to vaccination, we performed viral microneutralization assays. The majority of neutralization activity to seasonal influenza vaccinations was specific to the pandemic virus H1N1 and IgG responses (Fig. 3A and data not shown). As expected, vaccination significantly induced neutralizing titers against H1N1, B and H3N2 strains in both controls and patients (P < 0.05). Consistent with the ELISA results, anti-H1N1 neutralizing activity was higher in patients than controls on D7 and D14 but not on D0 (Fig. 3A). Baseline level of anti-H3N2 neutralizing titer was higher in patients relative to controls, and vaccine-induced neutralizing titers against the H3N2 strain were higher in patients compared with controls on D0 and D7 but not on D14 (Fig. 3B). There was no difference in neutralizing titers against the B strain virus in patients and controls at each visit (Fig. 3C, P > 0.05). Furthermore, vaccine-induced fold changes (D14 versus D0) of neutralizing activity to H1N1, B, and H3N2 virus were similar in controls and patients (Fig. 3D). These results suggest that vaccine-induced neutralizing titers against H1N1 virus were greater in patients relative to controls.
Figure 3.
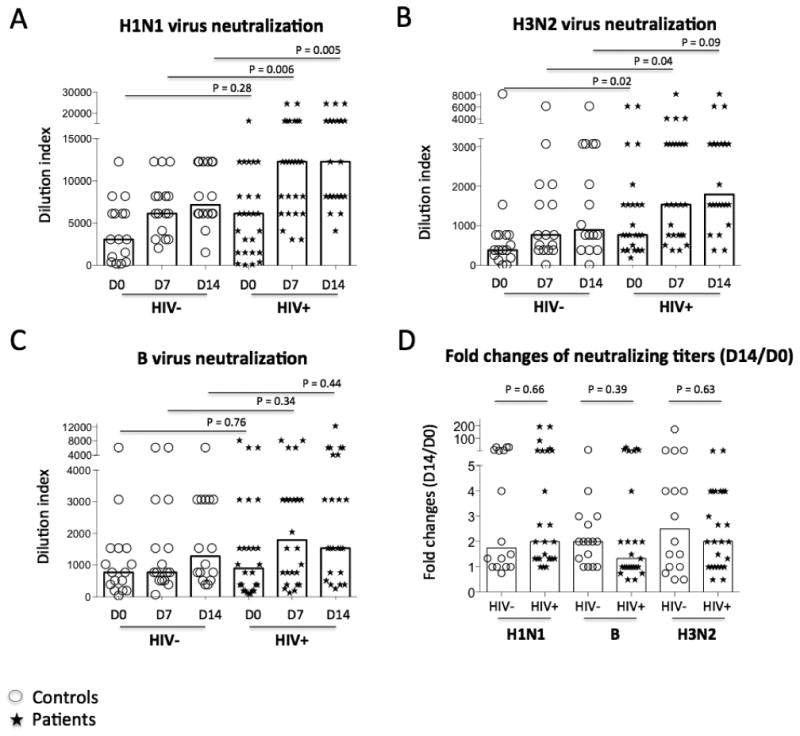
Strain-specific neutralizing Ab titers. Serial dilutions of plasma sample were tested for neutralizing activity against H1N1 (A), H3N2 (B), and B (C) strains. (D) Fold changes of neutralizing titers (D14 versus D0). Illustrated here are the median dilution indices providing 50% protection (IC50) in controls (circles) and patients (stars).
HI titers of Ag-specific recall Abs
HI titers were similar in subjects at each visit between controls and patients (P > 0.05, Fig. 4A). All participants had protective titers (≥ 1:40) against the type A H1N1 viruses at each visit (Fig. 4A). None had fold changes ≥ 4 against H1N1 virus in either controls or patients (data not shown). Moreover, 1 of 16 and 2 of 16 of the controls had fold changes ≥ 4 against the type B and H3N2 viruses respectively; 6 of 26 and 7 of 26 of the patients had fold change ≥ 4 against the type B and H3N2 viruses respectively (data not shown, D14 versus D0). When HI titers were evaluated by geometric mean (GMT), controls and patients had increased GMT titers post-vaccination except HI titers against H1N1 virus in patients (Fig. 4B-4D). There was no difference in HI titers between controls and patients. No correlations were found between the CD4+ T cell counts and HI titers against the H1N1, H3N2 or B influenza viruses in either controls or patients (P > 0.05).
Figure 4.
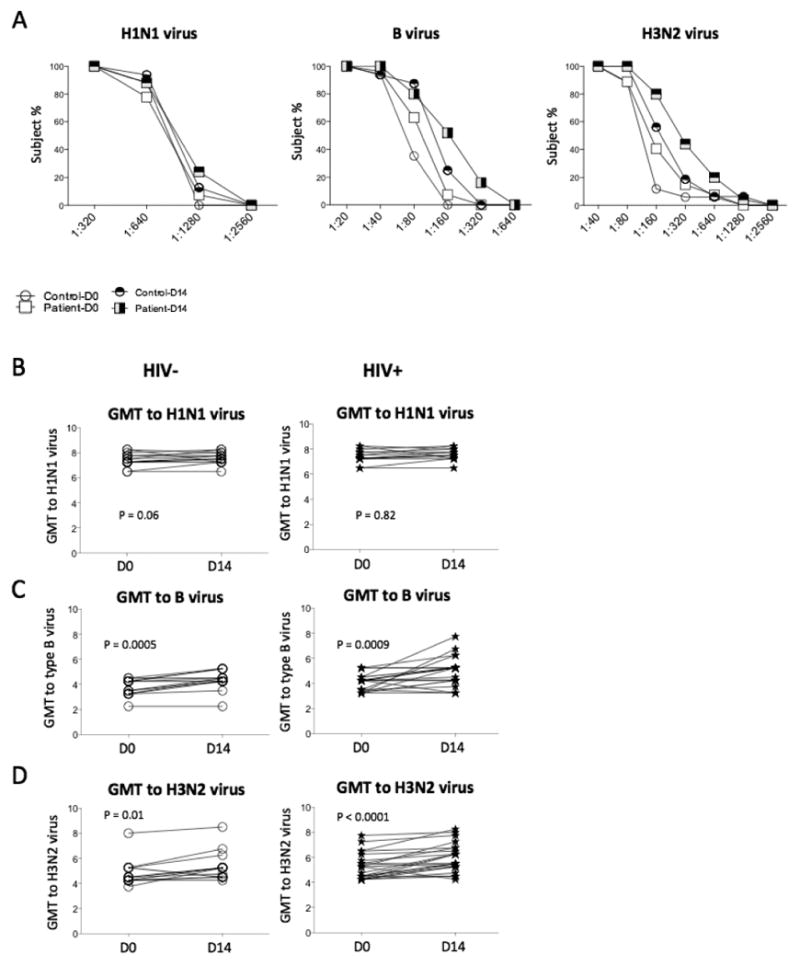
Ab responses to influenza vaccination by HI assay. Median frequencies of subjects attaining different dilutions of titers were shown on D0 and D14. Most participants achieved protective titers (≥ 1:40) at baseline. Geometric mean (GMT) Ab titers specific for H1N1 (B), B (C), and H3N2 (D) stains were determined at D0 and D14 by HI assay in plasma in controls and patients.
Similar frequencies of ASCs in response to vaccination in patients and controls
To further investigate vaccine-specific B cells (plasmablasts or plasma cells) that were circulating in the peripheral blood pre- and post-vaccination in vivo, ASCs were tested by ELISPOT among sorted B cells cultured with vaccine Ags without stimulation with cytokines or CpG ODNs. IgG+ ASCs were quantified on D0 and D7 (Fig. 5). D7 was chosen because it represented the peak transient responses of plasmablasts in the periphery in response to the influenza vaccine [5]. As expected, the frequencies of IgG+ ASCs on D0 were low but were clearly detectable in some donors (Fig. 5). Unexpectedly, we found that patients had comparable frequencies of IgG+ ASCs in response to vaccination compared with controls, suggesting that B cell responses to seasonal influenza vaccine Ag stimulation were restored in the setting of successful viral suppression by ART treatment. Furthermore, Ag-specific IgA+ and IgM+ ASCs were low or undetectable on D0 and D7 (data not shown), indicating that IgG-producing B cells are the predominant B cells involved in cellular recall responses in the periphery.
Figure 5.
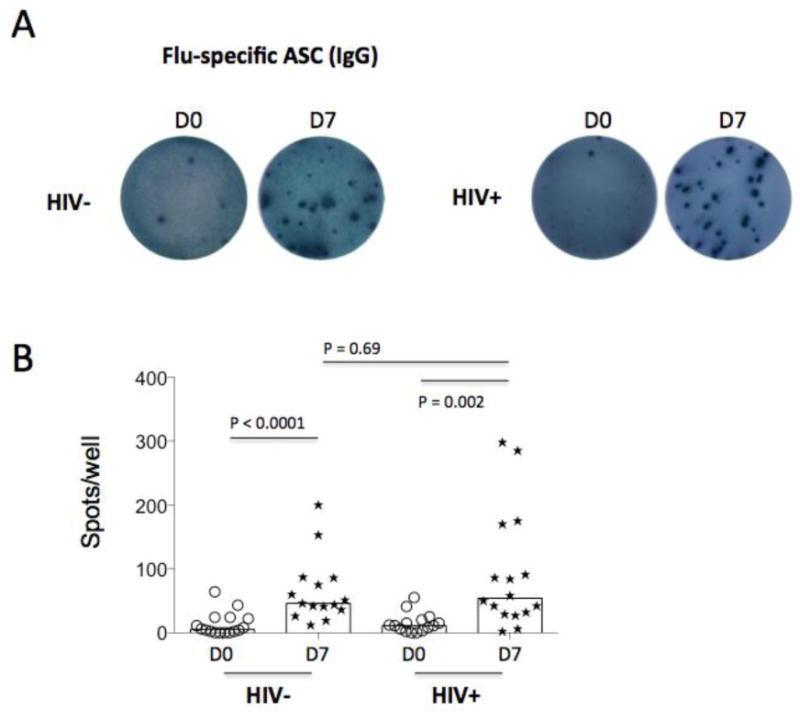
Cellular responses to influenza vaccination. Ag-specific Ab-secreting cell (ASC) was assessed by ELISPOT assay in freshly isolated B cells on D0 and D7. (A) One representative participant and the numbers of spot (flu-specific IgG+ ASCs, 0.5 million freshly isolated B cells per well) on D0 and D7 from controls and patients. (B) The median numbers of ASCs are shown from ELISPOT results.
Correlations between vaccine responses and parameters of CD4+ T cells and B cells and microbial translocation
Anti-H1N1-neutralizing activities were the primary Ab responses compared with anti-B- and anti-H3N2-neutralizing activities (Fig. 3). In subsequent analysis, we therefore used neutralizing titers on D14 and fold-change of anti-H1N1 neutralizing titers on D14 versus D0 as markers of the serologic vaccine responses. To assess the predictors of vaccine responses, we performed correlation tests between the parameters of CD4+ T cells and B cells and serologic Ab responses (anti-H1N1 IgG neutralizing activities). Parameters related to CD4+ T cells, including cell count, frequency of apoptosis, and activation (CD38 expression), were not related to any vaccine responses at any visit in either the controls or patients (P > 0.05, data not shown). There were no direct correlations between the proportion of CD4+ T cells out of the total T cells and the number of ASCs on D7 in controls and in patients (r = 0.46, P = 0.10 and r = -0.05, P = 0.85, respectively, data not shown), and between frequency of CD4+ T cells and the anti-H1N1-neutralizing titers in controls and in patients (r = 0.28, P = 0.28 and r = -0.34, P = 0.13, respectively, data not shown). Because similar Ab responses were found in both controls and patients and among HIV-infected patients regardless of their relative success with CD4+ T cell recovery, we were interested in analyzing B cell functioning, including baseline apoptosis, the proportion of total lymphocytes and the various subsets, and ki67 expression (Fig. 6A-6D, and data not shown). Interestingly, vaccine-mediated frequency of B cell apoptosis was inversely correlated with neutralizing titers (r = -0.47, P = 0.04, Fig. 6A) but not with the fold changes of neutralizing titers (D14 versus D0, r = -0.22, P = 0.42, data not shown) in controls. Moreover, vaccine-mediated frequency of B cell cycling was directly correlated with neutralizing titers (r = 0.53, P = 0.05, Fig. 6C) but not with the fold changes of neutralizing titers (D14 versus D0, r = -0.17, P = 0.52, data not shown) in controls. These relationships between neutralizing titers and B cell function in controls did not hold true in patients (P > 0.05, Fig. 6B and 6D). Notably, baseline levels of neutralizing titers were inversely correlated with the fold changes of neutralizing titers post-vaccination in both controls and patients (Fig. 6E and 6F, P < 0.0001). This observation may reflect a plateau effect of vaccine-induced fold changes of Ab responses based on baseline Ab titers. Interestingly, baseline plasma bacterial 16S rDNA levels were directly correlated with both baseline and D14 neutralizing titers only in patients but not in controls (Fig. 7). As expected [33], there was a significant correlation between level of 16S rDNA and CD4+ T cell activation (%CD38+ on memory CD4+ T cells, r = 0.45, P = 0.04), but not between plasma level of 16S rDNA and the percentage of ki67+ B cells (r = 0.27, P = 0.20) in patients. These correlations were not demonstrable in controls (data not shown).
Figure 6.
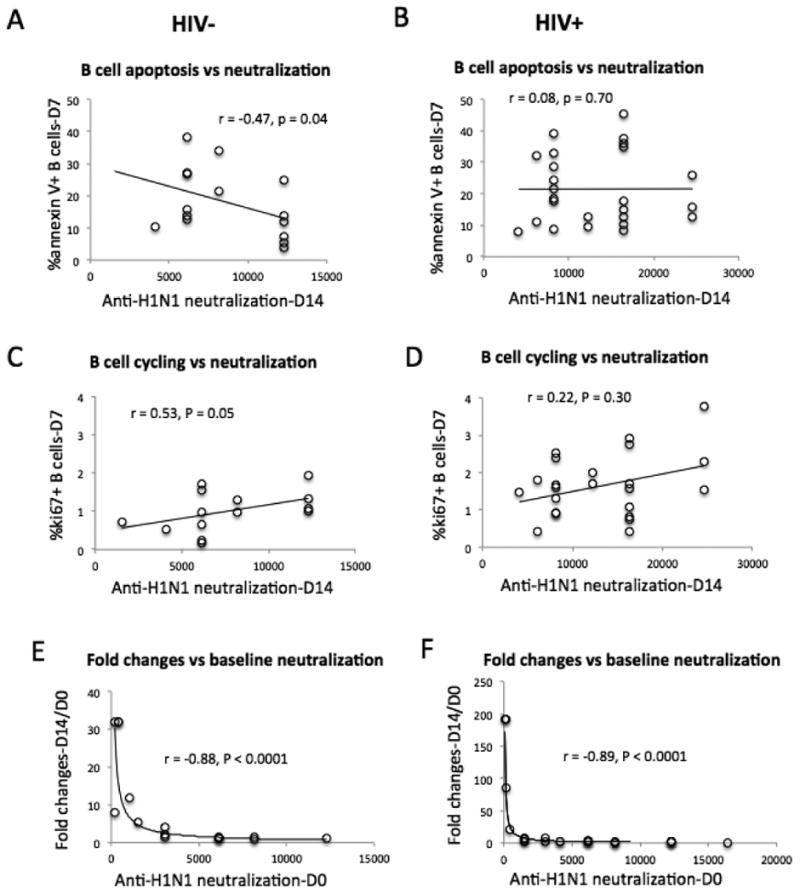
Correlation analyses between neutralizing titers and B cell function. (A, B) Correlation analysis between vaccine-induced anti-H1N1 neutralizing activities and B cell apoptosis. (C, D) Correlation analysis between vaccine-induced anti-H1N1 neutralizing activities and B cell cycling. (E, F) Correlation analysis between vaccine-induced anti-H1N1 neutralizing activities and fold changes of anti-H1N1 neutralizing activities (D14 versus D0).
Figure 7.
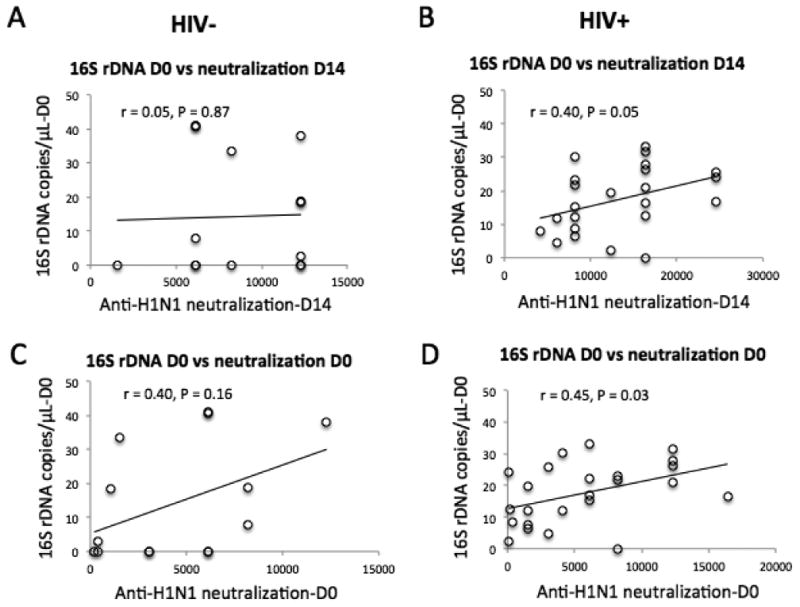
Correlation analyses between neutralizing titers and baseline level of microbial translocation. (A, B) Correlation analysis between vaccine-induced anti-H1N1 neutralizing activities and baseline plasma levels of 16S rDNA. (C, D) Correlation analysis between baseline anti-H1N1 neutralizing activities and baseline plasma levels of 16S rDNA.
In summary, aviremic ART-treated HIV-infected patients had comparable or greater overall responses against influenza vaccination, but important differences were observed in terms of B cell function and activation states. In T-cell dependent responses to serial annual influenza vaccinations, unlike healthy controls, B cell hyperactivation is not consistently correlated with the strength of the immunologic response in the setting of treated HIV infection. The marker of microbial translocation is directly correlated with the strength of the immunologic response in HIV-infected patients but not in controls. Our results suggest that loss of CD4+ T cells and baseline B cell dysfunction can be overcome by repeated immunization in those ART-treated aviremic HIV-infected subjects.
Discussion
Long- and short-term impairments in Ab responses to some vaccines and Ags have been reported in patients with HIV [4,24,37], but the extent to which impairment is observed in the setting of HIV suppression with ART is unclear. In the current study, we characterized the immune response to influenza vaccine in ART-suppressed HIV-infected subjects relative to a cohort of healthy volunteers. To our surprise, we found that HIV-infected patients tended to have a similar or even higher quantity and quality of Ag-specific Abs post-vaccination. The plasma levels of anti-H1N1 neutralization titers were correlated with post-vaccination frequencies of ki67+ B cells and B cell apoptosis in controls, but not in patients.
Although some studies demonstrated that HIV+ patients have similar or greater responses to the influenza vaccine [31,38,39], most reported that these patients have compromised responses to this vaccine [5,17-19,22,40], in disagreement with our results. Potential reasons for this discrepancy may include differences in patient populations. While impaired influenza vaccine responses have been reported in ART-naïve and viremic HIV+ patients, our patients were aviremic and ART-treated [5]. In addition, all control and patient participants in this study have baseline Ab protection levels above 1:40 (HI titers against H1N1 virus, Fig. 4), which is higher compared with other studies [24]. This difference may be due to more frequent yearly vaccinations among the HIV+ study participants. Varying rates of pre-vaccination protection based on unknown history of past influenza vaccinations could be responsible for some of the differences between studies. Our results suggest that although B and CD4+ T cells were not fully recovered after ART treatment in patients relative to controls (Table 1 and Fig. 1), serologic vaccine Ab responses could overcome cell dysfunction and reach normal levels in treated HIV-infected patients. Despite differences in the levels of B activation and apoptosis, three different serologic Ab assays (ELISA, HI, and neutralization) and one antigen-specific B cell assay (ELISPOT) were performed and showed consistent results. Therefore, we believe that serial annual influenza vaccination of aviremic ART-treated patients is immunogenic similar to controls after receiving repeated vaccination.
Impaired class switching has been reported in HIV disease [41]. In the current study, we found that the vaccine-induced fold change of Ag-specific IgM was higher in patients as compared to controls, but this was not true for IgA or IgG (Fig. 2). This suggests a possible defect in class switching perhaps as a result of elevated apoptosis and dysfunctional T follicular helper cells (data not shown).
The potential mechanisms of greater vaccine responses observed in patients compared with controls could be accounted for by several different mechanisms. First, elevated activation of innate immunity pre-vaccination could contribute to enhanced humoral immune responses. As an example of this principal, TLR9 ligands CpG ODNs are used as vaccine adjuvants to enhance Ab responses [42]. Interestingly, in the current study, we found that baseline plasma levels of bacterial 16S rDNA were directly correlated with neutralizing activity in patients (Fig. 7, P < 0.05) but not in controls (Fig. 7, P > 0.05). As such, this provides evidence that increased innate immune activation in patients could help account for heightened vaccine responses in patients. Of note, plasma level of bacterial 16S rDNA in aviremic and treated patients was lower than those observed in ART-naïve patients [33], and heightened microbial translocation has been shown to play a role in B cell dysfunction [43]. Whether intermediate levels of plasma microbial products promote B cell responses to vaccination in these patients or higher vaccine Ab responses in these patients reflect B cell polyrelativities is not known, and the exact mechanism requires further study. Greater vaccine responsiveness in HIV-infected patients could relate to plasma cell function. HIV protease inhibitors have been shown to activate the unfolded protein response (UPR) and enhance function of hepatocytes and macrophages [44-46]. The same mechanism could contribute to increased plasma cell function from HIV-infected patients (26.9% patients in the current study were having protease inhibitors in their ART treatment). Theoretically, increased plasma cell number and decreased CD4+ T regulatory cells (Treg cells) could contribute to heightened B cell vaccine responses. However, in the current study this is unlikely because ART-treated patients have similar number of Treg cells and plasma cells compared with controls (data not shown) [47-49]. In addition, we did not characterize potential differences in Treg and plasma cell functionality.
Limitations in the present study include: 1) because the data in the present study was obtained from viral-suppressed HIV-infected patients (plasma HIV-RNA < 50 copies/mL), therefore the results may not be applicable to all HIV-infected patients; 2) past vaccination status of HIV- controls and HIV+ subjects needs to be matched; 3) due to the restriction of a small number of participants in this study, further investigation in a large sample size is warranted to increase our understanding of the differences in immunological response between these two groups.
In summary, we report that aviremic ART-treated HIV-infected patients had similar or increased Ab responses to recall influenza vaccinations. This observation was made in the setting of elevated levels of B-cell apoptosis, B-cell ki67 expression, and reduced CD4+ T cell counts at baseline compared to control subjects. Post-vaccination frequencies of B cell apoptosis and intracellular ki67+ B cells were associated with vaccine responses only in controls. Baseline plasma level of microbial translocation was directly correlated with vaccine responses only in patients. The correlations between B cell function of cycling and apoptosis and vaccine-induced Ab responses in controls but not in patients suggest that the recovery of B cell function in patients is not consistent with its Ab production after ART treatment, and repeated vaccination can overcome B cell dysfunction in treated HIV-infected patients. These results provide valuable insights into the characteristics of the humoral immune response against an in vivo recall T cell-dependent Ag in HIV-treated individuals that contribute to our understanding of vaccination strategies and B cell pathogenesis.
Acknowledgments
This work was supported by NIH grants AI091526 and AI084816, the International Science and Technology Cooperation Program of China (2011DFR30420), the 12th Five Year Research Project of People's Liberation Army (CWS11J160), Beijing Key Laboratory for HIV/AIDS (BZ0089), and the National High Technology Research and Development Program (863 program 2012AA02A404-7).
The authors thank the Bad Boys of Cleveland/Cleveland Immunopathogenesis Consortium (AI 076174) for the helpful comments and discussion related to this project.
Footnotes
Author contributions: W.J. designed the study and wrote the article; Z.L., L.M., P.B., and L.Z. performed experiments; Z.W., Z.L, H.W., L. H., and A.K analyzed data; L.M. provided samples, clinical data, and contributed to article preparation; Y.G., Z.L., E.G.M., G.L., and J.M.K. provided study design and contributed to article preparation. None of the authors have any competing interests in the manuscript.
References
- 1.Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 2.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 4.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 5.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 6.Levesque MC, Moody MA, Hwang KK, Marshall DJ, Whitesides JF, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 8.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frasca D, Diaz A, Romero M, Phillips M, Mendez NV, et al. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int Immunol. 2012;24:175–182. doi: 10.1093/intimm/dxr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, et al. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J Immunol. 2011;186:6173–6181. doi: 10.4049/jimmunol.1100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cagigi A, Rinaldi S, Santilli V, Mora N, E CM, et al. Premature ageing of the immune system relates to increased anti-lymphocyte antibodies (ALA) after an immunization in HIV-1-infected and kidney-transplanted patients. Clin Exp Immunol. 2013;174:274–280. doi: 10.1111/cei.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egli A, Santer DM, O'Shea D, Barakat K, Syedbasha M, et al. IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza. PLoS Pathog. 2014;10:e1004556. doi: 10.1371/journal.ppat.1004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Y, Tamayo P, Nakaya H, Pulendran B, Mesirov JP, et al. Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response. Eur J Immunol. 2014;44:285–295. doi: 10.1002/eji.201343657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014;157:499–513. doi: 10.1016/j.cell.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JP, Macauley C. Susceptibility to influenza A in HIV-positive patients. JAMA. 1989;261:245. doi: 10.1001/jama.1989.03420020097023. [DOI] [PubMed] [Google Scholar]
- 16.Aberg JA, Gallant JE, Anderson J, Oleske JM, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39:609–629. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- 17.Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 18.Cagigi A, Nilsson A, Pensieroso S, Chiodi F. Dysfunctional B-cell responses during HIV-1 infection: implication for influenza vaccination and highly active antiretroviral therapy. Lancet Infect Dis. 2010;10:499–503. doi: 10.1016/S1473-3099(10)70117-1. [DOI] [PubMed] [Google Scholar]
- 19.Bickel M, Wieters I, Khaykin P, Nisius G, Haberl A, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–35. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 20.Fuller JD, Craven DE, Steger KA, Cox N, Heeren TC, et al. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 21.Valour F, Cotte L, Voirin N, Godinot M, Ader F, et al. Vaccination coverage against hepatitis A and B viruses, Streptococcus pneumoniae, seasonal flu, and A(H1N1)2009 pandemic influenza in HIV-infected patients. Vaccine. 2014;32:4558–4564. doi: 10.1016/j.vaccine.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 22.George VK, Pallikkuth S, Parmigiani A, Alcaide M, Fischl M, et al. HIV infection worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine. J Infect Dis. 2015 doi: 10.1093/infdis/jiu840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez LA, Daniel A, Frank I, Tebas P, Boyer JD. Seroprotection of HIV-infected subjects after influenza A(H1N1) vaccination is directly associated with baseline frequency of naive T cells. J Infect Dis. 2014;210:646–650. doi: 10.1093/infdis/jiu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, et al. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One. 2013;8:e79816. doi: 10.1371/journal.pone.0079816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pallikkuth S, Kanthikeel SP, Silva SY, Fischl M, Pahwa R, et al. Innate immune defects correlate with failure of antibody responses to H1N1/09 vaccine in HIV-infected patients. J Allergy Clin Immunol. 2011;128:1279–1285. doi: 10.1016/j.jaci.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cagigi A, Pensieroso S, Ruffin N, Sammicheli S, Thorstensson R, et al. Relation of activation-induced deaminase (AID) expression with antibody response to A(H1N1)pdm09 vaccination in HIV-1 infected patients. Vaccine. 2013;31:2231–2237. doi: 10.1016/j.vaccine.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–993. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas JM, Pos Z, Reinboth J, Wang RY, Wang E, et al. Differential responses of plasmacytoid dendritic cells to influenza virus and distinct viral pathogens. J Virol. 2014;88:10758–10766. doi: 10.1128/JVI.01501-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frasca D, Blomberg BB. B cell function and influenza vaccine responses in healthy aging and disease. Curr Opin Immunol. 2014;29:112–118. doi: 10.1016/j.coi.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonduelle O, Yahia N, Siberil S, Benhabiles N, Carrat F, et al. Longitudinal and integrative biomodeling of effector and memory immune compartments after inactivated influenza vaccination. J Immunol. 2013;191:623–631. doi: 10.4049/jimmunol.1203483. [DOI] [PubMed] [Google Scholar]
- 31.Siegrist CA, van Delden C, Bel M, Combescure C, Delhumeau C, et al. Higher memory responses in HIV-infected and kidney transplanted patients than in healthy subjects following priming with the pandemic vaccine. PLoS One. 2012;7:e40428. doi: 10.1371/journal.pone.0040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cagigi A, Cotugno N, Giaquinto C, Nicolosi L, Bernardi S, et al. Immune reconstitution and vaccination outcome in HIV-1 infected children: present knowledge and future directions. Hum Vaccin Immunother. 2012;8:1784–1794. doi: 10.4161/hv.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigano A, Bricalli D, Trabattoni D, Salvaggio A, Ruzzante S, et al. Immunization with both T cell-dependent and T cell-independent vaccines augments HIV viral load secondarily to stimulation of tumor necrosis factor alpha. AIDS Res Hum Retroviruses. 1998;14:727–734. doi: 10.1089/aid.1998.14.727. [DOI] [PubMed] [Google Scholar]
- 35.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 36.Badley AD, Parato K, Cameron DW, Kravcik S, Phenix BN, et al. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ. 1999;6:420–432. doi: 10.1038/sj.cdd.4400509. [DOI] [PubMed] [Google Scholar]
- 37.Tuaillon E, Tabaa YA, Baillat V, Petitjean G, Reynes J, et al. Long-term persistence of memory B cells specific for hepatitis B surface antigen in HIV-1-infected patients. AIDS. 2007;21:2343–2345. doi: 10.1097/QAD.0b013e32827035b5. [DOI] [PubMed] [Google Scholar]
- 38.Agrati C, Gioia C, Castilletti C, Lapa D, Berno G, et al. Cellular and humoral immune responses to pandemic influenza vaccine in healthy and in highly active antiretroviral therapy-treated HIV patients. AIDS Res Hum Retroviruses. 2012;28:1606–1616. doi: 10.1089/aid.2011.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yam KK, Gipson E, Klein M, Walmsley S, Haase D, et al. High level antibody avidity is achieved in HIV-seropositive recipients of an inactivated split adjuvanted (AS03A) influenza vaccine. J Clin Immunol. 2014;34:655–662. doi: 10.1007/s10875-014-0054-z. [DOI] [PubMed] [Google Scholar]
- 40.Fabbiani M, Sidella L, Corbi M, Martucci R, Sali M, et al. HIV-infected patients show impaired cellular immune response to influenza vaccination compared to healthy subjects. Vaccine. 2013;31:2914–2918. doi: 10.1016/j.vaccine.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Iwajomo OH, Finn A, Ogunniyi AD, Williams NA, Heyderman RS. Impairment of pneumococcal antigen specific isotype-switched Igg memory B-cell immunity in HIV infected Malawian adults. PLoS One. 2013;8:e78592. doi: 10.1371/journal.pone.0078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krieg AM, Davis HL. Enhancing vaccines with immune stimulatory CpG DNA. Curr Opin Mol Ther. 2001;3:15–24. [PubMed] [Google Scholar]
- 43.Zhang L, Luo Z, Sieg SF, Funderburg NT, Yu X, et al. Plasmacytoid dendritic cells mediate synergistic effects of HIV and lipopolysaccharide on CD27+ IgD- memory B cell apoptosis. J Virol. 2014;88:11430–11441. doi: 10.1128/JVI.00682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta AK, Li B, Cerniglia GJ, Ahmed MS, Hahn SM, et al. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia. 2007;9:271–278. doi: 10.1593/neo.07124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H, Gurley EC, Jarujaron S, Ding H, Fang Y, et al. HIV protease inhibitors activate the unfolded protein response and disrupt lipid metabolism in primary hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1071–1080. doi: 10.1152/ajpgi.00182.2006. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Pandak WM, Jr, Lyall V, Natarajan R, Hylemon PB. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol. 2005;68:690–700. doi: 10.1124/mol.105.012898. [DOI] [PubMed] [Google Scholar]
- 47.Epple HJ, Loddenkemper C, Kunkel D, Troger H, Maul J, et al. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108:3072–3078. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- 48.Lim A, Tan D, Price P, Kamarulzaman A, Tan HY, et al. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. AIDS. 2007;21:1525–1534. doi: 10.1097/QAD.0b013e32825eab8b. [DOI] [PubMed] [Google Scholar]
- 49.Tsunemi S, Iwasaki T, Imado T, Higasa S, Kakishita E, et al. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS. 2005;19:879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]


