Abstract
Neural models of major depressive disorder (MDD) posit that over-response of components of the brain’s salience network (SN) to negative stimuli plays a crucial role in the pathophysiology of MDD. In the present proof-of-concept study, we tested this formulation directly by examining the affective consequences of training depressed persons to down-regulate response of SN nodes to negative material. Ten participants in the real neurofeedback group saw, and attempted to learn to down-regulate, activity from an empirically identified node of the SN. Ten other participants engaged in an equivalent procedure with the exception that they saw SN-node neurofeedback indices from participants in the real neurofeedback group. Before and after scanning, all participants completed tasks assessing emotional responses to negative scenes and to negative and positive self-descriptive adjectives. Compared to participants in the sham-neurofeedback group, from pre- to post-training, participants in the real-neurofeedback group showed a greater decrease in SN-node response to negative stimuli, a greater decrease in self-reported emotional response to negative scenes, and a greater decrease in self-reported emotional response to negative self-descriptive adjectives. Our findings provide support for a neural formulation in which the SN plays a primary role in contributing to negative cognitive biases in MDD.
Keywords: major depressive disorder, neurofeedback, functional magnetic resonance imaging, salience network, information processing biases
1. Introduction
Over the last two decades, neuroimaging investigations of Major Depressive Disorder (MDD) have been instrumental in increasing our understanding of this prevalent and debilitating condition (Kessler and Wang, 2009). Sufficient data have now accumulated from functional neuroimaging investigations of depression that, through meta-analytic integration, we have been able to identify the neural abnormalities that have been found most reliably to characterize this disorder. Specifically, in a recent meta-analysis of studies using task-based functional magnetic resonance imaging (FMRI), we found reliably increased response in fronto-insular cortex, amygdala, and dorsal anterior cingulate cortex (dACC) to negative stimuli relative to neutral stimuli; importantly, we did not observe this pattern with respect to response to positive relative to neutral stimuli in MDD (Hamilton et al., 2012). Based on these findings, we presented a neural account of the well-documented heightened response to negative stimuli in MDD, which has been hypothesized to play a significant role in the etiology and maintenance of this disorder (Beck, 1976; Gotlib and Joormann, 2010). In this formulation, we posit that through monosynaptic projections to the amygdala, dACC, and fronto-insular cortex (Jones and Burton, 1976; Mufson and Mesulam, 1984; Padmala et al., 2010), heightened baseline activity in the pulvinar nucleus in depression (Hamilton et al., 2012) potentiates response of these primary limbic nodes to affective information.
In this model, fronto-insular cortex, dACC, and amygdala — primary nodes in the brain’s salience network (SN), which is postulated to undergird perception of and response to personally relevant stimuli (SN; Seeley et al., 2007) — play a crucial role in biasing the processing of negative information in MDD. This and similar formulations proposed by clinical neuroscientists (e.g., Menon, 2011) are difficult to test directly using traditional functional neuroimaging paradigms, which typically identify only neural correlates of cognitive or emotional activity. Given this limitation of traditional FMRI paradigms in making causal attributions, in the present study we used an FMRI-based neurofeedback system that allows individuals to see and learn to modulate regional brain responses. The implementation of this method permits investigators to examine the effects on behavior of modulating neural activation (Weiskopf et al., 2004), as opposed to the more typical examination of the effects on the brain of manipulating behavior. Such FMRI neurofeedback systems have now been used successfully to teach healthy individuals to volitionally alter response in sensorimotor (DeCharms et al., 2004), and limbic regions (Caria et al., 2007; Hamilton et al., 2011), and to reduce the experience of pain (deCharms et al., 2005).
Recent studies using FMRI neurofeedback in MDD have explored the therapeutic utility of this method in depression. A seminal, non-placebo-controlled study showed that it is possible to teach depressed persons to increase idiographic neural activity associated with positive affect and, in doing so, decrease depressive symptomatology (Linden et al., 2012). Another recent study showed that teaching depressed persons to increase amygdala activity during recall of happy autobiographical memories increases happiness and decreases anxiety in MDD (Young et al., 2014). While not controlling for placebo effects, these studies have provided strong preliminary support for the clinical efficacy of FMRI neurofeedback paradigms.
In the current proof-of-concept study, we take a different perspective relative to previous, clinically oriented work by using FMRI neurofeedback as a tool to test and develop neural models of MDD. For this study, we constructed an experimental paradigm for testing the role of SN node over-response in producing the negative affective biases implicated reliably in MDD. In designing this study, we reasoned that if the SN plays a crucial role in producing negative affective biases in MDD, then teaching depressed persons to decrease responding in SN nodes to negative affective information should decrease affective responding to negative but not positive information. If, on the other hand, learned down-modulation of SN response to negative information has no effect on response to negative information, the hypothesis that the SN plays a critical role in negative affective biases in MDD will be disconfirmed.
To measure the effects of SN-node neurofeedback training (NFT), we assessed responses to both negative and positive stimuli before and after a regimen of NFT. In order to determine effects on affective functioning attributable to NFT as distinct from placebo effects, we also included a group of depressed participants who received sham NFT; that is, the neurofeedback they received was not veridical but, instead, was feedback from other depressed participants who had received real neurofeedback. We predicted, first, that receiving real NFT would lead to successful down-modulation of SN node response; thus, we predicted that, compared with depressed participants who received sham NFT, depressed participants who received real NFT would show reduced response of their most reactive SN node to negative stimuli following NFT. Further, and in accord with the model we present above, we predicted that, relative to their sham NFT counterparts, depressed persons who received real SN NFT would also exhibit reduced affective responding to negative affective challenge. Finally, given that the SN has been conceptualized as part of a negative valence system (Insel et al., 2010) in MDD (Hamilton et al., 2012), we predicted that the effects of real NFT would not generalize to affective responses to positive stimuli.
2. Methods and Materials
2.1. Participants
Twenty-two adults diagnosed with MDD initially participated in this study. All participants met criteria for a DSM-IV diagnosis of MDD based on their responses to the Structured Clinical Interview for DSM (SCID; First et al., 2001), administered by trained diagnostic interviewers. Depressed individuals with a current comorbid diagnosis of any Axis-I disorder other than Social Anxiety Disorder (SAD) were not included in the study. Given that we were comparing two groups of depressed participants (real versus sham NFT), we included depressed individuals who were taking antidepressant medication in this study because we would not be confounding medication status with psychiatric diagnosis and, importantly, because this would bolster the generalizability of our findings to the general population of depressed persons, over half of whom take psychotropic medications (Pratt et al., 2011). At the end of the interview session, all participants completed the Beck Depression Inventory-II (BDI-II; Beck et al., 1979) and the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). The BDI-II and PANAS are frequently used and well validated self-report measures of the severity of depressive symptoms and levels of positive and negative affect, respectively. All stages of the research presented here were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
To assess cognitive, behavioral, and neural effects of NFT, we assigned participants to one of two groups: REAL, in which participants saw real-time neural response data from a component of their own SN, and SHAM, in which participants saw, in the context of an otherwise equivalent neurofeedback procedure, neural response data from participants in the REAL group instead of their own neural data. We elected to use a sham NFT control group — as opposed to a control group seeing neurofeedback from a control region not implicated in depression — because this form of experimental control is the only way to ensure that the positive and negative reinforcement provided from neurofeedback, and the potential effects of this feedback on subsequent task performance, were precisely controlled. Importantly, two researchers were involved in NFT scanning: one who interacted with participants and was blind to group assignment and one that ran the NFT interface and who was not blind to group assignment. Further, in keeping with the objective of this study was to investigate the role of the SN in the pathophysiology of MDD, we focused only on the effects of learned modulation of this network.
Given pilot data indicating that 5 out of 6 individuals were successful in using neurofeedback to learn how to control neural response, we first ran 12 participants through the REAL neurofeedback protocol and identified 10 who were successful in using NFT to learn to regulate responding of a functionally defined SN-node (we include performance data for the two non-learners in a supplement to this article). Even though we excluded only a small proportion of our recruited REAL NFT sample, to ensure that this process did not bias our sample (i.e., that selecting successful neuromodulators did not inadvertently also select for factors such as age or severity of depression that might affect performance), we selected and ran through the SHAM NFT protocol depressed individuals who were matched to participants from the REAL group with respect to age, education, medication, symptom severity, and comorbidity with SAD. Importantly, all participants provided consent for participation in the NFT procedure knowing that there would be a chance that they would see sham neurofeedback.
2.2. Neurofeedback Scanning Protocol
2.2.1. Overview
Participants were first shown the neurofeedback interface and neuromodulation task outside of the scanner. In addition, before and after scanning, participants completed two computer-based tasks (described below) to assess response to affective challenge. After entering the scanner, participants underwent a functional-localizer/pre-training assessment scan, three NFT scans, and a post-training assessment scan. Finally, following scanning and assessment, all participants were interviewed about whether they believed the NFT signal was real and, for exploratory purposes, what technique they used to control the NFT signal.
2.2.2. Pre- and post-scanning assessments
To assess changes in affective responding due to NFT, participants completed, both before and after training, an out-of-scanner picture-rating task in which they viewed and rated on a scale of 1 to 9 the intensity of 15 novel, negatively valenced pictures from the International Affective Picture System (IAPS; Lang and Greenwald, 1993). We used a total of 78 novel, negative IAPS pictures (mean intensity: 4.18; SE: 0.17; mean arousal: 5.11; SE: 0.15) for the behavioral testing and scanning portions of the study (15 for each rating task, 15 for each of the pre- and post-training scans, and 6 for each of three NFT scans). To ensure their appropriateness for use with depressed participants, the negative pictures were all rated by two trained clinicians on a 1-to-9 scale as reflecting higher levels of sadness than of fear or disgust (mean sadness: 7.5; mean fear: 2.5; mean disgust: 2.0). In addition, before and after NFT, participants completed a brief version of the self-referent encoding task (SRET; Ramel et al., 2007). In this task, participants rated on a 1-to-9 scale the self-relevance of ten positive and ten negative adjectives from the Affective Norms for English Words list (ANEW; Bradley and Lang, 1999), matched with respect to frequency and intensity. For both the picture-rating task and the SRET, stimuli were presented pseudorandomly and counterbalanced such that the words and pictures that were presented before NFT for half of participants were presented after NFT for the other half of the participants.
2.2.3. Scanner and pulse sequence
We conducted FMRI scans using a 3.0 Tesla General Electric Signa MR 750 scanner with an eight-channel, whole-head quadrature imaging coil at the Richard M. Lucas Center for Imaging at the Stanford University School of Medicine. Following whole-brain shimming and high-resolution in-plane anatomical scans, we conducted five FMRI scans [28 sagittal slices with 3.44 mm2 in-plane and 4 mm through-plane resolution, TE = 30 ms, flip angle = 80°, FOV = 22 cm, acquisition time (TR) = 2000 ms per frame, number of frames = 170 per run for each of two assessment scans and 119 per run for each of three neuromodulation scans] using a spiral in/out pulse sequence (Glover and Law, 2001). We used a photoplethysmograph fitted over the left toe to measure heart-rate and a pneumatic respiratory belt fitted around the abdomen to measure respiratory fluctuations.
2.2.4. rtfMRI Software Configuration
Processing and analysis of blood-oxygen-level dependent (BOLD) data in real time was conducted with an in-house software suite comprising C/C++ programs and Matlab scripts running on a Linux computer with a socket connection to the scanner for image transfer. At the end of each volume repetition, scanner software reconstructed spiral in-out data (Glover and Law, 2001) from k-space to native brain space and sent it to the rtfMRI computer. The neurofeedback program then calculated a voxel-wise weighted-average of spiral-in and spiral-out acquisitions to optimize signal-to-noise ratio. Reconstructed data were then passed to a module for signal processing and converting data to graphical renderings. For all functional scans, we used this program to co-register individual images to the second functional image of the scan and used multiple regression to factor out effects on BOLD time series of three translational and three rotational motion covariates (x-, y-, and z- dimensions) and two physiological noise covariates reflecting the influence of changes in respiration and cardiac rate on the BOLD signal (Chang and Glover, 2009).
2.2.5. Prescan preparation
Prior to scanning, participants were introduced to the neurofeedback interface and the task design of the study. They were told that they would see a neurofeedback signal from a brain structure involved in emotional response and that they should try to adopt strategies that brought about a decrease in the response of this structure. Consistent with previous research (Caria et al., 2007), the results of pre-testing indicated that NFT with the SN progressed slowly if participants were not given a set of possible strategies. Therefore, at the outset of scanning, we provided participants in both the REAL and SHAM groups with strategies involving cognitive reappraisal (Ochsner et al., 2002), attentional redirection (Kalisch et al., 2006), and imagery (Jarvinen and Gold, 1981) to try at their discretion (see Supplement to Methods and Materials for details). Further, participants were reminded that the neurofeedback signal was there to help teach them, and that if one strategy did not produce the desired change in the neurofeedback signal, they should try another.
2.2.6. Functional-localizer/pre-training assessment scan
For their first functional scan —designed both to identify a region of interest (ROI) for NFT (REAL group) and to assess pre-training response in this ROI (REAL and SHAM groups) — participants engaged in 15 trials of viewing negative IAPS pictures. Participants were instructed to attend to and stay focused on each picture for the six seconds that it was presented and then to engage in an active control task for 16 seconds following the offset of each picture. Given that response in neural regions that subserve affect can persist beyond the duration of an affective challenge (Siegle et al., 2002), we used a simple, active control task — pressing a different button (1–4) every two seconds on an MR-compatible keypad — to distract participants from any persisting, ruminative thoughts following the offset of affective stimuli. Our goal in administering this control task was to make analysis of BOLD time-series data more tractable by facilitating deactivation in neural structures activated by emotional stimuli following emotional provocation, thereby increasing coherence between BOLD data and task covariates.
To identify the SN ROI for each participant we used multiple regression to identify voxels in the SN — SN structures were identified during the scanning session in native brain space by an experienced neuroanatomist (JPH) — with corrected time series data that correlated with a gamma-function-convolved boxcar covariate reflecting the onset and offset of negative IAPS pictures. Directly following the localizer/pre-training assessment scan, we consulted an automatically generated statistical map (overlaid on brain structural data) showing the degree of correlation between voxel time series data and the task covariate. Using this map with statistical threshold set at p = .05 for each voxel, we identified the single voxel within the SN (dACC, amygdala, or fronto-insular cortex) with a time series that had the highest correlation with the task covariate. We then selected this peak SN voxel and its eight within-plane neighbors as the ROI for NFT (REAL group) or for comparison to the REAL NFT group (SHAM group). We decided to use as the ROI for each participant the most responsive node of the SN rather than the entire SN both to accommodate individual differences in SN responding and to provide the most stable and reliable training signal to the REAL NFT participants. As an additional quality check of this ROI selection process, on the brain-wide BOLD data collected for the localizer scan we conducted simple functional connectivity analysis using the ROI BOLD data for each participant as the seed time series. We hypothesized that if we were, in fact, identifying SN regions with the localizer scan, then we should see significant SN connectivity, across one or more nodes of this network when we combined connectivity maps at the group level. Please see Supplementary Figure 1 for a summary of these findings.
2.2.7. NFT scans
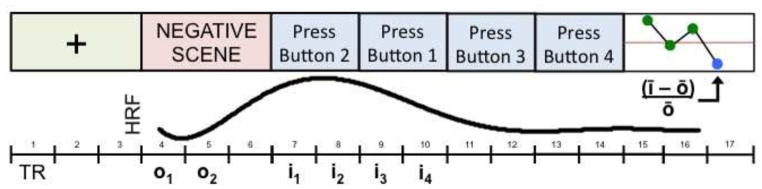
Each participant completed three NFT scans, each of which consisted of six, 34-second trials. Each trial began with six seconds of passive viewing of a fixation cross followed by six seconds of attempting to decrease emotional response while viewing a novel, negative IAPS picture. Following this, participants engaged in the active, button-pressing control task for 16 seconds before seeing a line graph for six seconds showing the degree of SN response to the negative picture they just viewed, in addition to the SN response on previous trials for that training scan. We defined SN-node response for each trial as the average, noise-covariate corrected BOLD signal for the 4th–7th TRs (i1–i4) following onset of the picture — corresponding to the epoch of peak hemodynamic response resulting from six seconds of picture presentation — minus the average BOLD signal during a baseline epoch comprising the first two TRs (o1–o2) following picture onset, divided by the average of o1 and o2 (see Figure 1 for a depiction of the trial structure and calculation of SN-node response for NFT scans).
Figure 1.
Trial structure and calculation of SN-node response for NFT scans.
2.2.8. Post-training assessment scan
Following the three NFT scans, participants engaged in a final scan equivalent in structure to the pre-training scan with the exceptions that novel IAPS pictures were used and that upon seeing the negative pictures, participants were asked to invoke the affective regulatory strategy that had proven most effective in reducing the neurofeedback signal they saw during NFT runs; no neurofeedback signal was provided during this post-training scan.
2.3. Analysis
2.3.1. ROI and behavioral data
Given that participants were trained using the neurofeedback index described above, we used this index as the primary dependent variable for assessing the effects of NFT on SN-node response. We first calculated for each participant the average response across trials of the SN ROI for the pre-training and post-training scans and converted these data to a single percent-change index:
We then compared this index of SN-node response change in the REAL and SHAM groups. Similarly, for ratings of the intensity of the IAPS pictures and of the self-relevance of the negative and positive adjectives that were obtained before and after NFT training, we calculated percent-change scores and examined differences in these scores between participants in the REAL and the SHAM groups. Given our a priori directional predictions, we used one-tailed t-tests to compare the REAL and SHAM groups. Further, we calculated effect sizes of NFT effects (Cohen, 1988). Finally, given that motion can affect BOLD signal, to ensure that participants in the REAL relative to the SHAM group were not learning to move in order to influence their neurofeedback signal, we also compared the REAL and SHAM groups on percent-change indices of the Euclidean norm of the derivatives of their motion covariates and on percent change of correlations between motion and the task timing.
2.3.2. Functional connectivity
To examine whether depressed participants in the REAL group, relative to participants in the SHAM group, recruited additional brain regions in the service of learning to modulate their respective NFT ROIs, we conducted a functional connectivity (FC) analysis — via a procedure described elsewhere (Hamilton and Gotlib, 2008) — using as the seed region each participant’s ROI. Following normalization of each participant’s FC coefficient maps to standard space (Talairach et al., 1988) we conducted a voxel wise, mixed model, two-by-two analysis of variance (ANOVA) of Group (REAL versus SHAM) repeated over Training (PRE versus POST NFT) using a family-wise error corrected α = .05. Importantly, this analysis was conducted for exploratory purposes and, given the small sample size and the degrees of freedom used in the two-by-two factorial model, was relatively insensitive to detect effects.
3. Results
3.1. Demographic and clinical measures
As shown in Table 1, the REAL and SHAM groups did not differ significantly with respect to age, years of education, depressive symptomatology, or levels of negative or positive affect (all t-tests p > 0.15); further, the two groups also did not differ significantly in the proportion of participants who had comorbid Social Anxiety Disorder or who were taking psychotropic medications (all chi-square p > 0.15).
Table 1.
Group demographic and clinical data
| Sample Size | Age | Years Education | Proportion Receiving Psychotropic Medication | Proportion with Comorbid Social Anxiety Disorder | BDI-II | PANAS-Negative | PANAS-Positive | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| REAL | 10 | 31.2 (±2.9) | 16.4 (±0.83) | 60% | 30% | 33.3 (±2.3) | 36.7 (±3.8) | 25.1 (±2.1) |
|
| ||||||||
| SHAM | 10 | 34.5 (±3.7) | 17.0 (±0.75) | 40% | 20% | 34.6 (±4.0) | 31.3 (±3.6) | 22.8 (±2.5) |
Note: all between-group comparison p > .15; BDI = Beck Depression Inventory; PANAS = Positive and Negative Affect Schedule
3.2. Neuromodulation
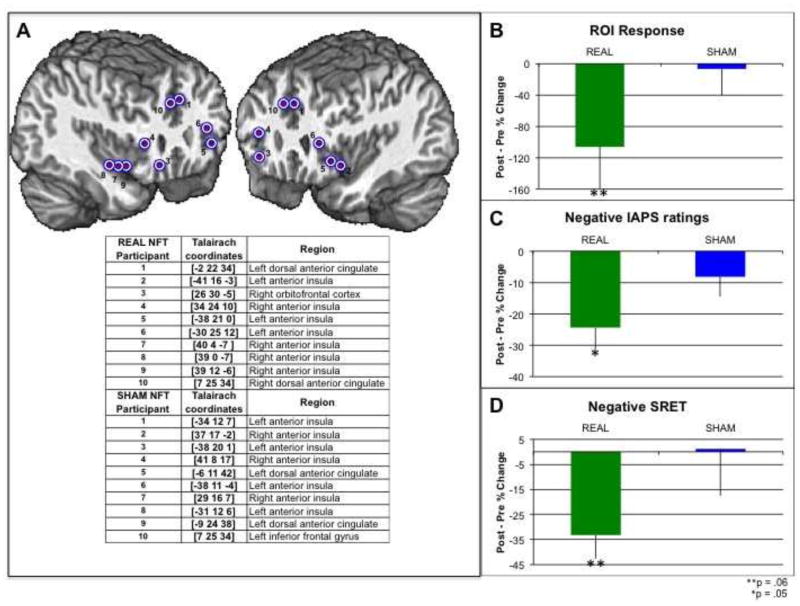
We present in Figure 2.A. the loci of the SN-node ROIs — both in Talairach coordinates and in the context of a brain map — for each of the ten REAL neurofeedback participants. The SN region showing the strongest response during the localizer/pre-training assessment scan was typically in fronto-insular cortex (8 of 10 participants); two participants, however, showed the highest response in the dACC. For comparison purposes, we also identified in the SHAM group the component of the SN that was most responsive during the pre-training scan; as in the REAL group, 8 of the 10 SHAM participants showed the strongest response in fronto-insular cortex and two participants showed the greatest response in the dACC (Talairach coordinates presented in Figure 2.A.). As expected, participants in the REAL NFT group exhibited a greater decrease in SN-node ROI response from pre- to post-NFT than did participants in the SHAM NFT group (p = 0.06; Cohen’s d = 0.70; see Figure 2B). More specifically, REAL and SHAM groups did not differ with respect to ROI response before NFT (p > 0.10), ROI response did not change from pre- to post-NFT in the SHAM group (p > 0.10), but ROI response did decrease significantly in the REAL NFT group (p < 0.05). Importantly, in none of the three NFT runs did the REAL and SHAM groups differ with respect to ROI response; this is perhaps not surprising, however, given that several participants reported trying both to increase and decrease the NFT signal during NFT runs in order to get a better sense for cause and effect relations between NFT strategies and ROI activity. Finally, the REAL and SHAM groups did not differ in pre- to post-NFT either in absolute motion or in the correlation between motion and task timing (both p > 0.15).
Figure 2.
A. Loci of functionally defined SN-node ROIs for the REAL (brain map and Talairach coordinates) and SHAM (Talairach coordinates) NFT groups. B. Change from pre-to post-NFT in SN-node ROI response in REAL and SHAM groups. C. Change from pre-to post-NFT in self-reported response to negative IAPS pictures in REAL and SHAM groups. D. Change from pre-to post-NFT in self-reported response to negative self-descriptive adjectives in REAL and SHAM groups.
3.3. Affective responses
Compared with the SHAM NFT group, the REAL NFT group showed a greater decrease from pre- to post-NFT in emotional response to negative IAPS pictures (p < 0.05; Cohen’s d = 0.78; see Figure 2C) and in ratings of self-relevance of the negative adjectives (p = 0.06; Cohen’s d = 0.73; see Figure 2D); the two groups did not differ in the change from pre- to post-NFT in their ratings of the self-relevance of the positive adjectives (p > 0.15; p > .15; Cohen’s d = 0.24). In neither group did changes in IAPS or self-relevance ratings correlate significantly with changes in SN response. See Supplemental Figure 2 for performance data from the REAL and SHAM groups in addition to the two EXCLUDED participants.
3.4. Functional connectivity
Our FC analysis identified no regions that changed connectivity with participant ROIs as a function of NFT training in the REAL group relative to the SHAM group. To augment this analysis, we also calculated at all voxels the degree of response to the emotion regulation task in all participants for both the pre- and post-training scans and then entered these estimates into a two- (Group) -by-two (Time) factorial analysis of variance. See Supplemental Figure 3 for the results of this analysis.
3.5. Post NFT and assessment debriefing
All participants from both the REAL and SHAM groups believed that the NFT signal they saw was a real NFT signal from their own brain.
4. Discussion
The present study was designed to test the hypothesis that the SN plays a critical role in the negative affective bias reliably associated with MDD (Beck, 1976; Gotlib and Joormann, 2010). To test this hypothesis, we examined whether learning to control reactivity of nodes of the SN using NFT during negative affective provocation affected emotional response in MDD. Consistent with our hypotheses, we found that compared with depressed persons who received SHAM NFT, depressed persons who received REAL NFT learned to control reactivity in components of the SN and, as a result of NFT, showed decreased emotional response to negative, but not to positive, stimuli. These findings have important implications for neural conceptualizations of depression.
Theorists have postulated that cognitive biases facilitating the processing of negative material in depression contribute to the etiology and maintenance of this disorder (Beck, 1976; Gotlib et al., 2004). In a synthesis of functional neuroimaging investigations of MDD, we identified a small number of structures showing reliable over-response to negative stimuli in MDD and postulated that, among these, the nodes of the SN play a primary role in contributing to negative processing biases in depression (Hamilton et al., 2012). In the present study we showed that teaching depressed individuals to decrease SN-node reactivity leads to a reduction in emotional reactivity to negative stimuli. Importantly, we found no evidence of involvement of non-SN structures in this SN-NFT effect. These findings, considered alongside the putative functional role of the SN (Seeley et al., 2007), are consistent with the formulation that nodes of the SN play a key role in negative processing biases in MDD. It is important that we note here that participants learned to control as opposed to reduce NFT ROI response given that several participants reported trying both to decrease and increase NFT reactivity during the training runs. The neural and behavioral effects of NFT, therefore, may be best conceptualized as resulting from general mastery of ROI response as opposed simply from learning to decrease ROI reactivity.
We should note four limitations of the present study. First, we did not include a healthy control group in this study that could allow us to replicate the documented finding of heightened SN response to negative affective challenge in MDD. Rather, based on meta-analytic results presented above (Hamilton et al., 2012), we assume here that elevated response to negative stimuli in nodes of the SN is reliable in MDD. Second, like recent preliminary investigations of neurofeedback effects in MDD (Linden et al., 2012; Young et al., 2014), our proof-of-concept investigation of SN NFT effects on negative processing biases in MDD incorporated a relatively small sample. While we observed large effects of NFT both on changes in SN node responding and on responses to negative affective challenge — with Cohen’s d scores all .70 or higher (Cohen, 1988) — it will be important in subsequent work to examine NFT effects in larger depressed samples. Further, we hasten to point out that our objectives in this study were to examine short-term neural and behavioral changes resulting from NFT; thus, the data we present are equivocal with respect to any long-term effects of learning to modulate the SN. Finally, based on results from early piloting that healthy males were relatively ineffective at learning to control neurofeedback signals from regions involved in affective responding, we recruited only female MDD participants for this study; our results, therefore, my not generalize to the full population of individuals with MDD.
While we designed this study as a critical test of the hypothesis that SN over-response plays a vital role in negative affective biases in MDD, the present results do have potential implications for non-pharmacological neural interventions for depression. Specifically, along with recent and more clinically oriented work (Linden et al., 2012; Young et al., 2014) our findings indicate that NFT may be a useful therapeutic tool for depression. In this context, however, it is important to acknowledge that we did not examine the effects of NFT on depressive symptomatology, but instead, given our formulation that SN over-response affects acute but not chronic emotional expression in MDD, we assessed its effects on affective response. Subsequent investigations should test this formulation more explicitly by examining the effects of multiple NFT sessions on mood and diagnostic status in MDD, and by assessing the effectiveness of NFT as a supplement to, or in lieu of, more traditional cognitive-behavioral therapies for depression. Finally, given that FMRI-based neural interventions have a number of exclusion criteria (e.g., claustrophobia, weight restrictions) it will be important in future work to identify peripheral biomarkers of effective neural regulation (e.g., changes in electroencephalographic or cardiac measures) for use in more traditional biofeedback paradigms.
Supplementary Material
Highlights.
We examine the salience network’s role in depressotypic information processing biases.
We present an FMRI neurofeedback system for teaching control over brain activity.
We find that real versus sham neurofeedback training decreases salience network activity.
We find that learned down-modulation of salience network response decreases depressotypic biases.
Acknowledgments
The research presented here was made possible by National Institutes of Health grants P41 EB15891 (GHG), F32 MH079651 (JPH), and R01 MH59259 (IHG). The authors gratefully acknowledge the support of Mirra Schwartz, Meghan Vinograd, Arkadiy Maksimovskiy, Melissa Henry, and Becka Johnson for their assistance in collecting the data presented here. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
None of the authors has a conflict of interest to report pertaining to the data presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT. Cognitive Therapy And The Emotional Disorders. International Universities Press; New York: 1976. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy Of Depression. The Guilford Press; New York: 1979. [Google Scholar]
- Bradley MM, Lang PJ. Affective Norms for English Words (ANEW): Instruction Manual and Affective Ratings. The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Caria A, Veit R, Sitaram R, Lotze M, Welskopf N, Grodd W, Birbaumer N. Regulation Of Anterior Insular Cortex Activity Using Real-Time fMRI. Neuroimage. 2007;35:1238–1246. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects Of Model-Based Physiological Noise Correction On Default Mode Network Anti-Correlations And Correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JDE. Learned Regulation Of Spatially Localized Brain Activation Using Real-Time fMRI. Neuroimage. 2004;21:436–443. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JDE, Mackey SC. Control Over Brain Activation And Pain Learned By Using Real-Time Functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM–IV–TR Axis I disorders. NY State Psychiatric Institute, Biometrics Research; New York: 2001. [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI For Increased SNR And Reduced Susceptibility Artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and Depression: Current Status and Future Directions. 2010:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional Biases For Negative Interpersonal Stimuli In Clinical Depression. Journal of Abnormal Psychology. 2004;113:127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional Neuroimaging Of Major Depressive Disorder: A Meta-Analysis And New Integration Of Baseline Activation And Neural Response Data. American Journal of Psychiatry. 2012:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation Of Subgenual Anterior Cingulate Cortex Activity With Real-Time Neurofeedback. Human Brain Mapping. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural Substrates Of Increased Memory Sensitivity For Negative Stimuli In Major Depression. Biological Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jarvinen PJ, Gold SR. Imagery as an Aid in Reducing Depression. Journal of Clinical Psychology. 1981;37:523–529. doi: 10.1002/1097-4679(198107)37:3<523::aid-jclp2270370314>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. Projection From Medial Pulvinar To Amygdala In Primates. Brain Research. 1976;104:142–147. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural Correlates Of Self-Distraction From Anxiety And A Process Model Of Cognitive Emotion Regulation. Journal of Cognitive Neuroscience. 2006;18:1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. In: The Epidemiology of Depression. 2. Gotlib IH, Hammen CL, editors. Guildord Press; New York: 2009. pp. 5–22. [Google Scholar]
- Lang PJ, Greenwald MK. International Affective Picture System Standardization Procedure And Results For Affective Judgments: Technical Reports 1A–1C. University of Florida., Gainesville: Center for Research in Psychophysiology; 1993. [Google Scholar]
- Linden DE, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Sorger B, Healy D, Goebel R. Real-Time Self-Regulation Of Emotion Networks In Patients With Depression. Plos One. 2012;7:e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-Scale Brain Networks And Psychopathology: A Unifying Triple Network Model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic Connections Of The Insula In The Rhesus-Monkey And Comments On The Paralimbic Connectivity Of The Medial Pulvinar Nucleus. Journal of Comparative Neurology. 1984;227:109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking Feelings: An fMRI Study Of The Cognitive Regulation Of Emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Padmala S, Lim S, Pessoa L. Pulvinar And Affective Significance: Responses Track Moment-To-Moment Visibility. Frontiers in Human Neuroscience. 2010:1–9. doi: 10.3389/fnhum.2010.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ, Quiping G. Antidepressant Use In Persons Aged 12 And Over; United States, 2005–2008. National Center for Health Statistics Data Brief. 2011;76:1–8. [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala Reactivity And Mood-Congruent Memory In Individuals At Risk For Depressive Relapse. Biological Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable Intrinsic Connectivity Networks For Salience Processing And Executive Control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t Shake That Feeling: Assessment Of Sustained Event-Related Fmri Amygdala Activity In Response To Emotional Information In Depressed Individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, Rayport M. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development And Validation Of Brief Measures Of Positive And Negative Affect - The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Scharnowski F, Veit R, Goebel R, Birbaumer N, Mathiak K. Self-Regulation Of Local Brain Activity Using Real-Time Functional Magnetic Resonance Imaging (fMRI) Journal of Physiology-Paris. 2004;98:357–373. doi: 10.1016/j.jphysparis.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J. Real-Time fMRI Neurofeedback Training of Amygdala Activity in Patients with Major Depressive Disorder. Plos One. 2014:9. doi: 10.1371/journal.pone.0088785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.