Abstract
Large population studies have shown that living at higher altitudes, which lowers ambient oxygen exposure, is associated with reduced cardiovascular disease mortality. However, hypoxia has also been reported to promote atherosclerosis by worsening lipid metabolism and inflammation. We sought to address these disparate reports by reducing the ambient oxygen exposure of ApoE−/− mice. We observed that long term adaptation to 10% O2 (equivalent to oxygen content at ~5000 m), compared to 21% O2 (room air at sea level), resulted in a marked decrease in aortic atherosclerosis in ApoE−/− mice. This effect was associated with increased expression of the anti-inflammatory cytokine interleukin-10 (IL-10), known to be anti-atherogenic and regulated by hypoxia-inducible transcription factor-1α (HIF-1α). Supporting these observations, ApoE−/− mice that were deficient in IL-10 (IL10−/− ApoE−/− double knockout) failed to show reduced atherosclerosis in 10% oxygen. Our study reveals a specific mechanism that can help explain the decreased prevalence of ischemic heart disease in populations living at high altitudes and identifies ambient oxygen exposure as a potential factor that could be modulated to alter pathogenesis.
Keywords: oxygen, hypoxia, atherosclerosis, anti-inflammatory, IL-10
Introduction
Various epidemiologic studies over the course of decades have shown that living in high altitude environments is associated with decreased cardiovascular disease incidence, in particular a lower risk of coronary artery disease (CAD) [1, 2]. The Swiss National Cohort study involving 14.5 million person-years of follow up showed a significant risk reduction in both CAD and stroke of 22% and 12% per 1000 meter elevation, respectively [2]. A number of reasons have been proposed for this observation, including the physiological changes associated with adaptation to higher altitudes, increased physical activity, better dietary habits, lower CAD risk factors, and environmental factors such as UV, vitamin D and air pollutants. A more specific delineation of the molecular mechanism(s) underlying the association between higher altitude and lower atherosclerotic heart disease mortality could provide insights for developing new strategies for maintaining cardiovascular health.
In contrast to human observational studies, hypoxia, associated with higher altitudes, has been reported to accelerate atherosclerosis in animal models. In one study, ApoE−/− mice exposed to 10% ambient oxygen for a relatively short period of time (3 wk) showed increased lipid levels in blood and in aortic tissue [3]. In another study that investigated the association between hypoxia and cardiovascular disease, blood lipid levels and aortic plaque growth of ApoE−/− mice were increased upon being exposed to intermittent hypoxia as a model of obstructive sleep apnea that consisted of cycling at 1 min intervals between room air and 6.5% O2 over a 4 to 12 wk period [4]. In these studies, the stress response to relatively acute or intermittent hypoxia could have contributed to the exacerbation of atherosclerosis and may not necessarily apply to physiologic adaptation to reduced ambient oxygen such as in humans residing at higher altitudes over a lifetime.
It is well-established that vascular inflammation involving both innate and adaptive immunity plays a critical role in atherosclerosis and that there is evidence of pathologic necrosis due to tissue ischemia in atherosclerotic plaques [5]. Although hypoxia and HIF-1α pathways are known to control the immune systems [6], the role of oxygen and its sensing mechanism in atherosclerosis is less clear. The conditional knockout of HIF-1α in myeloid cells showed that it is essential for the pro-inflammatory activity of macrophages which contributes to atherosclerotic plaque development [7]. In contrast, mice with HIF-1α deficient T lymphocytes display a stronger inflammatory response to bacterial sepsis, suggesting that HIF-1α can also have immune suppressive effects [8]. Indeed, the overexpression of HIF-1α in the lymphocytes of ApoE−/− mice decreased interferon γ (IFN-γ) expression and atherosclerosis in association with increased anti-inflammatory and anti-atherogenic cytokine IL-10 [9, 10]. Moreover, another body of work suggests that hypoxia can also suppress immune response through a HIF-activated signaling pathway involving adenosine receptors [11].
Given the strength of the human epidemiologic data, we performed a reverse translational study investigating whether atherosclerosis progression can be altered in ApoE−/− mice physiologically adapted to low ambient oxygen from a young age. Here, we report that chronic adaptation to hypoxia protects ApoE−/− mice from atherosclerosis via increased anti-inflammatory activity. Using in vitro and genetic mouse models, we further show that the expression of IL10 is directly regulated by HIF-1α and that this cytokine plays an important role in mediating the anti-atherogenic effect of low ambient oxygen.
Materials and Methods
Study approval
All mice were maintained and handled in accordance with the NHLBI Animal Care and Use Committee. All human samples were obtained from healthy volunteers after informed consent as approved by the NHLBI Institutional Review Board.
Antibodies and western blotting
Antibodies were as follows: control rabbit IgG (Santa Cruz, sc-2027); rabbit polyclonal anti-HIF-1α (Cayman Chemical, cat. #10006421); and monoclonal anti-β-actin (Sigma, AC-15) antibodies. Protein samples were solubilized in loading buffer, resolved by Tris-glycine SDS PAGE, and transferred to Immobilon-P membrane (Millipore) for standard ECL western blotting.
Cell culture
Raw 264.7 murine macrophage cell line was obtained from the American Type Culture Collection and maintained as recommended. Mouse CD4+ T cells were isolated from spleens using a CD4+ isolation kit (Miltenyi) and human T cells were prepared from peripheral blood mononuclear cells (PBMCs). T cells were expanded by activating on immobilized anti-CD3 and anti-CD28 antibodies in a 21% or 5% O2 tissue culture incubator for 3 d as previously described [12]. RPMI medium (Invitrogen) supplemented with 10% FBS and 2 μM β-mercaptoethanol was used for culturing T cells. Bone marrow-derived macrophages (BMDM) were isolated from mouse femora and tibia, cultured using standard protocols, and stimulated with 10 ng/ml lipopolysaccharide (LPS, Sigma) for the indicated time [13]. Hypoxia mimetic deferoxamine for tissue culture experiments was obtained from Sigma.
Mice and hypoxia chamber
The ApoE−/− and IL10−/− mice (>10 generations backcrossed to C57BL6, ApoE−/− stock # 002052 and IL10−/− stock # 002251) were obtained from Jackson Laboratories and maintained according to recommended animal care conditions. IL10−/− ApoE−/− double knockout mice were obtained after 3 generations of crossing the individual gene knockout mice. Mice were fed normal chow diet (NIH-31, Envigo). Sterilized mouse cages, food and water were used in experiments that involved the IL10−/− genotype as previously described [14]. For chronic hypoxia experiments, 5–6 wk old female mice in standard sized mouse cages were placed in a large hypoxia chamber (Coy Laboratory Product Inc.) set at 10% O2 and 30–70% humidity at room temperature as previously described [15, 16]. CO2 was set at ≤0.5% and all set parameters were continuously monitored with internal and external probes through a tele-alarm service (Rees Scientific). Mice were exposed to 10% O2 for 22–23 wk prior to aortic atherosclerotic plaque analyses. The control normoxia mice were kept long term in room air (Fig. 1) or in the chamber with identical settings as described above except that O2 was maintained at 21% (Fig. 4). For in vivo LPS stimulation studies, ApoE−/− mice were exposed to hypoxia for 5 wk and then intraperitoneally injected with 0.1 μg of LPS (E. coli 0127:B8, Sigma) per gram of body weight.
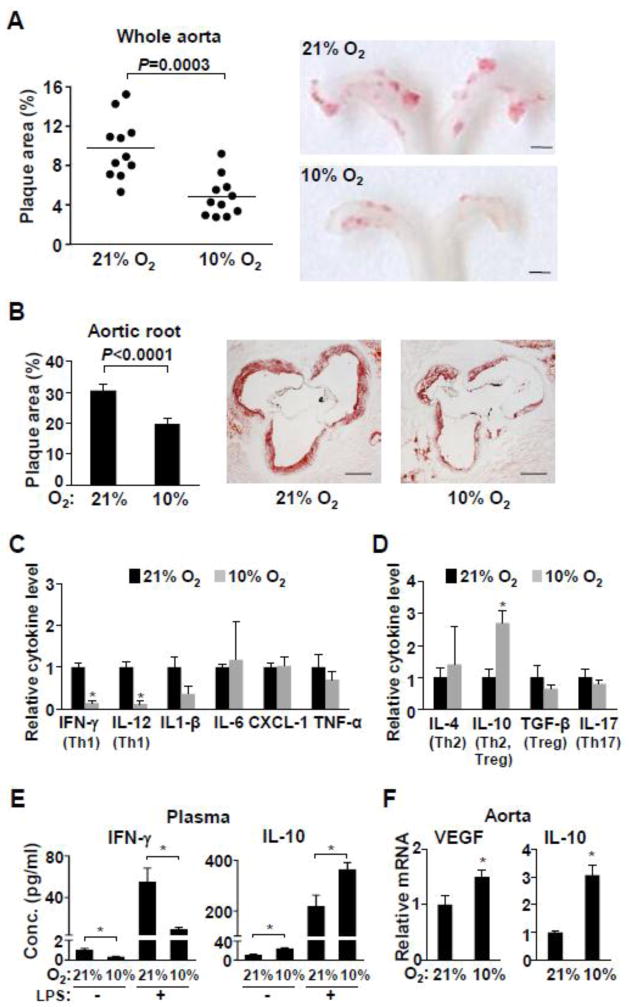
Fig. 1.
Low ambient oxygen reduces atherosclerosis and inflammation in ApoE−/− mice. ApoE−/− mice (5–6 wk old) were maintained in room air (21% O2) or placed in a hypoxia chamber (10% O2) for 22 wk prior to analyses (except for 1e). a The aortas were longitudinally dissected and atherosclerotic plaque areas (red) were quantified after staining with Sudan IV. Shown are representative images of the aortic arch where most of the plaques develop (n=11). Scale bars: 1 mm. b Aortic root cross sections were stained with Oil Red O and the stained plaque areas were measured (n=10–11). Representative aortic root sections are shown. Scale bars: 200 μm. c ApoE−/− mice were exposed to the indicated ambient oxygen for 22 wk and the proinflammatory cytokine level in plasma were measured by multiplex immunoassay kit (Meso Scale Discovery) (n=5). Cytokines that can serve as Th1 markers are indicated in brackets. d Levels of plasma cytokines that can serve as markers of other Th subtypes (indicated in brackets) were measured by individual immunoassay kits (Thermo and eBioscience) (n=5). e Effect of hypoxia on both basal and LPS-stimulated plasma cytokine levels in ApoE−/− mice. These mice were exposed to the indicated ambient oxygen for 5 wk prior to i.p. injection of LPS (n=5–11). f Hypoxia responsive VEGF and anti-inflammatory cytokine IL10 gene expression in aortic tissue were measured by real-time RT-PCR (n=6). Values shown as mean ± SEM, *p < 0.05.
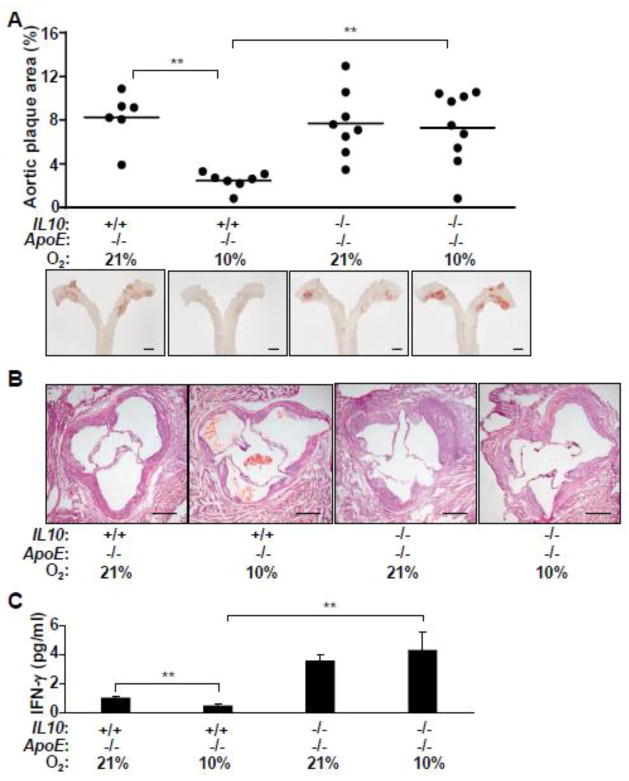
Fig. 4.
IL-10 is necessary for the decrease in atherosclerosis in low ambient oxygen. a Effect of IL-10 on aortic atherosclerotic plaque development in ApoE−/− mice chronically adapted to hypoxia. Mice of the indicated genotypes were maintained in either 21% O2 or 10% O2 for 22 wk. Atherosclerosis was quantified by staining whole aorta with Sudan IV (red, representative images). Scale bars: 1 mm. b Representative cross sections of the aortic root at the valve leaflet level were stained with H&E to show differences in the arterial wall (neointima and smooth muscle layers) depending on ambient oxygen exposure and IL-10 genotype. Scale bars: 200 μm. c Plasma IFN-γ levels were measured as marker of inflammatory activity in mice exposed to 10% or 21% O2 for 22 wk (n=5–7). **p< 0.01, one-way ANOVA with a Tukey posttest.
Cytokine and lipid measurements
Cytokine levels in plasma and media were measured using the following immunoassay kits: multiplex immunoassay kit (Meso Scale Discovery); IL-10 ELISA kit (Thermo Scientific); IL-4, IL-17, TGF-β and IFN-γ ELISA kit (eBioscience). Plasma lipids were measured using an enzymatic assay (Wako Chemicals USA, Inc.) on a ChemWell 2910 analyzer (Awareness Technology, Inc). Oxidized LDL cholesterol was measured using the OxLDL ELISA kit (CUSABIO Life Science).
Flow cytometry analysis
Blood, spleen and bone marrow cells were incubated with ACK to lyse the red blood cells, stained with conjugated CD11b antibody (Clone M1/70, BD-Biosciences), and analyzed using a Canto II flow cytometer (BD).
Lentiviral transduction and shRNA knockdown
Plasmids containing the sequences of non-specific shRNA and mouse HIF-1α shRNA (Open Biosystems) were used to prepare lentivirus according to manufacturer’s protocol (Sigma-Aldrich). CD4+ T cells were activated on immobilized anti-CD3 and anti-CD28 antibodies for 18 h prior to transduction with lentivirus as follows. The lentivirus and polybrene (6 μg/ml) were added to the T cell culture and centrifuged at 1000 × g at 30 °C for 1 h. The transduced cells were grown for 2 d followed by puromycin (2.5 μg/ml) selection.
mRNA quantification by real-time reverse transcriptase PCR (RT-PCR)
Total RNA from tissue was isolated using the RNeasy Kit (QIAGEN). mRNA from total RNA or cultured cell lysate was purified by binding to poly(dT) magnetic beads (Invitrogen), reverse transcribed using Superscript III (Invitrogen), and quantified by real-time PCR using SYBR green fluorescence on the 7900HT Sequence Detection System (Applied Biosystems) as previously described [17]. Cycle threshold (Ct) values were normalized to the housekeeping gene eukaryotic translation initiation factor EIF3F (TIF). Primer sequences are provided in the list of PCR primers (Online Methods).
Chromatin immunoprecipitation (ChIP) analysis
Hypoxia response elements (HRE consensus sequence, Pu(A/G)CGTG) were searched using the mouse IL10 gene sequence (NM_010548) from the promoter region (defined as −2000 bp upstream of the transcription start site) to the 3′ end of the gene (+702 bp from the stop codon) [18]. ChIP assay was carried out using ChIP-IT Express kit (Active Motif) according to the manufacturer’s protocol. Briefly, mouse CD4+ cells (5 × 106 cells) were cultured in 5% O2 incubator for 4 d and fixed in 1% formaldehyde for 10 min at room temperature, after which the nuclei were isolated, sonicated, immunoprecipitated (4 μg control rabbit IgG or anti-HIF1α antibody), and bound-DNA quantified by real-time PCR. The primer sequences of APOE (nonspecific genomic control) and IL10 HREs are provided in the list of PCR primers (Online Methods).
Telemetry and body mass composition analysis
Body composition (fat and muscle) of non-anesthetized mice was measured using the Bruker Minispec NMR analyzer (Bruker Optics). For activity and blood pressure measurements using telemetry, transmitters were implanted in 14 wk old mice and allowed to recover from the surgery for 2 wk. Blood pressure and physical activity were measured every 5 sec for 10 min (120 data points) at 3 a.m. (dark cycle) and 3 p.m. (light cycle), 3 d/wk for 5 wk.
Atherosclerotic plaque quantification and histology
For atherosclerotic plaque quantification, the whole aorta or aortic root at the base of the heart was dissected and stained with Sudan IV or Oil Red O solution, respectively, as previously described [13, 19]. The base of the heart was fixed in 10% formalin, frozen in OCT Compound (Tissue Teck), cross sections cut at the level of the aortic valves, and stained with either hematoxylin and eosin (H&E) for histology or Oil Red O for plaque area measurements. Paraffin-embedded aortic root cross sections were also used for H&E and Masson trichrome collagen staining. Plaque quantification was performed using the Image-J software (NIH) and confirmed by a second investigator blinded to the sample identities. The measurements were expressed as a fraction of the aortic wall surface area.
Statistical analysis
Data are reported as mean ± SEM and were analyzed by the two-tailed unpaired Student’s t test. One-way ANOVA with Tukey posttest analysis was performed for data containing more than two experimental groups.
Results
Lowering ambient oxygen reduces atherosclerosis in ApoE−/− mice
To examine the effect of lowering ambient oxygen on atherosclerosis, 5 wk old female ApoE−/− mice were housed in a large temperature- and humidity-controlled chamber in which the oxygen concentration was set at 10%, a level equivalent to that at ~5000 meters above sea level and compatible with long-term adaptation [15, 20]. Acclimation to this degree of hypoxia was reflected by the resumption of normal activity level, normalization of body weight, and increased blood hematocrit after a period of ~3–5 wk (Supplemental Fig. 1). Female mice were used because they have been reported to develop larger and more advanced atherosclerotic lesion when fed a normal chow diet over an extended period of time [21, 22]. Remarkably, exposure of the ApoE−/− mice to 10% O2 over a significant fraction of their lives reduced the aortic plaque area by approximately 40–50% of the control mice in 21% O2 (equivalent to room air at sea level) (Fig. 1a,b). Qualitative examination of stained aortic root cross sections showed decreased neointima formation and fibrotic collagen deposition in 10% O2 that was consistent with the decrease in lipid staining plaques (Supplemental Fig. 2).
Given the established role of inflammation in atherosclerosis, a plasma cytokine screen was performed, and it revealed significant changes in the levels of IFN-γ, IL-12 and IL-10 in ApoE−/− mice chronically exposed to 10% O2 for 22 wk (Fig. 1c,d). The pro-inflammatory cytokines IFN-γ and IL-12, which are associated with type 1 T helper (Th1) cells, were significantly lower in hypoxia. In contrast, the anti-inflammatory cytokine IL-10, known to be regulated by hypoxia and to inhibit Th1 cells and atherosclerosis [9, 23–25], was significantly higher in 10% O2. No significant difference was detected in the plasma levels of TGF-β and IL-17, which can serve as Treg and Th17 markers, respectively (Fig. 1d). To confirm the reciprocal response of IFN-γ and IL-10 in mice adapted to hypoxia, ApoE−/− mice were acclimated to 10% O2 for 5 wk and then challenged with lipopolysaccharide (LPS) which can elicit both Th1 and Th2 responses (Fig. 1e) [26]. Even with the shorter exposure time, the IFN-γ level was decreased while IL-10 level was increased either before or after LPS treatment, confirming the cytokine screen data of chronically (22 wk) adapted mice (Fig. 1c–e). Additionally, VEGF and IL10 mRNA expression in aortic tissue were significantly increased suggesting relative tissue hypoxia and suppression of localized inflammatory activity at the disease site (Fig. 1f).
Effect of lowering ambient oxygen on cardiometabolic factors in ApoE−/− mice
Given this marked effect on aortic plaque development, we examined whether some of the known cardiac risk factors associated with human atherosclerosis may be altered in ApoE−/− mice by low ambient oxygen. There was no significant change in body weight, fat content, systemic blood pressure (both in dark and light cycles), or basal activity that could contribute to the decrease in atherosclerosis (Table 1 and Supplemental Fig. 1a). Notably, despite the decrease in atherosclerosis, cholesterol (total and LDL/VLDL fractions) and triglyceride levels were significantly increased in 10% O2 while the level of pro-atherogenic oxidized LDL was unchanged (Table 1). This pattern of increased total serum lipid under hypoxia has been reported in mice exposed to hypoxia and may be consistent with the expected decrease in fatty acid oxidation under oxygen limiting conditions [3, 4]. The apparent lack of a positive relationship between atherosclerosis and serum lipid levels or the relative fraction of myeloid cells (Supplemental Fig. 3), suggested that other factors may be involved in mediating the anti-atherogenic effect of low ambient oxygen.
Table 1.
Effect of ambient oxygen on cardiometabolic factors in ApoE−/− mice
| Parameters | 21% O2 | 10% O2 | P-value |
|---|---|---|---|
| Body weight (g) | 24.2 ± 0.3 | 24.0 ± 0.4 | 0.7 |
| Fat Mass (%) | 10.2 ± 0.8 | 9.0 ± 0.2 | 0.4 |
| Systolic blood pressure (mmHg) | |||
| Dark | 131.9 ± 10.3 | 131.9 ± 4.8 | 1 |
| Light | 110.3 ± 3.6 | 118.9 ± 4.7 | 0.2 |
| Diastolic blood pressure (mmHg) | |||
| Dark | 99.6 ± 7.0 | 105.4 ± 4.0 | 0.5 |
| Light | 79.4 ± 5.5 | 91.5 ± 3.3 | 0.1 |
| Total cholesterol (mg/dL) | 384.4 ± 19.1 | 536.5 ± 35.4 | 0.009 |
| HDL (mg/dL) | 166.9 ± 9.6 | 178.5 ± 12.5 | 0.5 |
| LDL/VLDL (mg/dL) | 247.2 ± 15.0 | 351.7 ± 38.0 | 0.049 |
| Triglycerides (mg/dL) | 55 ± 10.7 | 157 ± 33.9 | 0.001 |
| OxLDL (μmole/ml) | 6.2 ± 0.1 | 6.3 ± 0.2 | 0.6 |
Female ApoE−/− mice were exposed to the indicated oxygen concentration for 22 wk after which plasma lipids, body weight and composition were determined (n = 5–22). Blood pressure was monitored by telemetry during 12-h dark and light cycles after a 5-wk acclimation period to 10% O2 (n = 4).
Lowering oxygen exposure increases anti-inflammatory IL-10 expression
The increased plasma level of IL-10 in 10% O2 caught our attention because the IL10 gene has been reported to have HIF-1α binding sequences and its expression is induced by HIF-1α [10, 24]. IL-10 plays diverse immune regulatory roles and is expressed by various cell types, but its release by CD4+ Th2 cells, which suppresses inflammatory Th1 cells, appeared pertinent to our current observation [23, 25]. In fact, a comprehensive survey of mouse atherosclerosis studies concluded that IL-10 was the most consistently anti-atherogenic cytokine [9]. We therefore isolated CD4+ T cells from the spleen of mice adapted to 21% or 10% O2 and measured the gene expression of the respective T helper cell markers. Consistent with elevated IL-10 in mouse plasma, the mRNA levels of Th2 cytokines IL-4 and IL-10 were significantly increased under the low ambient oxygen condition (Fig. 2a). The Th17 and Treg markers represented by IL-17 and FOXP3, respectively, were unchanged and consistent with the plasma cytokine levels in hypoxia adapted mice (Fig. 2a). The unchanged levels of FOXP3 mRNA and plasma TGF-β levels make it less likely that the IL-10 increase originates from the Treg population under hypoxia.
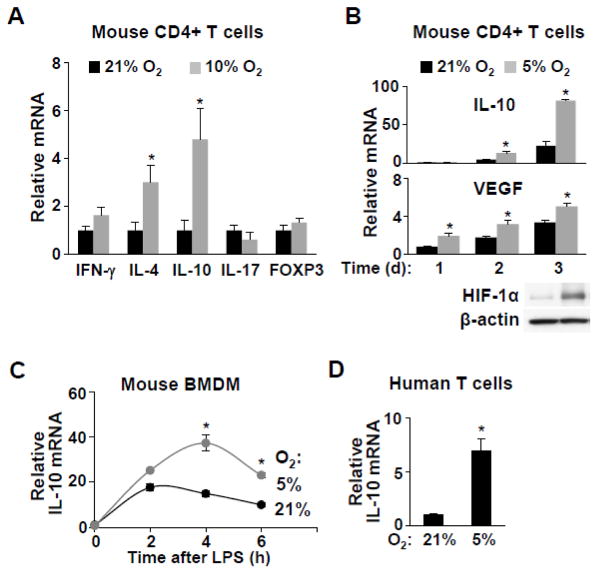
Fig. 2.
Hypoxia increases anti-inflammatory cytokine IL-10 expression in vivo and in vitro. a Mouse CD4+ T cells were isolated from spleens of ApoE−/− mice chronically adapted to the indicated ambient oxygen condition and the mRNA levels of the following genes were measured as markers of the respective T cell subtypes: INF-γ (Th1); IL-4 (Th2); IL-10 (Th2 and Treg); IL-17 (Th17); and FOXP3 (Treg) (n=6). b Effect of hypoxia (5% O2, 1 to 3 d exposure) on IL-10 mRNA levels in cultured ApoE−/− mouse CD4+ T cells. Relative hypoxia was confirmed by the increase in HIF-1α protein and the expression of its target gene VEGF (n=3). c Effect of hypoxia on the time course of IL-10 expression in cultured mouse bone marrow-derived macrophages (BMDM) after lipopolysaccharide (LPS) stimulation (n=3). d IL-10 expression in human peripheral blood T cells cultured for 3 d under the indicated oxygen conditions (n=3). Values shown as mean ± SEM, *p < 0.05.
To replicate the in vivo observation in a more defined system, mouse CD4+ T cells were cultured in 5% O2, which is considered to be more physiologic, and relative hypoxia was confirmed by the increase in HIF-1α protein and its target gene VEGF mRNA expression (Fig. 2b) [27]. As observed in vivo, culturing T cells in 5% O2 increased IL10 expression by mRNA as well as protein release into culture media (Fig. 2b, Supplemental Fig. 4). Hypoxia-induced IL10 expression was also confirmed in ApoE−/− mouse bone marrow derived macrophages (BMDM) and in human T cells (Fig. 2c,d). As ApoE itself has been reported to have effects on immune function, we used ApoE+/+ mice to show further that hypoxia-inducible IL-10 expression was not limited to the mouse atherosclerosis model both in vivo and in vitro (Supplemental Fig. 5) [28].
IL10 expression is regulated by HIF-1α in CD4+ T cells
IL10 gene expression is known to be regulated by various transcription factors including HIF-1α [10, 24, 29]. Accordingly, in primary mouse splenic CD4+ T cells, the HIF-1α stabilizing agent deferoxamine (DFO) dose-dependently increased the mRNA levels of IL10 as well as VEGF, a marker of HIF-1α activity (Fig. 3a). Conversely, knockdown of HIF-1α in both mouse CD4+ T cells and Raw 264.7 macrophages attenuated the expression of IL10, indicating that HIF-1α is a general mediator of IL10 transcription under hypoxia (Fig. 3b,c).
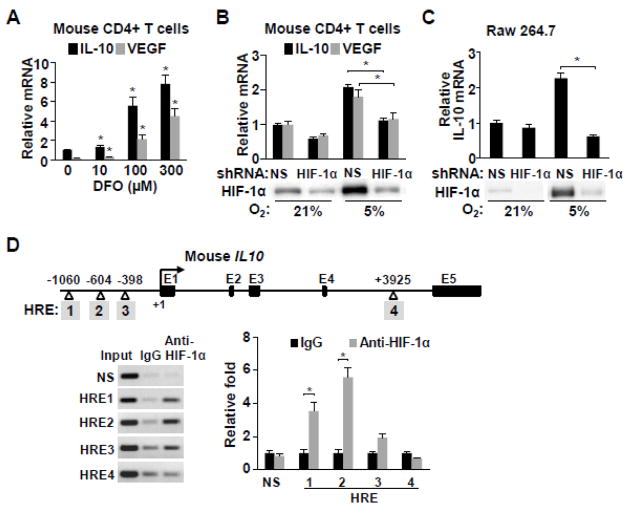
Fig. 3.
IL10 gene is regulated by HIF-1α. a HIF-1α protein was stabilized by deferoxamine (DFO) in mouse CD4+ T cells in a dose-dependent manner and IL-10 mRNA levels quantified by real-time RT-PCR (n=3). b Hypoxia (5% O2) induced HIF-1α protein was knocked down in mouse CD4+ T cells using shRNA and IL-10 mRNA levels were measured. Nonspecific shRNA (NS) was used as control (n=3). c Hypoxia (5% O2) induced HIF-1α protein was knocked down in the Raw 264.7 macrophage cells using shRNA and IL-10 mRNA levels were measured. Nonspecific shRNA (NS) was used as control (n=3). d The sites of candidate hypoxia response elements (HRE, consensus sequence Pu(A/G)CGTG) in the promoter and intron 4 sequences of the mouse IL10 gene are numbered in shaded boxes. HIF-1α interaction with the putative HRE sites was examined by chromatin immunoprecipitation and quantified using real-time PCR in mouse CD4+ cells cultured in a 5% O2 incubator. Values shown as mean ± SEM, *p < 0.05.
Genomic sequence analysis revealed four putative HIF-1α-interacting hypoxia response elements (HRE consensus sequence, Pu(A/G)CGTG) in the promoter and intron regions of the mouse IL10 gene, one of which had been reported (HRE3) (Fig. 3d) [24]. Chromatin immunoprecipitation (ChIP) analysis of mouse CD4+ cells cultured in 5% O2 showed that HIF-1α interacts with HRE1 and HRE2 but less strongly with HRE3 sequence (Fig. 3d). HRE4 in intron 4 did not interact with HIF-1α, although another HRE located 3′ of the last IL10 exon has been reported to interact with both HIF-1α and HIF-1β in mouse C2C12 myocytes subjected to cyclic hypoxia-reoxygenation for modeling remote ischemic preconditioning (Fig. 3d) [24]. This suggests that the HRE elements of IL10 may be differentially utilized depending on the type of cell and hypoxic stimulus. Taken together, the current results indicate that the protection against atherosclerosis under reduced ambient oxygen may be mediated in part by the anti-inflammatory activities of IL10 transactivated by HIF-1α.
IL-10 is necessary for preventing atherosclerosis in low ambient oxygen
To examine the role of IL-10 in atherosclerosis under low ambient oxygen, we generated IL10−/− mice in an ApoE−/− genetic background (IL10−/− APOE−/− double knockout (DKO) mouse). Littermates of these DKO mice were maintained in 10% or 21% O2 for approximately 22 wk. Unlike in ApoE−/− mice, the reduction in aortic plaque development associated with chronic hypoxia was no longer evident in DKO mice, indicating that IL-10 is necessary for mediating this decrease in atherosclerosis (Fig. 4a,b). It should be noted here that the level of atherosclerosis in relatively young female ApoE−/− mice has been reported to be increased in the absence of IL-10 but that this effect was not evident in older mice as also observed here in the current study (Fig. 4a) [30]. The measurement of plasma IFN-γ as a marker of systemic inflammatory activity also showed loss of its suppression by hypoxia in the absence of IL-10 (Fig. 4c). Decreased IFN-γ in hypoxia likely contributes to the reduction in atherosclerosis, but it is unlikely to be the only factor as IL-10 can regulate various immune cell types and activities [14, 25, 31].
Discussion
In the current study, we have demonstrated protection from aortic plaque development in a mouse model of atherosclerosis by long-term adaptation to a low ambient oxygen environment. This observation parallels human epidemiologic data showing decreased coronary artery disease at high altitudes for which the molecular basis is unclear. ApoE−/− mice adapted to 10% oxygen displayed evidence of increased anti-inflammatory activity as measured by higher IL-10 levels, at both the systemic and localized levels. Mechanistically, we have identified multiple HIF-1α binding motifs in the IL10 gene and showed that its transactivation under hypoxia is dependent on HIF-1α in both mouse and human immune cells, complementing previous studies [10, 24, 29]. As genetic evidence that IL-10 mediates the anti-atherogenic effect of low ambient oxygen, ApoE−/− mice that are deficient in IL-10 are not protected against atherosclerosis by chronic hypoxia.
Although oxygen deprivation sufficient to cause cellular injury is clearly pro-inflammatory, physiologically adaptable low ambient oxygen appears to have markedly different outcomes likely depending on various factors including the degree and duration of hypoxia. Mountain sickness has often been cited as evidence of hypoxia promoting inflammation, but it is a phenomenon that occurs relatively acutely with altitude change and only in a subset of exposed individuals [32]. In the absence of such an overt pro-inflammatory clinical syndrome or acute fluctuations in tissue oxygenation, the long term consequences on the immune state of individuals who are chronically adapted to a less severe degree of hypoxia may be quite different.
Our current observation also differs from a report of increased plaque formation in ApoE−/− mice exposed to 10% O2, but it should be noted that the mice were followed for only 3 wk (age 6 to 9 wk) which is the minimum time required for physiological acclimation as indicated by activity level and growth (Supplemental Fig. 1a,b) [3]. Analysis of our mice on a normal (non-high fat) chow diet after 3 wk of hypoxia exposure did not reveal significant difference in plaque formation while the longer exposure (22 wk) ameliorated atherosclerosis progression (Supplemental Fig. 6). On the other hand, supraphysiologic levels of oxygen (95%) have been reported to decrease necrotic core size, but this phenomenon likely involves mechanisms different from those in our current study [33]. Because atherosclerosis in different arterial trees such as the innominate artery can be more under the influence of the immune system and model the human coronary arteries, it will be useful to confirm the effects of long-term reduced ambient oxygen exposure in this model [34]. Furthermore, here we have identified HIF-1α-inducible IL-10 as a mediator of the phenomenon in ApoE−/− mouse, but the effect of other factors that may also contribute to the immune suppressive effects of high altitude adaptation such as hypoxia-adenosine signaling and even erythropoietin cannot be ruled out [35–37].
It is also tempting to speculate that some of the counterintuitive, clinical observations regarding the benefits of reduced oxygen exposure may be related to our study. Patients with asthma, an inflammatory airway disease, have been reported to benefit from high altitude exposure beyond the effect of allergen avoidance and to show trends of increased anti-inflammatory markers including IL-10 [38]. In both acute myocardial infarction and heart failure where insufficient oxygenation and inflammation play critical roles in their pathogenesis, there are reports of the benefits of limiting oxygen supplementation in these conditions [39, 40]. Recently published work in the immunology field further confirms the profound importance of hypoxia regulated IL-10 in the immune suppression of T cells [41, 42]. Taken together, our study suggests that physiologically adaptable hypoxia may have beneficial effects by promoting an anti-inflammatory effect via IL-10 with potential clinical implications for preventing or delaying atherosclerosis and other inflammatory diseases.
Supplementary Material
KEY MESSAGES.
Chronic low ambient oxygen exposure decreases atherosclerosis in mice.
Anti-inflammatory cytokine IL-10 levels are increased by low ambient O2.
This is consistent with the established role of HIF-1α in IL-10 transactivation.
Absence of IL-10 results in the loss of the anti-atherosclerosis effect of low O2.
This mechanism may contribute to decreased atherosclerosis at high altitudes.
Acknowledgments
We wish to thank members of our laboratory Cory U. Lago, William M. Kamp, Jerry J. Li and Jie Zhuang for helpful assistance and critical comments. We also thank the mouse facility staff for hypoxia chamber maintenance and Elias Gonzalez and Bruce Bishop for technical assistance.
Sources of Funding
Research supported by the Division of Intramural Research, National Heart, Lung, and Blood Institutes (NHLBI), National Institutes of Health.
Footnotes
Disclosures
None.
Author contributions
Concept and design: JGK, HS, JYK, PYW, PH
Contributed data: JGK, HS, MJA, MP, MDA, AN, DS, JC, JP
Analysis and interpretation of data: JGK, AR, AN, JP, JC, PYW, PH
Drafting of manuscript: JGK, PYW, PH
References
- 1.Mortimer EA, Jr, Monson RR, MacMahon B. Reduction in mortality from coronary heart disease in men residing at high altitude. N Engl J Med. 1977;296:581–585. doi: 10.1056/NEJM197703172961101. [DOI] [PubMed] [Google Scholar]
- 2.Faeh D, Gutzwiller F, Bopp M. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation. 2009;120:495–501. doi: 10.1161/CIRCULATIONAHA.108.819250. CIRCULATIONAHA.108.819250 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Nakano D, Hayashi T, Tazawa N, Yamashita C, Inamoto S, Okuda N, Mori T, Sohmiya K, Kitaura Y, Okada Y, et al. Chronic hypoxia accelerates the progression of atherosclerosis in apolipoprotein E-knockout mice. Hypertension research: official journal of the Japanese Society of Hypertension. 2005;28:837–845. doi: 10.1291/hypres.28.837. [DOI] [PubMed] [Google Scholar]
- 4.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P, Ohta A, Lentsch AB, Lukashev D, Sitkovsky MV. Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS One. 2007;2:e853. doi: 10.1371/journal.pone.0000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Shoshan J, Afek A, Maysel-Auslender S, Barzelay A, Rubinstein A, Keren G, George J. HIF-1alpha overexpression and experimental murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:665–670. doi: 10.1161/ATVBAHA.108.183319. [DOI] [PubMed] [Google Scholar]
- 11.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 12.Wang PY, Ma W, Park JY, Celi FS, Arena R, Choi JW, Ali QA, Tripodi DJ, Zhuang J, Lago CU, et al. Increased oxidative metabolism in the Li-Fraumeni syndrome. N Engl J Med. 2013;368:1027–1032. doi: 10.1056/NEJMoa1214091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang JG, Amar MJ, Remaley AT, Kwon J, Blackshear PJ, Wang PY, Hwang PM. Zinc finger protein tristetraprolin interacts with CCL3 mRNA and regulates tissue inflammation. J Immunol. 2011;187:2696–2701. doi: 10.4049/jimmunol.1101149. jimmunol.1101149 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 15.Sung HJ, Ma W, Starost MF, Lago CU, Lim PK, Sack MN, Kang JG, Wang PY, Hwang PM. Ambient oxygen promotes tumorigenesis. PLoS One. 2011;6:e19785. doi: 10.1371/journal.pone.0019785. PONE-D-11-00018 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci U S A. 2012;109:1239–1244. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patino WD, Kang JG, Matoba S, Mian OY, Gochuico BR, Hwang PM. Atherosclerotic plaque macrophage transcriptional regulators are expressed in blood and modulated by tristetraprolin. Circ Res. 2006;98:1282–1289. doi: 10.1161/01.RES.0000222284.48288.28. [DOI] [PubMed] [Google Scholar]
- 18.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 19.Maganto-Garcia E, Tarrio M, Lichtman AH. Mouse models of atherosclerosis. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 15. Chapter 15. 2012. pp. 24pp. 11–23. [DOI] [PubMed] [Google Scholar]
- 20.West JB. Highest permanent human habitation. High Alt Med Biol. 2002;3:401–407. doi: 10.1089/15270290260512882. [DOI] [PubMed] [Google Scholar]
- 21.Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Caligiuri G, Nicoletti A, Zhou X, Tornberg I, Hansson GK. Effects of sex and age on atherosclerosis and autoimmunity in apoE-deficient mice. Atherosclerosis. 1999;145:301–308. doi: 10.1016/s0021-9150(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354:1166–1176. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 24.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110:17462–17467. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X, Boisvert WA. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb Haemost. 2015;113:505–512. doi: 10.1160/TH14-06-0509. [DOI] [PubMed] [Google Scholar]
- 26.McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Critical reviews in immunology. 2008;28:281–299. doi: 10.1615/critrevimmunol.v28.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkuri KR, Herzenberg LA, Niemi AK, Cowan T. Importance of culturing primary lymphocytes at physiological oxygen levels. Proc Natl Acad Sci U S A. 2007;104:4547–4552. doi: 10.1073/pnas.0611732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali K, Middleton M, Pure E, Rader DJ. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ Res. 2005;97:922–927. doi: 10.1161/01.RES.0000187467.67684.43. [DOI] [PubMed] [Google Scholar]
- 29.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 30.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 31.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsch E, Theelen TL, Demandt JA, Jeurissen M, van Gink M, Verjans R, Janssen A, Cleutjens JP, Meex SJ, Donners MM, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–2553. doi: 10.1161/ATVBAHA.114.304023. [DOI] [PubMed] [Google Scholar]
- 34.Getz GS, Reardon CA. Use of Mouse Models in Atherosclerosis Research. Methods Mol Biol. 2015;1339:1–16. doi: 10.1007/978-1-4939-2929-0_1. [DOI] [PubMed] [Google Scholar]
- 35.Meehan RT. Immune suppression at high altitude. Annals of emergency medicine. 1987;16:974–979. doi: 10.1016/s0196-0644(87)80743-6. [DOI] [PubMed] [Google Scholar]
- 36.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and redirection of the immune response. Trends in immunology. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Lu KY, Ching LC, Su KH, Yu YB, Kou YR, Hsiao SH, Huang YC, Chen CY, Cheng LC, Pan CC, et al. Erythropoietin suppresses the formation of macrophage foam cells: role of liver X receptor alpha. Circulation. 2010;121:1828–1837. doi: 10.1161/CIRCULATIONAHA.109.876839. [DOI] [PubMed] [Google Scholar]
- 38.Karagiannidis C, Hense G, Rueckert B, Mantel PY, Ichters B, Blaser K, Menz G, Schmidt-Weber CB. High-altitude climate therapy reduces local airway inflammation and modulates lymphocyte activation. Scandinavian journal of immunology. 2006;63:304–310. doi: 10.1111/j.1365-3083.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- 39.Saeed O, Bhatia V, Formica P, Browne A, Aldrich TK, Shin JJ, Maybaum S. Improved exercise performance and skeletal muscle strength after simulated altitude exposure: a novel approach for patients with chronic heart failure. Journal of cardiac failure. 2012;18:387–391. doi: 10.1016/j.cardfail.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, et al. Air Versus Oxygen in ST-Segment Elevation Myocardial Infarction. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 41.Shehade H, Acolty V, Moser M, Oldenhove G. Cutting Edge: Hypoxia-Inducible Factor 1 Negatively Regulates Th1 Function. J Immunol. 2015;195:1372–1376. doi: 10.4049/jimmunol.1402552. [DOI] [PubMed] [Google Scholar]
- 42.Vuillefroy de Silly R, Ducimetiere L, Yacoub Maroun C, Dietrich PY, Derouazi M, Walker PR. Phenotypic switch of CD8(+) T cells reactivated under hypoxia toward IL-10 secreting, poorly proliferative effector cells. Eur J Immunol. 2015;45:2263–2275. doi: 10.1002/eji.201445284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.