Abstract
Aim
Human periodontitis is associated with overactivation of complement, which is triggered by different mechanisms converging on C3, the central hub of the system. We assessed whether the C3 inhibitor Cp40 inhibits naturally-occurring periodontitis in non-human primates.
Materials and Methods
Non-human primates with chronic periodontitis were intra-gingivally injected with Cp40 either once (5 animals) or three times (10 animals) weekly for six weeks followed by a 6-week follow-up period. Clinical periodontal examinations and collection of gingival crevicular fluid and biopsies of gingiva and bone were performed at baseline and during the study. A one-way repeated measures ANOVA was used for data analysis.
Results
Whether administered once or three times weekly, Cp40 caused a significant reduction in clinical indices that measure periodontal inflammation (gingival index and bleeding on probing), tissue destruction (probing pocket depth and clinical attachment level) or tooth mobility. These clinical changes were associated with significantly reduced levels of pro-inflammatory mediators and decreased numbers of osteoclasts in bone biopsies. The protective effects of Cp40 persisted, albeit at reduced efficacy, for at least six weeks following drug discontinuation.
Conclusion
Cp40 inhibits pre-existing chronic periodontal inflammation and osteoclastogenesis in non-human primates, suggesting a novel adjunctive anti-inflammatory therapy for treating human periodontitis.
Keywords: periodontitis, inflammation, cytokines, complement, compstatin, non-human primates
Introduction
Periodontitis is a prevalent chronic oral disease that affects nearly half of adults in the U.S.A. and the U.K. and perhaps worldwide (Eke et al., 2015, White et al., 2012, Demmer and Papapanou, 2010). The disease is driven by exaggerated inflammation induced by dysbiotic microbial communities forming on subgingival tooth sites (Lamont and Hajishengallis, 2015) and can lead to tooth loss and impaired mastication and nutritional status (Chapple, 2014). Tooth loss may occur as a result of excessive destruction of periodontium. In its severe form that affects almost 10% of adults (Eke et al., 2012, White et al., 2012), chronic periodontitis is not merely a common cause of tooth loss but is also associated with certain systemic conditions, such as atherosclerosis, diabetes, rheumatoid arthritis, chronic obstructive pulmonary disease, and adverse pregnancy outcomes (Kebschull et al., 2010, Han et al., 2014, Hajishengallis, 2015, Tonetti et al., 2007, Koziel et al., 2014, Lalla and Papapanou, 2011). The serious public health impact of this oral disease and its economic burden (Beikler and Flemmig, 2011, Chapple, 2014) call for innovative treatments adjunctive to existing therapies (such as mechanical removal of the tooth-associated biofilm and antimicrobial treatment), which are not always sufficient to control periodontitis (Colombo et al., 2012, Armitage, 2002, Rams et al., 2014). In the present study, we have tested a complement-targeted therapeutic approach in a highly relevant preclinical model of periodontitis.
The complement system, a network of interacting fluid-phase and cell surface-associated molecules, is centrally involved in immunity and inflammation through direct effects on immune cells or crosstalk and regulation of other host signaling pathways, such as those activated by Toll-like receptors (TLRs) (Hajishengallis and Lambris, 2010). The various components of complement are produced systemically or locally in peripheral tissues and the system can be triggered via distinct cascade mechanisms (classical, lectin, or alternative), all of which converge at the third component (C3) (Ricklin et al., 2010). C3 cleavage and activation by convertases leads to the generation of effector molecules that mediate diverse functions, including recruitment and activation of inflammatory cells (induced by the anaphylatoxins C3a and C5a), microbial opsonization and phagocytosis (via opsonins such as C4b and C3b), and direct lysis of susceptible microbes (by the C5b-9 membrane attack complex) (Ricklin et al., 2010).
Early clinical and histological observations in human periodontitis have associated periodontal inflammation and tissue destruction with increased complement activity (Patters et al., 1989, Nikolopoulou-Papaconstantinou et al., 1987, Schenkein and Genco, 1977, Niekrash and Patters, 1986). Indeed, complement components and their activation products are readily detected in chronically inflamed gingiva and the gingival crevicular fluid (GCF) of patients, whereas they are undetected or present at lower levels in healthy control samples (Courts et al., 1977, Attstrom et al., 1975, Toto et al., 1978, Nikolopoulou-Papaconstantinou et al., 1987, Rautemaa and Meri, 1996, Schenkein and Genco, 1977, Lally et al., 1982). Moreover, induction of experimental gingivitis in human volunteers causes progressive C3 activation in the GCF (Patters et al., 1989). Conversely, a decrease in clinical parameters of inflammation upon successful periodontal therapy leads to reduced C3 activation in the GCF (Niekrash and Patters, 1985).
More recently, studies in animal models, including mouse strains with complement knockout mutations, have established a cause-and-effect relationship between complement activation and periodontitis and gleaned insights into the mechanisms whereby complement mediates periodontitis (Breivik et al., 2011, Liang et al., 2011, Hajishengallis et al., 2011, Maekawa et al., 2014a, Maekawa et al., 2014b). In this regard, we have shown that C3-dependent inflammation in mice is crucial for the long-term sustenance of the dysbiotic microbiota and for maximal induction of alveolar bone loss (Maekawa et al., 2014a). Consistent with these findings, local C3 inhibition in a model of ligature-induced periodontitis in young non-human primates (NHPs) prevents the development of gingival inflammation and alveolar bone loss (Maekawa et al., 2014a). The inhibitor we used was Cp40, an improved analog of compstatin, which is a peptidic compound that blocks C3 activation exclusively in humans and non-human primates (Ricklin and Lambris, 2013, Mastellos et al., 2015b, Qu et al., 2013). Cp40 and other compstatin analogs bind C3 and interfere with its binding to and cleavage by the C3 convertase, thereby blocking the generation of downstream effector molecules regardless of the initiation mechanism of complement activation (Ricklin and Lambris, 2013, Mastellos et al., 2015b).
However, whether Cp40 is effective in a therapeutic – rather than preventive – setting was not addressed in our previous publication. The main objective of the current study was therefore to determine whether local C3 inhibition could inhibit pre-existing chronic periodontal disease, which typically affects adults; in this context, aging is thought to affect the immunoinflammatory status of the periodontal tissue, thereby contributing to increased susceptibility to periodontitis (Hajishengallis, 2014a). This notion is consistent with recent studies in NHPs showing age-dependent differential expression of immune and inflammatory genes in the periodontium (Ebersole et al., 2015, Gonzalez et al., 2015). By screening a population of adult NHPs (cynomolgus monkeys) in the Simian Conservation Breeding and Research Center (SICONBREC; Makati, Philippines), we identified animals with naturally occurring chronic periodontitis and treated them with Cp40. Our results show that locally administered Cp40 can reverse pre-existing chronic periodontal inflammation in the absence of additional treatments, such as scaling and root planing, thus identifying an anti-inflammatory therapy that can potentially contribute to the treatment of human periodontitis.
Materials and Methods
Non-human primates
All animal procedures were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania and of the Simian Conservation Breeding and Research Center (SICONBREC; Makati City, Philippines), an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility where the NHP work was performed. Fifteen adult male cynomolgus monkeys (Macaca fascicularis) (7 – 15 years old; 5.0 – 7.6 kg body weight) were selected for the study after screening the SICONBREC breeding colonies for animals with chronic periodontitis. The inclusion criteria were the presence of at least 30% of sites with probing pocket depth and clinical attachment level ≥ 4 mm, associated with bleeding on probing, and radiographic evidence of bone loss (using a digital X-ray dental system; Vatech). The animals were socially housed in stainless steel cages and were used in the study after they were acclimatized to the protocol procedures for 4 weeks. Environmental enrichment was provided through daily handling by animal care technicians, environmental enrichment items, and visual contact with other study animals. Each animal was offered a measured amount of an approved feed mixture. Fresh, potable drinking water was available to the animals ad libitum. Clinical periodontal examinations, dental X-rays, collection of gingival crevicular fluid (GCF), and periodontal tissue biopsies were performed in a manner similar to a human clinical study, except that the animals were anesthetized during the procedures.
C3 inhibitor
The compstatin analog Cp40 (y-I[CV(1MeW)QDW-Sar-AHRC](NMeI)-NH2; with y = D-tyrosine; Sar = sarcosine/N-methyl glycine) was produced as a disulfide-bridged, cyclic peptide by solid-phase peptide synthesis methodology as previously described (Qu et al., 2013).
Experimental design
The study involved a 6-week treatment period with Cp40 and a 6-week follow-up period without Cp40 treatment. Cp40 was administered either once a week (5 animals; “1X-treatment”) or three times per week (10 animals; “3x-treatment”). Specifically, using a 30-g short needle, Cp40 was injected locally into the gingiva (100 μg/site in 50-μl volume) of anterior and posterior teeth on both sides of the maxilla (15 sites total; 13 sites corresponding to palatal interdental papillae and 2 sites corresponding to the distal gingiva of the second molars). Clinical readings made before Cp40 administration served as baseline controls for each animal. The mandible was not treated with Cp40 but was monitored by clinical periodontal examination and sampling of biological specimens (see below) throughout the study, for comparative purposes. Clinical examinations, sample collection and standard laboratory techniques (immunofluorescence histochemistry, histological TRAP staining, and assessment of host immune responses) are described in Supplementary Methods.
Statistical analysis
For the comparison of mean values within the groups during the time–course studies, one-way repeated-measures ANOVA with Greenhouse-Geisser correction was performed using the GraphPad Prism program, version 6.0h (La Jolla, CA, USA). In case of significant differences, Bonferroni’s or Tukey’s multiple comparisons test was performed.
Results
Locally administered Cp40 inhibits clinical periodontal inflammation in NHPs
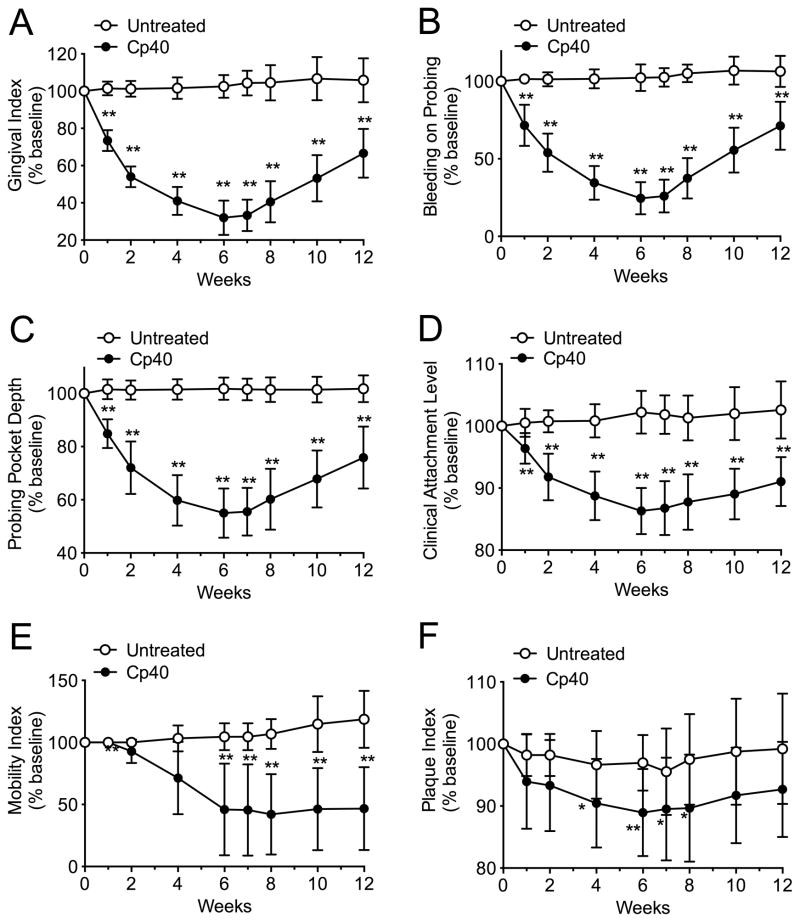
All animals enrolled in the study were systemically healthy and maintained good systemic health during the observation period. No adverse effects were noted during the course of the study. To determine whether complement is causally linked to inflammation associated with naturally occurring chronic periodontitis, we targeted the central complement component C3. Specifically, we locally injected the C3-specific inhibitor Cp40 into the maxillary gingiva of NHPs. Initially, the drug was administered for 6 weeks at a frequency of three times weekly (“3X-treatment”) and its clinical effects were monitored for 12 weeks. Cp40 caused a significant reduction in several clinical indices that measure periodontal inflammation (gingival index and bleeding on probing), tissue destruction (probing pocket depth and clinical attachment level) or tooth mobility often associated with bone loss (Fig. 1A–E). These data indicate that, at least by clinical criteria, Cp40 blocks inflammatory processes that drive periodontal tissue destruction. Interestingly, these protective effects were evident as early as one week after treatment initiation and progressively improved until week 6, when the drug was discontinued. Remarkably, the protective effects of Cp40 persisted for at least six additional weeks, since at the termination of the study (week 12) all indices remained at significantly lower levels relative to their corresponding baselines (Fig. 1A–E). Importantly, without any exception, all ten cynomolgus monkeys responded favorably to Cp40 with 57–87 % reduction in gingival inflammation and 31–58 % decrease in the depths of the periodontal pockets after 6 weeks of treatment (Supplementary figs. S1–S10). The plaque index, a clinical measure of biofilm accumulation on tooth surfaces, was modestly but significantly reduced by Cp40 at a few time points, immediately before and after week 6 (Fig. 1F). The aforementioned clinical indices were also monitored in the untreated jaw (mandible) during the same 12-week interval. In contrast to the improved clinical condition of the Cp40-treated maxilla, the clinical indices in the mandible did not show significant differences in the course of the study as compared to their baseline values (Fig. 1A–F).
Figure 1. Cp40 decreases inflammatory clinical parameters of naturally occurring chronic periodontitis in NHPs after local administration three times weekly.
Cp40 was injected – three times weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). Each animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. The data were expressed relative to the baseline values (at week 0), set as 100 (Raw data are shown for each animal in supplementary figures S1 to S10). Results are means ± SD (n =10 monkeys). *P < 0.05 and **P < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple comparisons test).
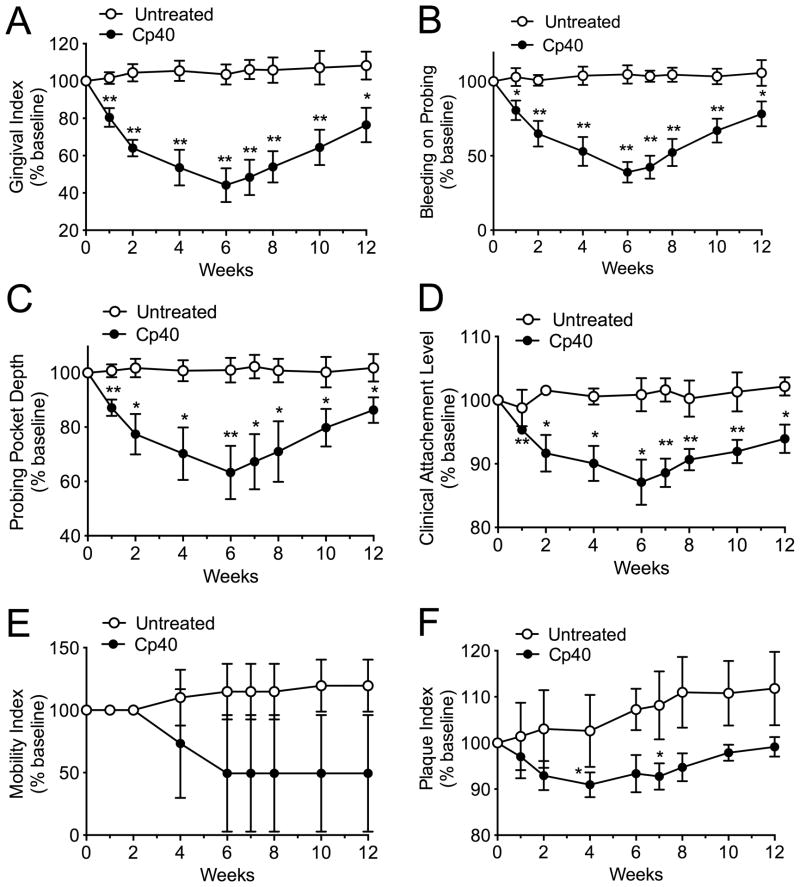
In a second, independent experiment, we investigated whether Cp40 could retain its efficacy if administered only once per week (“1X-treatment”). Similar clinical analyses revealed that a single weekly administration of Cp40 could significantly reduce indices of clinical inflammation and tissue destruction (Fig. 2), with almost comparable efficacy and similar time course pattern to that of the 3X-treatment (see superimposition of the data in Supplementary fig. S11). Moreover, similarly to the 3X-treatment, all five cynomolgus monkeys used in the 1X-treatment study responded favorably to the drug with no exception (Supplementary figs. S12–S16).
Figure 2. Single weekly administration of Cp40 decreases inflammatory clinical parameters of naturally occurring chronic periodontitis in NHPs.
Cp40 was injected – once weekly for 6 weeks – into the interdental papillae and the distal gingiva of the second molars of the maxilla (“Cp40”), whereas the mandible was not treated (“Untreated”). Each animal was clinically examined at the indicated time points and the following clinical parameters were recorded: (A) gingival index; (B) bleeding on probing; (C) probing pocket depth; (D) clinical attachment level; (E) mobility index; and (F) plaque index. The data were expressed relative to the baseline values (at week 0), set as 100 (Raw data are shown for each animal in supplementary figures S12 to S16). Results are means ± SD (n = 5 monkeys). *P < 0.05 and **P < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple comparisons test).
Decreased levels of pro-inflammatory mediators following local treatment with Cp40
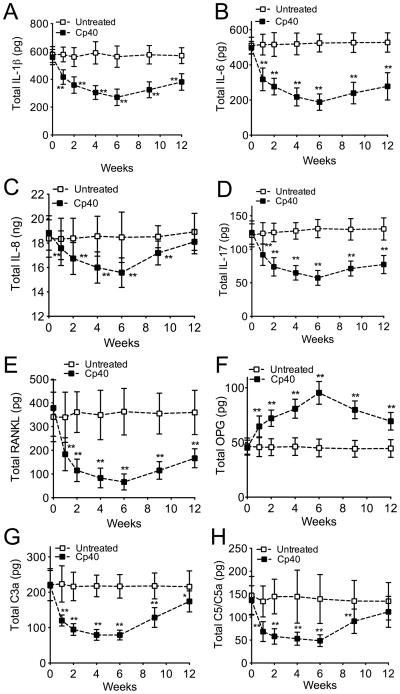
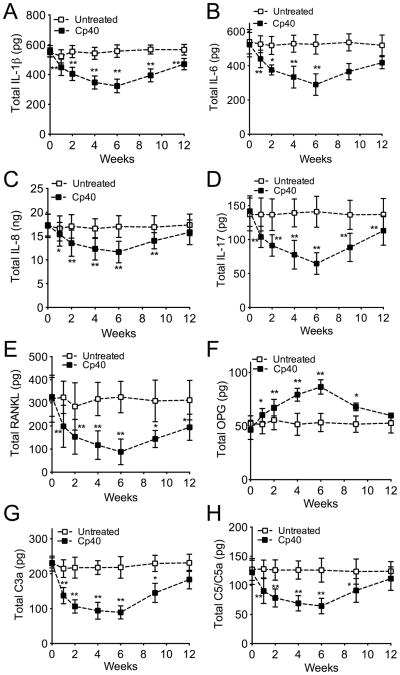
GCF was collected for monitoring changes in the cytokine and immune mediator levels during the 6-week course of Cp40 treatments, as well as during the follow-up period to week 12. Multi-cytokine analysis of the GCF revealed that the 3X-treatment with Cp40 resulted in significantly lower levels of pro-inflammatory and osteoclastogenic cytokines, as compared to their baseline values (Fig. 3). The pro-inflammatory cytokines measured included IL-1β, IL-6, IL-8, and IL-17 (Fig. 3A–D), all of which have been associated with periodontal inflammation in humans (Graves, 2008, Zenobia and Hajishengallis, 2015, Moutsopoulos et al., 2012), and receptor activator of NF-κB ligand (RANKL) (Fig. 3E), a key osteoclastogenic cytokine involved in bone loss disorders including periodontitis (Bostanci et al., 2007, Miossec and Kolls, 2012). In contrast, the GCF levels of osteoprotegerin (OPG), a natural antagonist of RANKL (Bostanci et al., 2007, Miossec and Kolls, 2012), were increased upon Cp40 treatment relative to baseline (Fig. 3F). Cp40 also caused a significant decrease in the GCF levels of C3a and C5/C5a as seen as early as one week after treatment (Figs. 3G and 3H, respectively). These favorable changes in the host response profile (inhibition of pro-inflammatory mediators and upregulation of OPG) were most pronounced at 6 weeks, although significant changes persisted for the entire or most of the study duration (12 weeks), despite drug withdrawal at week 6 (Fig. 3). The same mediators were monitored in GCF samples collected from the untreated jaw (mandible) during the same 12-week interval but did not show significant differences relative to baseline values (Fig. 3). Importantly, Cp40 retained its capacity to significantly suppress the GCF levels of pro-inflammatory mediators and upregulate OPG even when administered only once per week (Fig. 4).
Figure 3. Decreased GCF levels of pro-inflammatory and pro-osteoclastogenic mediators in NHPs with natural periodontitis after local treatment with Cp40 three times weekly.
At the indicated time-points, GCF was collected from monkeys with natural periodontitis which were treated three times weekly for 6 weeks with Cp40 in the maxilla (“Cp40”) but not in the mandible (“Untreated”). Total cytokine or mediator content (A, IL-1β; B, IL-6; C, IL-8; D, IL-17; E, RANKL; F, OPG; G, C3a; H, C5/C5a) in the eluted GCF samples was measured using a Bio-Plex system or ELISA (C3a only). Data are means ± SD (n = 10 monkeys). *P < 0.05; **P < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple comparisons test).
Figure 4. Single weekly administration of Cp40 decreases the levels of pro-inflammatory and pro-osteoclastogenic mediators in the GCF of NHPs with natural periodontitis.
At the indicated time-points, GCF was collected from monkeys with natural periodontitis which were treated once weekly for 6 weeks with Cp40 in the maxilla (“Cp40”) but not in the mandible (“Untreated”). Total cytokine or mediator content (A, IL-1β; B, IL-6; C, IL-8; D, IL-17; E, RANKL; F, OPG; G, C3a; H, C5/C5a) in the eluted GCF samples was measured using a Bio-Plex system or ELISA (C3a only). Data are means ± SD (n = 5 monkeys). *P < 0.05; **P < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple comparisons test).
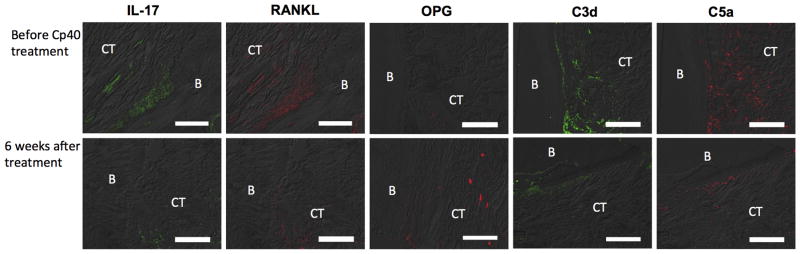
The anti-inflammatory action of Cp40 was also evident in the periodontal tissue (Fig. 5), thus confirming the GCF findings. Specifically, immunofluorescence histochemistry of biopsy specimens taken before (0 weeks) and after (6 weeks) treatment showed that Cp40 caused decreased expression of IL-17, RANKL, and elevated expression of OPG in the connective tissue adjacent to the alveolar bone at 6 weeks relative to baseline (Fig. 5). Moreover, Cp40 treatment caused a decrease in the complement cleavage fragments C3d and C5a (Fig. 5), further confirming its ability to inhibit complement activation.
Figure 5. Expression of inflammatory and osteoclastogenesis-related molecules in periodontal biopsy specimens from Cp40-treated NHPs.
Periodontal biopsy specimens from the maxillae of NHPs treated with Cp40 were processed for fluorescent microscopy. The specimens were taken before (week 0) and after (week 6) treatment with Cp40 locally administered three times weekly. Shown are representative overlays of differential interference contrast (DIC) and fluorescent images stained for the indicated molecules. B, bone; CT, connective tissue. Scale bar, 100 μm.
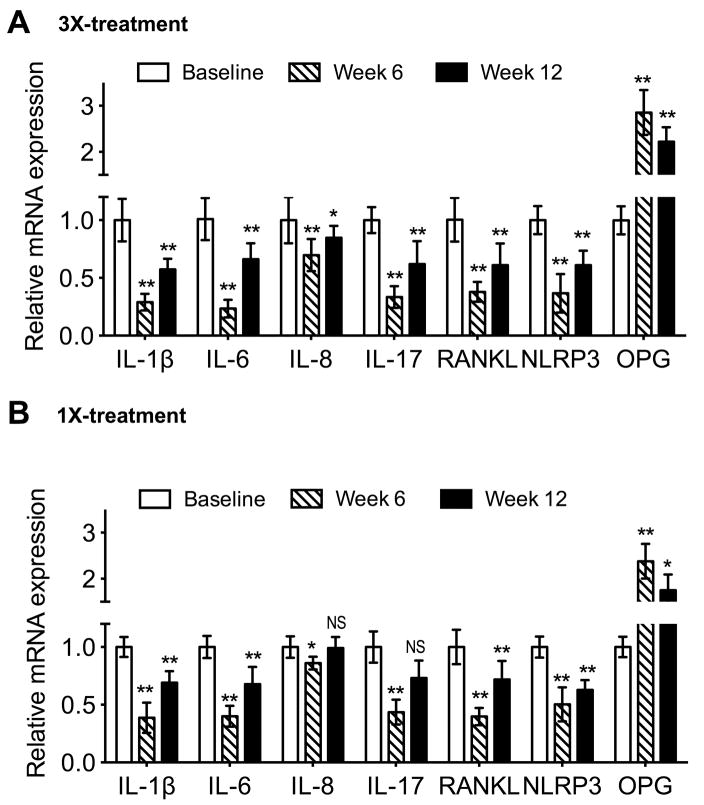
The inhibitory action of Cp40 on the various pro-inflammatory and/or pro-osteoclastogenic cytokines was mediated, at least in part, at the transcriptional level since the expression of gingival IL-1β, IL-6, IL-8, IL-17, and RANKL mRNA was significantly inhibited in animals with 3X or 1X weekly treatments (Fig. 6A and B, respectively). Conversely, and in accord with the protein data, OPG mRNA expression was increased (Fig. 6). The characteristic elevation of IL-1β in human periodontitis (compared to periodontal health) has been correlated with increased NLRP3 (NALP3) inflammasome mRNA expression levels (Bostanci et al., 2009). In this regard, Cp40 inhibited the gingival NLRP3 mRNA expression in NHPs (Fig. 6), suggesting its potential to interfere with NLRP3 inflammasome-dependent processing of IL-1β; this notion is consistent with the reduced levels of IL-1β protein in the GCF of Cp40-treated animals (Fig. 3A and 4A).
Figure 6. Cp40 inhibits mRNA expression of gingival inflammatory mediators in NHPs.
Gingival tissue biopsies were taken from the maxilla of NHPs treated three times (A) or once (B) weekly for 6 weeks and extracted RNA was subjected to real-time PCR processed to determine mRNA expression of the indicated molecules at the indicated times. Data were normalized to GADPH mRNA and are presented as fold change in the transcript levels relative to baseline levels (prior to treatment; week 0), set as 1. Data are means ± SD (A, n = 10 monkeys; B, n = 5 monkeys). *P < 0.05; **P < 0.01 compared to baseline (one-way repeated-measures ANOVA and Bonferroni’s multiple comparisons test). NS, not significant.
Cp40 causes a reduction in the numbers of periodontal osteoclasts in NHPs
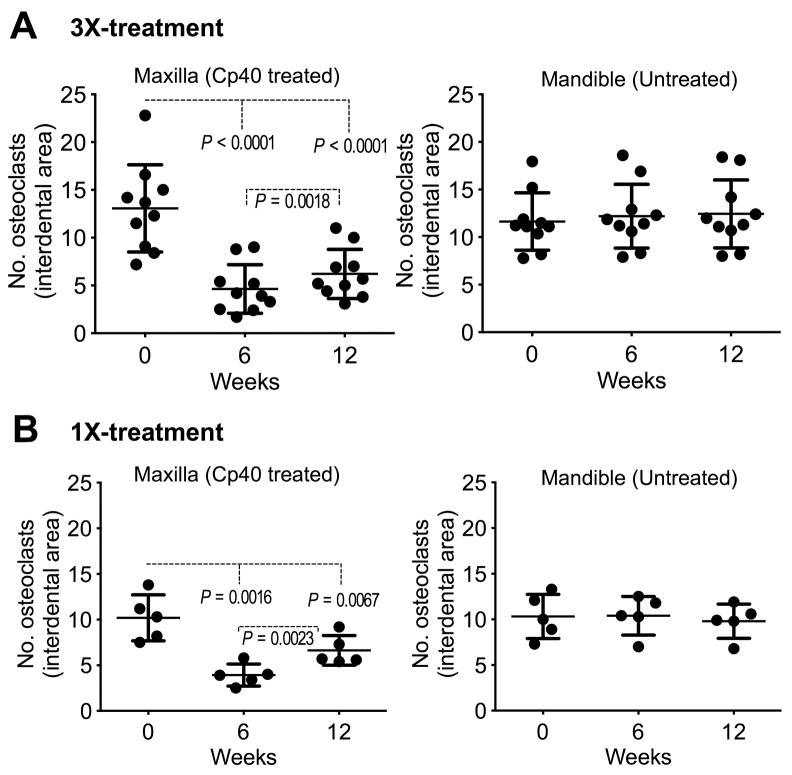
The RANKL/OPG ratio in the GCF is a potential indicator of periodontitis (Belibasakis and Bostanci, 2012, Bostanci et al., 2007). We therefore determined whether the ability of Cp40 to decrease the GCF levels of RANKL and enhance the levels of OPG (Figs. 3 and 4), hence to lower the RANKL/OPG ratio, could have an impact on osteoclastogenesis. To this end, we counted the numbers of osteoclasts (TRAP-positive multinucleated cells; Supplementary fig. S17) in bone biopsies taken at baseline and at 6 and 12 weeks following Cp40 treatment. We found that in the maxillae of the animals, Cp40 caused a significant decrease in the numbers of osteoclasts after 6 weeks of 3X or 1X weekly treatments (Fig. 7, A and B [left panels]). This favorable effect persisted through to week 12, even though the drug was not administered in the last 6 weeks (Fig. 7, A and B, left panels). In contrast, the numbers of osteoclasts in the mandibles, which were not treated, did not display significant differences in the course of the study (Fig. 7, A and B, right panels).
Figure 7. Decreased numbers of osteoclasts after treatment of natural NHP periodontitis with Cp40.
At the indicated time-points, alveolar bone biopsies were obtained from monkeys with natural periodontitis. The animals were treated three times (A) or once (B) weekly for 6 weeks with Cp40 in the maxilla but not in the mandible (“Untreated”). TRAP-positive multinucleated cells (osteoclasts) were enumerated in 10 randomly selected sections for each bone biopsy specimen from the maxilla or mandible of each of the animals. The numbers of osteoclasts were averaged for each biopsy specimen and are represented by dots. Scatter dot plots with mean ± SD are shown for each group of monkeys (A, n = 10; B, n = 5 animals). Indicated P values determined by one-way repeated-measures ANOVA and Tukey’s multiple comparisons test.
Discussion
To the best of our knowledge, this study marks the first time that a pharmacological intervention – whether host-modulation or antimicrobial – is shown to inhibit inflammation in the context of naturally occurring periodontitis in NHPs. Previously conducted studies, including our own, have used inducible models of the disease involving placement of ligatures with or without exogenous inoculation of periodontal pathogens (Page et al., 2007, Assuma et al., 1998, Cappelli et al., 2000, Maekawa et al., 2014a, Moritz et al., 1998, Nisengard et al., 1989, Persson et al., 1994, Li et al., 1996, Offenbacher et al., 1987, Roberts et al., 2004, Pierce and Lindskog, 1987, Shin et al., 2015). The immune system and periodontal anatomy of the cynomolgus monkey is similar to that of humans, and periodontitis in these animals exhibits clinical, microbiological, and immunohistological features that are highly similar to those observed in human periodontitis (Brecx et al., 1985, Page and Schroeder, 1982, Kornman et al., 1981, Ebersole et al., 2014, Ebersole et al., 2002). Therefore, the cynomolgus model, especially in the setting of naturally occurring periodontitis, is considerably more predictive of drug efficacy in humans compared to widely used models, such as those in rodents, rabbits, or dogs. The successful Cp40 inhibition of natural periodontal inflammation and osteoclastogenesis in NHPs additionally shows unequivocally that C3 is critical for the pathogenesis of this oral disease, as suggested by earlier correlative human clinical studies (Toto et al., 1978, Schenkein and Genco, 1977, Patters et al., 1989, Niekrash and Patters, 1985, Attstrom et al., 1975, Nikolopoulou-Papaconstantinou et al., 1987, Hajishengallis, 2010). In conjunction with the earlier human studies, the present work places periodontitis to a growing list of diseases with substantial complement involvement, including paroxysmal nocturnal hemoglobinuria (PNH), age-related macular degeneration, atypical hemolytic uremic syndrome, and rheumatoid arthritis (Ricklin and Lambris, 2013). Consistent with the role of C3 as a potential therapeutic target in periodontitis, a recent report has identified C3 among the top 21 most promising candidate genes involved in periodontitis, by using an integrative gene prioritization method and databases from genome-wide association studies and microarray experiments (Zhan et al., 2014).
The complement-inhibition capacity of Cp40 was confirmed by findings of decreased GCF levels of C3a and C5/C5a. The detecting antibody utilized in the latter assay does not distinguish between C5 and the activation product C5a and, therefore, C5 levels could be reduced indirectly by Cp40 through inhibition of inflammation (Zou et al., 2013). However, the ability of Cp40 to reduce C3a definitely confirmed its potential to inhibit complement activation in our study. In this regard, it is uncertain if intra-gingivally administered Cp40 inhibited complement activation in the gingival tissue resulting in lower levels of available C3a to transudate to the periodontal pocket or whether Cp40 also diffused to the periodontal pocket where it could additionally block complement activation in the GCF. Although both possibilities are likely, the ability of Cp40 to block complement in the gingiva was confirmed by immunohistochemistry that showed decreased levels of the complement cleavage products (C3d and C5a) in the connective tissue in the vicinity of the bone.
Cp40 is the first compstatin analog with subnanomolar target affinity (KD = 0.5 nM) and features a plasma half-life that exceeds expectations for most peptidic drugs (Qu et al., 2013, Mastellos et al., 2015b). The relatively long half-life of the Cp40 peptide was attributed to a “target-driven” model, according to which an initial rapid clearance of excess free peptide (i.e., not bound to C3) is followed by slow clearance of C3-bound peptide. As the measured half-life values of different compstatin analogs correlate positively with their binding affinities for C3 (Qu et al., 2013), it can be inferred that the tight binding of Cp40 to C3 delays its clearance. Similarly, the expected tight binding of Cp40 to abundant C3 in the inflamed periodontium could contribute to delayed elimination of the drug from the tissues, in turn accounting– at least in part– for the sustained protective effect of Cp40 observed in this study. Alternatively, or additionally, it is likely that the observed suppression of inflammation by Cp40 tips the balance towards tissue homeostasis, which might then resist pathological processes for some time even in the absence of the drug. In this regard, the clinical periodontal indices, the GCF levels of pro-inflammatory mediators, and the numbers of periodontal osteoclasts persisted at lower levels than their corresponding baseline values for at least 6 weeks following drug withdrawal.
Given that complement is not a linear cascade of events but essentially a hub-like network that is tightly connected to other components of the immune system (Ricklin et al., 2010), it is plausible that C3 inhibition has a far-reaching impact above and beyond complement itself. For instance, C3a- and C5a-induced signaling pathways cross-talk with and amplify TLR-mediated inflammatory responses in various tissues including the periodontium (Abe et al., 2012, Hajishengallis and Lambris, 2010, Zhang et al., 2007). Complement inhibition, therefore, is likely to mitigate inflammation initiated through TLR activation, either by microbial ligands (e.g., bacterial lipopolysaccharide or lipoproteins) or by endogenous molecules acting as danger signals upon their release due to inflammatory tissue destruction (e.g., biglycan, hyaluronan fragments, and heparan sulfate fragments) (Miyake, 2007, Schaefer, 2010). These considerations may in part explain why the inhibition of a single molecule, C3, has a strong influence on the course of natural periodontal inflammation.
It was also recently shown that, in human monocytes, C3a regulates the release of intracellular ATP into the extracellular space, thereby controlling NLRP3 inflammasome activation and subsequent secretion of IL-1β, which in turn promotes human CD4+ T cell production of IL-17 (Asgari et al., 2013). In this regard, a clinical study has shown enhanced expression of NLRP3 (NALP3) inflammasome in periodontitis correlating with increased IL-1β expression levels (Bostanci et al., 2009). Moreover, the formation of sublytic C5b-9 complex on human epithelial cells induces intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation and IL-1β release (Triantafilou et al., 2013). Both of these complement-dependent inflammatory mechanisms can be blocked by Cp40, potentially accounting – at least in part – for the reduced IL-1β and IL-17 levels after Cp40 treatment. In fact, the ability of Cp40 to reduce the levels of IL-1β protein in the GCF might, in part, be related to its capacity to inhibit the expression of NLRP3 which is involved in caspase-1–mediated processing and release of IL-1β protein (Lamkanfi and Dixit, 2014).
Cp40-mediated inhibition of IL-17 could in turn contribute to the observed suppression of RANKL, since IL-17 upregulates RANKL by acting on lymphocytes and stromal cells (e.g., fibroblasts and osteoblasts) (Miossec and Kolls, 2012). Besides inhibiting RANKL expression, Cp40 also caused a concomitant increase in the levels of OPG, both in the gingival tissue and the GCF. Although the underlying mechanisms are uncertain, RANKL and OPG were shown to be conversely regulated by several stimuli (Boespflug et al., 2014, Lee and Lorenzo, 1999, Devi et al., 2013). For instance, IL-17 was shown to induce RANKL and suppress OPG expression in human periodontal ligament cells (Devi et al., 2013). Consistently, the ability of Cp40 to decrease the RANKL/OPG ratio was associated with decreased osteoclastogenesis in bone biopsy samples. Therefore, Cp40 has the potential to inhibit naturally occurring bone loss, in accord with its capacity to block ligature-induced bone loss in NHPs (Maekawa et al., 2014a). Although induction of measurable bone loss can be observed within a few weeks in the relatively acute ligature-induced model, bone loss is a relatively slow process in naturally occurring chronic periodontitis (hence changes in bone loss could not be detected radiographically in the current study due to its relatively short duration).
By inhibiting complement at the level of C3, Cp40 (and earlier compstatin analogs) does not interfere with C4b-mediated opsonization of bacteria via the classical and lectin pathways (Kirjavainen et al., 2008). Nevertheless, for increased safety, Cp40 treatments in disease settings requiring long-term systemic intervention (e.g., in PNH (Risitano et al., 2014)) would necessitate vaccination against encapsulated bacteria (e.g., meningococci) to minimize the risk of potential infections (Mastellos et al., 2015a). Importantly, these potential safety considerations are unlikely to apply to the treatment of periodontitis through local Cp40 administration. In this regard, C3-deficient mice display reduced periodontal bacterial load compared to C3-sufficient controls in the course of experimental periodontitis (Maekawa et al., 2014a), suggesting that impaired complement activation does not predispose to defective immune surveillance in the periodontium. These findings are in accord with the notion that inflammation generates tissue breakdown products (e.g., degraded collagen peptides or heme-containing compounds) that serve the nutritional needs of periodontitis-associated bacteria (Hajishengallis, 2014b, Marsh, 2003). Indeed, studies in mouse and rabbit models of periodontitis indicate that the control of inflammation also reduces the bacterial load (Hasturk et al., 2007, Eskan et al., 2012, Abe et al., 2014, Moutsopoulos et al., 2014). Conversely, and consistently, the bacterial biomass of human periodontitis-associated biofilms increases with escalating periodontal inflammation (Abusleme et al., 2013).
Although Cp40 was successfully applied as a stand-alone treatment in the current NHP study, it can be envisioned as an adjunctive therapy to the management of human chronic periodontitis. Future clinical trials could investigate the potential of Cp40 to inhibit periodontal inflammation and bone loss compared to scaling and root planing, whereas in very severe cases of the disease, Cp40 could be combined with scaling and root planing and compared to periodontal surgery, in an effort to obviate the need for a surgical approach. It should be noted that future host-modulation interventions, such as Cp40, would not necessarily be implemented in a therapeutic setting but could also be provided on a preventive basis to high-risk individuals, such as cigarette smokers and diabetic patients (Heitz-Mayfield, 2005, Genco and Genco, 2014), before the onset of periodontitis. A clinically developed Cp40-based drug (AMY-101; Amyndas Pharmaceuticals) is currently under evaluation as a potential treatment of complications of ABO-incompatible kidney transplantation and PNH (Mastellos et al., 2015b). Whether Cp40/AMY-101 can find application for the treatment of human periodontitis is a possibility that– based on the results of this NHP study– merits investigation in future clinical trials.
Supplementary Material
Clinical Relevance.
Scientific rationale for study
Most interventional studies in animal models of periodontitis are performed in a preventive (rather than therapeutic) setting and involve inducible models of the disease. To increase the predictive value of preclinical intervention for drug efficacy in humans, we used non-human primates with naturally occurring periodontitis to test the therapeutic potential of a complement inhibitor (Cp40).
Principal findings
Cp40 inhibited pre-existing periodontal inflammation (determined by both clinical and laboratory assessment) and osteoclastogenesis in non-human primates, a clinically relevant model of human periodontitis.
Practical implications
These findings pave the way for testing Cp40 in future clinical trials for the treatment of human periodontitis.
Acknowledgments
We thank Dr. Niki M. Moutsopoulos (NIDCR/NIH) for comments and suggestions.
Footnotes
Conflict of interest and source of funding statement
G.H. and J.D.L. have a joint patent application that describes the use of complement inhibitors for therapeutic purposes in periodontitis. J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. This work was supported by grants from the National Institutes of Health (AI068730 and AI030040 to J.D.L.; DE015254, DE017138, DE021685, and DE024716 to G.H.) and the European Commission (FP7-DIREKT 602699 to J.D.L.).
References
- Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, Hajishengallis G. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Shin J, Hosur K, Udey MC, Chavakis T, Hajishengallis G. Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8. J Immunol. 2014;193:1383–1391. doi: 10.4049/jimmunol.1400970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage GC. Classifying periodontal diseases: a long-standing dilemma. Periodontol 2000. 2002;30:9–23. doi: 10.1034/j.1600-0757.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, Cook HT, Kemper C. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Attstrom R, Laurel AB, Lahsson U, Sjoholm A. Complement factors in gingival crevice material from healthy and inflamed gingiva in humans. J Periodont Res. 1975;10:19–27. doi: 10.1111/j.1600-0765.1975.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Beikler T, Flemmig TF. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontol 2000. 2011;55:87–103. doi: 10.1111/j.1600-0757.2010.00360.x. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- Boespflug ND, Kumar S, McAlees JW, Phelan JD, Grimes HL, Hoebe K, Hai T, Filippi MD, Karp CL. ATF3 is a novel regulator of mouse neutrophil migration. Blood. 2014;123:2084–2093. doi: 10.1182/blood-2013-06-510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, Curtis MA, Belibasakis GN. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. 2009;157:415–422. doi: 10.1111/j.1365-2249.2009.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Toz H, Atilla G, Hughes FJ, Belibasakis GN. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: implications of their relative ratio. J Clin Periodontol. 2007;34:370–376. doi: 10.1111/j.1600-051X.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Brecx MC, Nalbandian J, Ooya K, Kornman KS, Robertson PB. Morphological studies on periodontal disease in the cynomolgus monkey. II. Light microscopic observations on ligature-induced periodontitis. J Periodontal Res. 1985;20:165–175. doi: 10.1111/j.1600-0765.1985.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Breivik T, Gundersen Y, Gjermo P, Taylor SM, Woodruff TM, Opstad PK. Oral treatment with complement factor C5a receptor (CD88) antagonists inhibits experimental periodontitis in rats. J Periodont Res. 2011;46:643–647. doi: 10.1111/j.1600-0765.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- Cappelli D, Holt SC, Singer RE, Pickrum HM, Ebersole JL. Effects of 0.12% chlorhexidine gluconate on experimental gingivitis in non-human primates: clinical and microbiological alterations. Oral Dis. 2000;6:124–131. doi: 10.1111/j.1601-0825.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Chapple IL. Time to take periodontitis seriously. BMJ. 2014;348:g2645. doi: 10.1136/bmj.g2645. [DOI] [PubMed] [Google Scholar]
- Colombo AP, Bennet S, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst FE, Paster BJ. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol. 2012;83:1279–1287. doi: 10.1902/jop.2012.110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courts FJ, Boackle RJ, Fudenberg HH, Silverman MS. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J Dent Res. 1977;56:327–331. doi: 10.1177/00220345770560032001. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, Hall P, Ooi JD, Sobey CG, Kitching AR, Hickey MJ. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19:107–112. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Cappelli D, Mathys EC, Steffen MJ, Singer RE, Montgomery M, Mott GE, Novak MJ. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann Periodontol. 2002;7:102–111. doi: 10.1902/annals.2002.7.1.102. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Kirakodu S, Novak MJ, Exposto CR, Stromberg AJ, Shen S, Orraca L, Gonzalez-Martinez J, Gonzalez OA. Effects of aging in the expression of NOD-like receptors and inflammasome-related genes in oral mucosa. Mol Oral Microbiol. 2015 doi: 10.1111/omi.12121. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole JL, Kirakodu S, Novak MJ, Stromberg AJ, Shen S, Orraca L, Gonzalez-Martinez J, Burgos A, Gonzalez OA. Cytokine gene expression profiles during initiation, progression and resolution of periodontitis. J Clin Periodontol. 2014;41:853–861. doi: 10.1111/jcpe.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco RJ, Genco FD. Common risk factors in the management of periodontal and associated systemic diseases: the dental setting and interprofessional collaboration. J Evid Based Dent Pract. 2014;14(Suppl):4–16. doi: 10.1016/j.jebdp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez OA, Nagarajan R, Novak MJ, Orraca L, Gonzalez-Martinez JA, Kirakodu SS, Ebersole JL. Immune system transcriptome in gingival tissues of young nonhuman primates. J Periodontal Res. 2015 doi: 10.1111/jre.12293. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Complement and periodontitis. Biochem Pharmacol. 2010;80:1992–2001. doi: 10.1016/j.bcp.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Aging and its impact on innate immunity and inflammation: Implications for periodontitis. J Oral Biosci. 2014a;56:30–37. doi: 10.1016/j.job.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014b;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Houcken W, Loos BG, Schenkein HA, Tezal M. Periodontal disease, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Adv Dent Res. 2014;26:47–55. doi: 10.1177/0022034514528334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ. Disease progression: identification of high-risk groups and individuals for periodontitis. J Clin Periodontol. 2005;32(Suppl 6):196–209. doi: 10.1111/j.1600-051X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- Kebschull M, Demmer RT, Papapanou PN. “Gum bug leave my heart alone”: Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen V, Jarva H, Biedzka-Sarek M, Blom AM, Skurnik M, Meri S. Yersinia enterocolitica serum resistance proteins YadA and ail bind the complement regulator C4b-binding protein. PLoS Pathog. 2008;4:e1000140. doi: 10.1371/journal.ppat.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman KS, Holt SC, Robertson PB. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J Periodont Res. 1981;16:363–371. doi: 10.1111/j.1600-0765.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 2014;16:408. doi: 10.1007/s11926-014-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- Lally ET, McArthur WP, Baehni PC. Biosynthesis of complement components in chronically inflamed gingiva. J Periodont Res. 1982;17:257–262. doi: 10.1111/j.1600-0765.1982.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology. 1999;140:3552–3561. doi: 10.1210/endo.140.8.6887. [DOI] [PubMed] [Google Scholar]
- Li KL, Vogel R, Jeffcoat MK, Alfano MC, Smith MA, Collins JG, Offenbacher S. The effect of ketoprofen creams on periodontal disease in rhesus monkeys. J Periodont Res. 1996;31:525–532. doi: 10.1111/j.1600-0765.1996.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, Li F, Tzekou A, Lambris JD, Hajishengallis G. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Abe T, Hajishengallis E, Hosur KB, DeAngelis RA, Ricklin D, Lambris JD, Hajishengallis G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014a;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, Hashim A, Hoch S, Curtis MA, Nussbaum G, Lambris JD, Hajishengallis G. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014b;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- Mastellos DC, Ricklin D, Hajishengallis E, Hajishengallis G, Lambris JD. Complement therapeutics in inflammatory diseases: promising drug candidates for C3-targeted intervention. Mol Oral Microbiol. 2015a doi: 10.1111/omi.12129. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos DC, Yancopoulou D, Kokkinos P, Huber-Lang M, Hajishengallis G, Biglarnia AR, Lupu F, Nilsson B, Risitano AM, Ricklin D, Lambris JD. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest. 2015b;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J Periodontol. 1998;69:686–697. doi: 10.1902/jop.1998.69.6.686. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S, Osorio M, Wahl SM. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012;39:294–303. doi: 10.1016/j.jaut.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, Uzel G, Chen W, Chavakis T, Holland SM, Hajishengallis G. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17–driven inflammatory bone loss. Sci Transl Med. 2014;6:229ra240. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekrash CE, Patters MR. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. J Periodont Res. 1985;20:268–275. doi: 10.1111/j.1600-0765.1985.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Niekrash CE, Patters MR. Assessment of complement cleavage in gingival fluid in humans with and without periodontal disease. J Periodont Res. 1986;21:233–242. doi: 10.1111/j.1600-0765.1986.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol Scand. 1987;45:187–193. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- Nisengard R, Blann D, Zelonis L, McHenry K, Reynolds H, Zambon J. Effects of immunization with B. macacae on induced periodontitis--preliminary findings. Immunol Invest. 1989;18:225–237. doi: 10.3109/08820138909112239. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Braswell LD, Loos AS, Johnson HG, Hall CM, McClure H, Orkin JL, Strobert EA, Green MD, Odle BM. Effects of flurbiprofen on the progression of periodontitis in Macaca mulatta. J Periodont Res. 1987;22:473–481. doi: 10.1111/j.1600-0765.1987.tb02058.x. [DOI] [PubMed] [Google Scholar]
- Page RC, Lantz MS, Darveau R, Jeffcoat M, Mancl L, Houston L, Braham P, Persson GR. Immunization of Macaca fascicularis against experimental periodontitis using a vaccine containing cysteine proteases purified from Porphyromonas gingivalis. Oral Microbiol Immunol. 2007;22:162–168. doi: 10.1111/j.1399-302X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Page RC, Schroeder HE. Periodontitis in man and other animals- A comparative review. Basel, Switzerland: Karger; 1982. [Google Scholar]
- Patters MR, Niekrash CE, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J Clin Periodontol. 1989;16:33–37. doi: 10.1111/j.1600-051x.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- Persson GR, Engel D, Whitney C, Darveau R, Weinberg A, Brunsvold M, Page RC. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect Immun. 1994;62:1026–1031. doi: 10.1128/iai.62.3.1026-1031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A, Lindskog S. The effect of an antibiotic/corticosteroid paste on inflammatory root resorption in vivo. Oral Surg Oral Med Oral Pathol. 1987;64:216–220. doi: 10.1016/0030-4220(87)90094-6. [DOI] [PubMed] [Google Scholar]
- Qu H, Ricklin D, Bai H, Chen H, Reis ES, Maciejewski M, Tzekou A, DeAngelis RA, Resuello RR, Lupu F, Barlow PN, Lambris JD. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rams TE, Degener JE, van Winkelhoff AJ. Antibiotic resistance in human chronic periodontitis microbiota. J Periodontol. 2014;85:160–169. doi: 10.1902/jop.2013.130142. [DOI] [PubMed] [Google Scholar]
- Rautemaa R, Meri S. Protection of gingival epithelium against complement-mediated damage by strong expression of the membrane attack complex inhibitor protectin (CD59) J Dent Res. 1996;75:568–574. doi: 10.1177/00220345960750010901. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190:3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M, Del Vecchio L, Pane F, Lupu F, Notaro R, Resuello RR, DeAngelis RA, Lambris JD. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–2101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts FA, Houston LS, Lukehart SA, Mancl LA, Persson GR, Page RC. Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infect Immun. 2004;72:1166–1168. doi: 10.1128/IAI.72.2.1166-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2010;10:185–190. doi: 10.1016/j.coph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J Periodontol. 1977;48:778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- Shin J, Maekawa T, Abe T, Hajishengallis E, Hosur K, Pyaram K, Mitroulis I, Chavakis T, Hajishengallis G. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci Transl Med. 2015;7:307ra155. doi: 10.1126/scitranslmed.aac5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Toto PD, Lin L, Gargiulo A. Identification of C3a, IgG, IgM in inflamed human gingiva. J Dent Res. 1978;57:696. doi: 10.1177/00220345780570050501. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- White DA, Tsakos G, Pitts NB, Fuller E, Douglas GV, Murray JJ, Steele JG. Adult Dental Health Survey 2009: common oral health conditions and their impact on the population. Br Dent J. 2012;213:567–572. doi: 10.1038/sj.bdj.2012.1088. [DOI] [PubMed] [Google Scholar]
- Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 2015;69:142–159. doi: 10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Zhang R, Lv H, Song X, Xu X, Chai L, Lv W, Shang Z, Jiang Y, Zhang R. Prioritization of candidate genes for periodontitis using multiple computational tools. J Periodontol. 2014;85:1059–1069. doi: 10.1902/jop.2014.130523. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Feng Y, Li Y, Zhang M, Chen C, Cai J, Gong Y, Wang L, Thurman JM, Wu X, Atkinson JP, Chao W. Complement Factor B Is the Downstream Effector of TLRs and Plays an Important Role in a Mouse Model of Severe Sepsis. J Immunol. 2013;191:5625–5635. doi: 10.4049/jimmunol.1301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.